Highly concentrated MoS2 nanosheets in water achieved by thioglycolic acid as stabilizer and used as biomarkers†
Rajeshkumar Anbazhagan‡
a,
Hsing-Ju Wang‡b,
Hsieh-Chih Tsai*a and
Ru-Jong Jeng*b
aGraduate Institute of Applied Science and Technology, National Taiwan University of Science and Technology, Taipei, Taiwan. E-mail: h.c.tsai.@mail.ntust.edu.tw; Tel: +886-2-27303779
bInstitute Polymer Science and Engineering, National Taiwan University, Taipei, Taiwan. E-mail: rujong@ntu.edu.tw; Tel: +886-2-33665884
First published on 29th August 2014
Abstract
In this work, we reported the synthesis of water soluble MoS2 quantum dots from the monolayer nanosheets of MoS2, using thioglycolic acid (TGA) with a sonication method. The gaps between the MoS2 layers were enlarged by the stirring process. Meanwhile TGA molecules strongly binding to the defect sites of MoS2 would reduce the van der Waals force between the MoS2 layers through sonication. This led to the improved dispersion of MoS2 monolayers in water. The addition of TGA molecules not only exfoliated the bulk of MoS2, but also modified the surface of MoS2 with carboxylic acid groups. As a result, highly concentrated MoS2 nanosheets were produced in water. Subsequently, heavy metal-free MoS2 quantum dots with sustained fluorescence emission, which were fabricated from hydrothermal treatment of MoS2 monolayers, can be utilized as cell biomarkers.
Introduction
Since the discovery of graphene, the two-dimensional inorganic materials like transition metal dichalcogenides (MoS2, MoSe2 and WS2) have attracted great attention; particularly molybdenum disulphide (MoS2) is of great interest in the areas of catalysis,1 nanotribology,2 optics3 and energy storage.4 MoS2 nanomaterials exhibit unique properties such as large intrinsic band gaps and high carrier mobility (1–40 cm2 V−1 s−1) when exfoliated as monolayers. Bulk MoS2 has a band gap about 1.2 eV. When it became monolayer, the band gap increased to 1.9 eV. The monolayers of MoS2 possessing strong photoluminescence can be applied as photothermal agents,5 bio-sensor6 and drug carrier.7 The MoS2 monolayers also have a very high surface to mass ratio with ultrathin structure. The large surface to mass ratio creates the possibility of loading a large amount of drug. When compared to the MoS2 multi-layer, the MoS2 monolayer possesses a higher luminescent efficiency. The luminescent efficiency increases with decreasing layer number.8 In addition, MoS2 quantum dots (MoS2-QD) can be obtained when the sizes of exfoliated MoS2 monolayers are controlled to be less than 100 nm. This would induce the quantum confinement effect of MoS2 monolayers, leading to photoluminescence emission.9 In cell or tissue bioimaging experiment, it requires the use of dye-containing particles for entering cells. Because of this, the particle sizes should be in nanometer (less than 100 nm).10 For example, in in vivo experiment, the particle size is more critical and preferably less than 100 nm to avoid the capture by the reticuloendotheial system. Despite some successes in the synthesis of MoS2 quantum dots,11 these methodologies have limitations to be overcome. The production of MoS2 quantum dots is typically based on bi- or multi-layer MoS2 precursors. Furthermore, excessive layer aggregation of MoS2 would further reduce the photoluminescence properties of MoS2. For the preparation of highly photoluminescent MoS2-QD, the exfoliation of bulk MoS2 into monolayer nanosheets is an important step.Several approaches had been attempted to produce the monolayer nanosheets. A micromechanical cleavage was reported to produce high quality monolayers with low yields.12 Moreover, liquid-phase exfoliation is one of the most widely used methods to achieve moderate yields.13 However; this process requires hazardous organic solvents and a long sonication time. Apart from that, ion intercalation method is also effective in preparing MoS2 monolayers, but these monolayers tend to comprise metallic MoS2.8 This is inappropriate for a range of applications such as, drug carrier, bio-probe, etc. MoS2 monolayers can also be achieved by hydrothermal exfoliation, but the issues of high temperature and oxygen sensitivity require special attention.14 In addition, surfactants are often used to exfoliate the two-dimensional materials, owing to the presence of van der Waals binding and electrostatic stabilization.15 Although the hydrophobic nature of bulk MoS2 can be tuned through the surfactant exfoliation, the colloid stability of surfactant-exfoliated MoS2 still presents a challenge to be solved.16 In fact, chemically reactive sites are present on the surface of bulk MoS2 (particularly sulphur vacancy).17 The number of reactive sites would further increase when bulk MoS2 are exfoliated to few layers or monolayers. The high molecular affinity of their active sites on MoS2 has attracted great attention.18 Because of the surface versatility on MoS2, one can introduce various functionalization strategies accompanied with exfoliation process to obtain well dispersed MoS2 monolayers in different solvents (including water). Based on the above, effective exfoliation and chemical functionalization of MoS2 monolayers would be advantageous and innovative for bio-applications.
In this paper, we report that concurrent liquid-phase exfoliation and functionalization of MoS2 was successfully achieved using thioglycolic acid (TGA) as an efficient bi-functional ligand.19 TGA plays the role as an exfoliation ligand to produce nanosheets. Moreover, TGA would link to MoS2 via thiol chemistry with carboxylic acid end-groups which are supposed to convert hydrophobic nanosheets into hydrophilic ones in an aqueous solution. To the best of our knowledge, this is the first work presenting the exfoliation of MoS2 in water and subsequent preparation of water soluble MoS2-QD. MoS2-QD is highly potential for bioimaging applications due to its hydrophilic functional groups with bio-compatibility and the quantum confinement effect with fluorescence characteristics.
Before the addition of TGA to the water–MoS2 system, the MoS2 was insoluble due to its hydrophobic nature. However, after the addition of TGA to the water–MoS2 system, the MoS2 monolayers became soluble (hydrophilic) and were well suspended in water because the hydrophobic surface of MoS2 was covered with acid (COOH) end group of TGA (Fig. 1a). The large amount of well suspended MoS2 monolayers in water was prepared by stirring at room temperature and simple ultra-sonication. Infrared spectroscopy was used to identify the structure of MoS2 with the addition of TGA. The presence of carbonyl and hydroxyl peaks at 1713 and 3400 cm−1, respectively, and the disappearance of thiol peak at 2561 cm−1 indicate that the surface of MoS2 was successfully modified with TGA (Fig. 1b). The prepared MoS2 was further analyzed by UV-visible spectroscopy. The UV-visible absorption spectrum of a dilute modified MoS2 solution rendered a peak at 420 cm−1 owing to convoluted excitonic peaks while the peaks emerged from the k point of Brillouin zone were observed at 620 and 670 cm−1. This result indicates that these suspended MoS2 monolayers exhibited pristine semiconductor 2H–MoS2 phase unlike the ones from the ion intercalation method (Fig. 1c).13 It is important to note that a high yield of MoS2 nanosheets could also be achieved in this work as confirmed by UV spectroscopy. The mass of MoS2 monolayer in the water solution (after dialysis) allows one to define the yields of MoS2 monolayers. The concentration (yield) of exfoliated MoS2 monolayers was 3.49 mg mL−1 in water. In fact, the concentration of MoS2 monolayers would increase with increasing sonication time (ESI†). The colloidal stability of dispersion was investigated by measuring zeta potential. The values (−88 mV for pristine MoS2 and −47 mV for TGA-modified MoS2) of MoS2 implies that the surface of MoS2 was successfully modified by TGA (Fig. 1d). This is consistent with the result reported in literature.20 Moreover, the colloidal stability of TGA–MoS2 was evaluated for more than 3 months; TGA–MoS2 monolayers remained well dispersed afterwards.
In Raman spectrum of bulk MoS2, a notable in-plane (E12g) peak at 383 cm−1 and an out-plane peak (A1g) at 407 cm−1 were observed. The former one is due to in-plane sulphur molybdenum vibration and the later one is due to out-plane sulphur vibrations. As MoS2 was exfoliated to monolayers, the frequency of in-plane mode increased and frequency of out-plane mode decreased. The gap between the frequencies of in-plane and out-plane modes depends on the method of exfoliation. A closing gap implies that the monolayer structure is more predominant. In the exfoliated TGA–MoS2 monolayers, in-plane and out-plane peaks were observed at 385 cm−1 and 406 cm−1, respectively (Fig. 1e). This indicates the presence of MoS2 monolayer and few layer structure.21 The gap of E12g, A1g peaks in Fig. 2a was somewhat broadening when compared to those of the mechanically exfoliated molybdenum disulphide monolayers.22 This might be due to the larger thicknesses of MoS2 monolayers caused by the presence of TGA on the surfaces. XRD was used to identify the crystal structure of the exfoliated MoS2 monolayers. The pristine molybdenite showed a very sharp peak at 14.8° (002), and peaks with lower intensity at 39.5° (103) and 49.8° (105). The XRD pattern of exfoliated MoS2 monolayers displayed a peak only at 14.8° corresponding to the (002) plane of 2H–MoS2. In addition, the absence of (001) peak at 32.8° indicates that the majority of exfoliated dispersions exhibited the single layer nanosheets (Fig. 1f).23
The morphology of the functionalized MoS2 was investigated by TEM. The results indicate the lengths of the nanosheets ranged from 100 to 500 nm. Moreover, most of the nanosheets were transparent to the electron beam (Fig. 2a). This is because of the ultrathin structure of exfoliated MoS2 monolayers. The electron diffraction pattern of the TEM image was utilized to confirm the symmetry of the exfoliated materials, indicating that each molybdenum disulphide monolayer retained its hexagonal symmetry just like bulk MoS2 crystals (Fig. 2b). These exfoliated materials possessing a honeycomb-like structure without any defects was observed in a HR-TEM image (inset image of Fig. 2b).24 In addition, a large number of nanosheets deposited in the holey carbon grid were also observed in Fig. 2c. TEM results imply that TGA played the role of a ligand for the effective exfoliation of MoS2. It is noteworthy that a robust and high yield production of MoS2 monolayers could be achieved via the thiol chemistry between TGA and MoS2. An AFM image of the TGA–MoS2 nanosheets is shown in Fig. 2d. The topographic height of the exfoliated MoS2 material was around 1 nm (Fig. 2e), indicating the presence of monolayers. Fig. 2f illustrates the size distribution of the quantum dots. The prepared MoS2-QD exhibited sizes ranged from 5 to 10 nm (the average diameter range is about 7 nm).
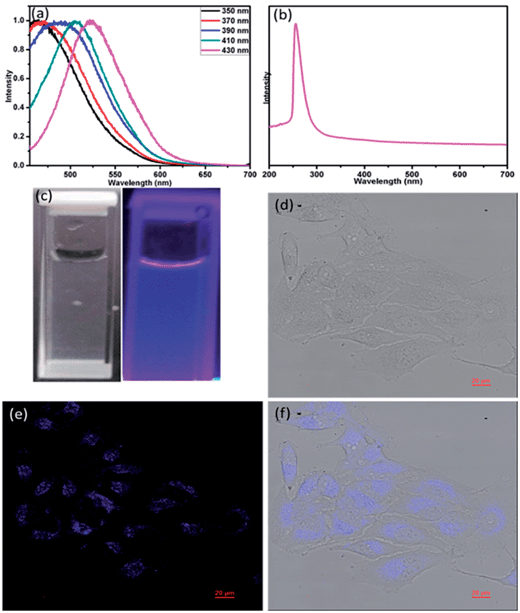
Due to the excellent optoelectronic properties of two-dimensional MoS2 nanomaterials, we first set out to explore the possibility of MoS2-QD bio-probe for human HeLa cancer cells. Fig. 3a presents the fluorescence spectrum of the MoS2-QD and the absorption spectrum was shown in the Fig. 3b. Different fluorescence emissions normally depend on the particle size of the quantum dot. This result implies the prepared MoS2-QD also exhibited polydispersity.8 The fluorescence emissions of the prepared quantum dots started from an excitation wavelength at 350 to 430 nm. In addition, the maximum fluorescence wavelength was around 440 nm with an excitation wavelength of 360 nm. The Fig. 3c shows the photograph of MoS2-QD the visible and UV light. MoS2-QD uptake and bio-imaging application were investigated by a confocal fluorescence microscope. The confocal image of MoS2-QD internalized by HeLa cells was observed in Fig. 3d–f. The presence of the blue color in the region inside the HeLa cell indicates the successful uptake of MoS2-QD through the cell membrane. Furthermore, MoS2-QD exhibited impressive biocompatible properties given the fact that the HeLa cell remained alive with the addition of MoS2-QD. This result implies that MoS2-QD could be biocompatible, used as biomarker and some other biomedical applications.
Conclusion
An effective exfoliation process of MoS2 into single layer nanosheets with high yields has been developed in this work. In this process, TGA not only played the role of an exfoliation ligand to produce MoS2 monolayers, but also linked to MoS2 via thiol chemistry with carboxylic acid end-groups. Because of this, bulk MoS2 could be exfoliated in water and subsequent preparation of water soluble MoS2-QD. Due to the low cytotoxicity and excellent bio-compatibility, MoS2-QD was demonstrated to be an eco-friendly material as well as a biolabeling agent.Experimental section
Preparation of MoS2 nanosheet
In a typical experiment, 1.86 gram of TGA and 400 mg of molybdenum disulphide were placed (the optimization concentration of TGA for this reaction was added in ESI†) in a 25 mL glass vial. The reaction mixture was then stirred for 24 h. Subsequently 20 mL of fresh water was added to the reaction mixture, which was sonicated for 2 h (Sonicator: DELTA, DC400H, 400 W). After sonication, the reaction mixture was centrifuged at the speed of 1500 rpm to remove undesirable aggregated materials. The supernatant was collected and placed in the dialysis membrane. Dialysis was performed for 48 h to remove the excess of TGA with a cut-off molecular weight at 1000 Da.Preparation of MoS2 quantum dot
MoS2-QD was prepared by refluxing of MoS2 monolayers in water for 24 h.8 The resulting reaction mixture was filtered off using a 0.43 micrometer membrane filter, and then a transparent MoS2-QD solution was obtained.Internalization
MoS2-QD internalized by HeLa cells were visualized using an LTCS SP5 confocal spectral microscopy imaging system (Leica Microsystems, Wetzlar, Germany). HeLa cells were cultured on cover slides for 24 h and treated with MoS2-QD. The concentration of MoS2-QD was fixed at 1 μg mL−1. After 2 h of incubation, cells were washed with a phosphate buffer solution and mounted on a slide with 4% w/w paraformaldehyde for confocal observation. Fluorescence was observed by confocal microscopy using 360 nm excitation and a long-pass filter of either 450 nm for MoS2-QD detection.Acknowledgements
The authors would like to thank Ministry of Science and Technology, Taiwan for financial support (NSC 100-2221-E-007-011-030-MY3).References
- H. I. Karunadasa, E. Montalvo, Y. Sun, M. Majda, J. R. Long and J. C. Chang, Science, 2012, 335, 698 CrossRef CAS PubMed.
- J. J. Hu and J. S. Zabinski, Tribol. Lett., 2005, 18, 173 CrossRef CAS PubMed.
- D. Sercombe, D. Schwarz, O. D. Pozo-Zamudio, F. Liu, B. J. Robinson, E. A. Chekhovich, I. I. Tartakovskii, O. Kolosov and A. I. Tartakovskii, Sci. Rep., 2013, 3, 3489 CAS.
- T. Stephenson, Z. Li, B. Olsen and D. Mitlin, Energy Enviro. Sci., 2014, 7, 209 RSC.
- S. S. Chou, B. Kaehr, J. Kim, B. M. Foley, M. De, P. E. Hopkins, J. Huang, C. J. Brinker and V. P. Dravid, Angew. Chem., Int. Ed., 2013, 52, 4160 CrossRef CAS PubMed.
- S. Wu, Z. Zeng, H. Qiyuan, Z. Wang, S. J. Wang, Y. Du, Z. Yin, X. Sun, W. Chen and H. Zhang, Small, 2012, 8, 2264 CrossRef CAS PubMed.
- T. Liu, C. Wang, X. Gu, H. Gong, L. Cheng, X. Shi, L. Feng, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 3433 CrossRef CAS PubMed.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. Chen and M. Chhowalla, Nano Lett., 2011, 11, 5111 CrossRef CAS PubMed.
- V. Stengl and J. Henych, Nanoscale, 2013, 5, 3387 RSC.
- S. Zhu, J. Zhang, C. Qiao, S. Tang, Y. Li, W. Yuan, B. Li, L. Tian, F. Liu, R. Hu, H. Gao, H. Wei, H. Zhang, H. Sun and B. Yang, Chem. Commun., 2011, 47, 6858 RSC.
- D. Gopalakrishnan, D. Damien and M. M. Shaijumon, ACS Nano, 2014, 8, 5297 CrossRef CAS PubMed.
- Z. Yin, H. Li, H. Li, L. Jiang, Y. Shi, Y. Sun, G. Lu, Q. Zhang, X. Chen and H. Zhang, ACS Nano, 2011, 6, 74 CrossRef PubMed.
- A. O'Neill, U. Khan and J. N. Coleman, Chem. Mater., 2012, 24, 2414 CrossRef.
- K. K. Liu, W. Zhang, Y. H. Lee, Y. C. Lin, M. T. Chang, C. Y. Su, C. S. Chang, H. Li, Y. Shi, H. Zhang, C. S. Lai and L. J. Li, Nano Lett., 2012, 12, 1538 CrossRef CAS PubMed.
- R. J. Smith, P. J. King, M. Lotya, C. Wirtz, U. Khan, S. De, A. O'Neill, G. S. Duesberg, J. C. Grunlan, G. Moriarty, J. Chen, J. Wang, A. I. Minett, V. Nicolosi and J. N. Coleman, Adv. Mater., 2011, 23, 3944 CrossRef CAS PubMed.
- S. D. Jiang, G. Tang, Z. M. Bai, Y. Y. Wang, Y. Hu and L. Song, RSC Adv., 2014, 4, 3253 RSC.
- T. F. Jaramillo, K. P. Jørgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100 CrossRef CAS PubMed.
- J. A. Spirko, M. L. Neiman, A. M. Oelker and K. Klier, Surf. Sci., 2004, 572, 191 CrossRef CAS PubMed.
- C. Altavilla, M. Sarno and P. Ciambelli, Chem. Mater., 2011, 23, 3879 CrossRef CAS.
- S. S. Chou, M. De, J. Kim, S. Byun, C. Dykstra, J. Yu, J. Huang and V. P. Dravid, J. Am. Chem. Soc., 2013, 135, 4584 CrossRef CAS PubMed.
- C. Lee, H. Yan, L. E. Brus, T. F. Heinz, J. Hone and S. Ryu, ACS Nano, 2010, 4, 2695 CrossRef CAS PubMed.
- T. S. Sreeprasad, P. Nguyen, N. Kim and V. Berry, Nano Lett., 2013, 13, 4434 CrossRef CAS PubMed.
- C. F. Castro-Guerrero, F. L. Deepak, A. Ponce, J. C. Reyes, M. D. Valle-Granados, S. F. Moyado, D. H. Galvan and M. J. Yacaman, Catal. Sci. Technol., 2011, 1, 1024 CAS.
- Y. Yao, L. Tolentino, Z. Yang, X. Song, W. Zhang, Y. Chen and J. P. Wong, Adv. Funct. Mater., 2013, 23, 3577 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07512a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |