Polybenzimidazole assisted fabrication of multiwalled carbon nanotube buckypapers and their silver nanoparticle hybrids
Yanli Zhanga,
Yan Wang*b,
Junrong Yua,
Lei Chenb,
Jing Zhub and
Zuming Hu*a
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, 201620, Shanghai, P. R. China. E-mail: hzm@dhu.edu.cn; Fax: +86-21-67792945; Tel: +86-21-67792944
bCollege of Material Science and Engineering, Donghua University, 201620, Shanghai, P. R. China. E-mail: wy@dhu.edu.cn; Fax: +86-21-67792945; Tel: +86-21-67792944
First published on 30th July 2014
Abstract
Buckypapers represent an attractive form of a macroscopic material of carbon nanotubes with promising applications in electronics, microfilters, and composites, etc. The deposition of metal nanoparticles on buckypaper can further endow buckypapers with multifunctionalities such as unique optical, magnetic and catalytic properties. The homogenous dispersion and appropriate surface functionalization of carbon nanotubes are key factors for realization of high-performance buckypapers and their multifunctional metal hybrids. To this goal, this work reports the fabrication of multi walled carbon nanotube (MWNT) buckypapers and their silver nanoparticle (AgNP) hybrids with the assistance of a high-performance polymer, polybenzimidazole (PBI) for the first time. By optimizing the dispersion conditions of MWNTs in organic solvent with PBI as a non-covalent modifier, highly concentrated dispersions of homogenously dispersed MWNTs can be obtained, thus facilitating the subsequent fabrication of buckypapers by filtration method. The resultant MWNT/PBI papers show good mechanical and thermal properties due to the excellent intrinsic properties of PBI and the strong interactions between MWNTs and PBI. The effect of processing parameters of MWNT dispersions on the final performance of buckypapers was also evaluated. Making use of the coordination effect of imidazole groups to metal ions, AgNPs with relatively uniform sizes were then deposited on surfaces of the MWNTs/PBI buckypaper. The Ag/buckypaper hybrid was found to be a highly active and reusable catalyst for reduction of 4-nitrophenol.
1. Introduction
The carbon nanotube (CNT) has gained extensive attention since its discovery in the 1990s due to its excellent mechanical properties, conductivity, thermal and chemical stability, etc. Since then, great efforts have been devoted to making use of these advantages of CNTs in real applications. In this respect, a prerequisite is to construct macroscopic materials based on CNTs. Incorporation of CNTs into a polymer matrix to fabricate CNT/polymer composites is a frequently adopted strategy to achieve this goal.1–3 However, since the homogeneous dispersion of CNTs in polymer composites is difficult to achieve, the addition amount of CNTs in the polymer matrix is usually very small, which poses a great challenge for exploitation of the full potential of CNTs.4–6 Assembled CNTs, such as two-dimensional CNT buckypaper, are another class of macroscopic CNT-based materials that have great potential in applications of electronics, filtration, and composites.7–10 As the major components in these materials are CNTs, they exhibit superior electrical or thermal properties as compared to CNT/polymer composites.11–15Solution-based filtration is a simple and economic way to make free standing CNT buckypapers with commercially available CNTs as starting materials. However, due to the inert surfaces of CNTs, it is hard to prepare homogeneous dispersion of CNTs in common solvents, as well as CNT papers with both good mechanical stability and electrical conductivity. Therefore, surface modification of CNTs prior to solution-processed assembly is necessary to prepare robust buckypapers.16–20 The surface modification could not only separate individual tubes from agglomerates that facilitate the fabrication of homogeneous CNT buckypapers, but also strengthen the intertube interactions. In this regard, non-covalent modification of CNTs with polymers is advantageous because: (1) in comparison to covalent modification, non-covalent method could preserve the conjugated structure of CNTs; (2) as compared to some modification agents of small molecules, polymers provide better stabilities to the modified buckypaper. As a result, many common surfactants21 or polymers have been used to reinforce CNT buckypaper, such as polyvinylpyrrolidone,22 poly(allylamine),23 and some biopolymers.24,25 However, from previous works, it can be seen that most of polymers used are water-soluble. Although water-soluble polymers could permit the fabrication of CNT buckypaper in environmentally-friendly aqueous solution, the susceptibility of these polymers to moisture would restrict the use of CNT buckypaper in wet conditions. Moreover, CNT buckypaper reinforced with water-soluble polymers could hardly be used in high-temperature or some harsh conditions because of the poor thermal and chemical stabilities of these water-soluble polymers. It is also noted that there were some reports dealing with non-water soluble polymers reinforced buckypaper, such as epoxy resin,7,26,27 polycarbonate,28,29 poly(phenylene sulphide) and poly(ether ether ketone).30,31 However, for these composites, buckypapers should be pre-synthesized by surfactant-aided dispersion and filtration, then the surfactant was removed by repeated washing and the polymers were infiltrated into the resultant buckypapers, which makes the fabrication process complex.
Decoration of CNT buckypapers with metal nanoparticles (MNPs) could further improve their electrical conductivity, and broaden the use of buckypapers in novel applications such as catalysis, energy, and sensors because of the unique optical, electronic, magnetic and catalytic properties of MNPs as compared to their bulk counterparts.32–35 With regard to MNPs, the physical and chemical properties directly related to their sizes and dispersion. However, MNPs tend to aggregate due to their high surface energy, which would compromise their excellent properties. Therefore, designing MNPs with long-term dispersion stability is highly important for their use in practical applications. Fixation of MNPs on solid supports, such as carbon spheres,36 electrospun nanofibers,37 or graphene papers,38 is a strategy that has been widely adopted to protect MNPs against aggregations and maintain their activity. In this respect, immobilizing MNPs onto CNT buckypapers could not only make these papers more conductive and multifunctional, but also make MNPs highly disperse and easy recovery in applications such as heterogeneous catalysis. Surface modification of CNT is again a crucial step to improve the interactions between CNTs and MNPs, and obtain highly integrated and stable MNP/CNT hybrid paper. In addition, mechanical, chemical and thermal stabilities of the paper substrate are equally highly important for reliable use of MNP/CNT hybrid paper.
Based on the above-mentioned consideration, herein, we report the fabrication of mechanically robust, chemically and thermally stable multiwalled carbon nanotube (MWNT) buckypaper, and their hybrids with silver nanoparticles (AgNPs). The key of this work is the selection of polybenzimidazole (PBI) as dispersant for non-covalent modification of MWNTs, and glues for buckypaper. PBI is a kind of high-performance heteroaromatic polymer with good mechanical properties as well as excellent thermal and chemical stabilities in comparison to traditional plastics. Some of us have previously demonstrated the advantages of the combination of PBI and carbon nanomaterials.39,40 Works conducted by Nakashima et al. also demonstrated that PBI could adhere to surfaces of MWNTs via π–π interactions,41–43 therefore, it is expected that the exploitation of the merits of PBI in buckypapers could enable us to prepare stronger and more stable buckypapers. In fact, we note that high-performance aromatic polymers were scarcely reported for this purpose. Another important reason for the choice of PBI is that in contrast to the non-polar characteristics of many other high-performance polymers, the active imidazole groups in PBI chains could coordinate with metal ions,44–46 thus facilitate the immobilization of MNPs on MWNT papers. AgNPs were chosen as model MNPs because they were cost-effective with good catalytic and antibacterial properties.47–49 In this paper, we first optimized the preparation condition for buckypapers and investigated their mechanical, electrical and thermal properties, then we have in situ deposited AgNPs on buckypapers and evaluated the use of Ag/buckypaper hybrid as reusable catalyst for reduction of 4-nitrophenol.
2. Experimental section
2.1. Materials
MWNTs with diameters of 10–15 nm and lengths of 10–20 μm were supplied by Chengdu Organic Chemicals Co. Ltd. 3,3′-Diaminobenzidine (DABz) and isophthalic acid (IPA) were purchased from J&K Chemical in Shanghai. Polyphosphoric acid (PPA), phosphorus pent-oxide (P2O5), N,N-dimethylacetamide (DMAc), silver nitrate (AgNO3), 4-nitrophenol (4-NP), sodium borohydride (NaBH4), and hydrazine hydrate were purchased from Alfa Aesar and used as received. PBI was synthesized by direct polycondensation of DABz and IPA in P2O5/PPA solution as previous reported,50 the chemical structure of PBI is shown in Fig. 1. The intrinsic viscosity of PBI was measured to be 1.0255 dL g−1 in 98 wt% sulfuric acid solution using an Ubbelohde viscometer at 30 °C. The molecular weight of PBI was thus calculated to be 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 based on Mark–Houwink–Sakurada equation.50
900 based on Mark–Houwink–Sakurada equation.50
2.2. Fabrication of MWNT-based buckypapers
Prior to the fabrication of buckypapers, MWNTs should be homogenously dispersed in solvents. Therefore, we first optimized the dispersion of MWNTs in DMAc with the aid of PBI. In typical experiments, PBI was dissolved in 10 mL DMAc completely, then required amount of MWNTs were added in the solution. The mixture was subjected to high-power tip sonication for 5 min and low-power bath sonication for 2 h. After centrifugation at 954 g for 10 min, homogenous dispersions of MWNTs were obtained. Buckypapers were prepared by traditional filtration method. The dispersion of MWNTs were filtrated through a polytetrafluoroethylene (PTFE) membrane with pore size of 0.22 μm and diameter of 47 mm. Then large amount of pure DMAc was filtrated through the formed buckypapers to get rid of loosely bounded PBI. After washing, the buckypapers were dried at 80 °C in vacuum overnight. The dried buckypapers were then carefully peeled off from the underlying PTFE membrane.2.3. Fabrication of Ag/buckypaper hybrids
In a typical synthesis, the buckypaper was immersed into aqueous solution of AgNO3 (2.5 mg mL−1) with slight shaking for a particular time at room temperature. After that, buckypaper was washed with water for several times and dipped into dilute hydrazine hydrate solution to reduce the adsorbed Ag ions into AgNPs.2.4. Characterization
UV-vis spectra were measured on a Perkin-Elmer Lambda 35 UV-vis spectrometer. Transmission electron microscopy (TEM) images were obtained using JEM-2100. SEM images were obtained using scanning electron microscope (FE-SEM) (S-4800, HITACHI Ltd, Japan). Element Analysis were taken with an Vario EL III CHNS analyzer (Elementar, Germany), samples were combusted at high temperature in oxygen-rich environment, the combustion gas were analyzed to measure the elements of C, H and N, element O was then calculated by difference. Raman spectra was taken with Renishaw Micro-Raman Spectroscopy (inVia-Reflex), equipped with a holographic grating of 1800 lines mm−1 and a He–Ne laser (633 nm) as excitation source. Thermogravimetric analysis (TGA) was performed in area with a Netzsch TGA 209 F1 instrument at a heating rate of 20 °C min−1. The tensile properties of buckypapers and Ag/buckypaper hybrids were measured with a WDW3020 instrument at room temperature with humidity about 50% at a crosshead speed of 1 mm min−1, the initial gauge length is 30 mm. The conductivities of papers were measured by a standard four-probe method. X-ray diffraction (XRD) patterns were collected in a D/max-2550 PC instrument.The catalytic activity of the Ag/buckypaper hybrid was tested by a model reaction, which is the reduction of 4-NP with NaBH4 as the reductant. After mixing the homogeneous solution of 4-NP (0.2 mL, 0.1 mM) and NaBH4 (0.8 mL, 1 mM) with de-ionized water (3 mL) in quartz cuvette, Ag/buckypaper hybrid (1 mg) was added. Meanwhile UV-vis spectra were recorded in the sequence of time to monitor the reduction of 4-NP.
3. Results and discussion
3.1. Optimizing the dispersion of MWNTs/PBI in DMAc
Homogeneous dispersion of MWNTs in solvent is necessary to prepare buckypaper with good properties, because the aggregations of MWNTs could act as stress concentration points that weaken the buckypaper. PBI has previously been used for non-covalent modification of MWNTs, the conjugated backbone of PBI was demonstrated to interact with MWNTs via π–π interactions.41–43 However, the solution behavior of MWNTs in PBI solution was not reported and the optimal condition for dispersion of MWNTs was unknown. Therefore, we first investigated the dispersion of MWNTs/PBI in DMAc, which was a good solvent for PBI.Fig. 2a visually demonstrated the dispersion ability of PBI to MWNTs. When unmodified MWNTs were dispersed in DMAc and allowed to stand for one week, most of the MWNTs aggregated and precipitated at the bottom of the vial even with concentration as low as 0.01 mg mL−1. The addition of PBI resulted in more homogeneous dispersions of MWNTs, as shown in Fig. 2a. At low MWNT concentration (0.01 mg mL−1), MWNTs/PBI dispersed homogenously in solution. As the concentration of MWNTs increased to 1 mg mL−1, thicker dispersion was obtained. However, we also noted that a portion of MWNTs were settled at the bottom, suggesting the necessity to optimize the dispersion condition of MWNTs to prepare concentrated dispersion of MWNTs, which would facilitate the subsequent fabrication of MWNT-based macroscopic materials.
To this end, we quantitatively evaluated the effect of PBI concentration and initial MWNT concentration on the final concentration of dispersed MWNTs in solution, while kept the sonication time and centrifugation force (954 g) constant. UV-vis absorbance measurements was used to measure the efficacy of PBI at dispersing MWNTs. MWNT concentrations could be determined by the Beer–Lambert law, A = εlc, where A was the absorbance at a given wavelength, ε was the extinction coefficient, l was the light path length, and c was the MWNT concentration. The ε was determined by plotting the absorbance at wavelength of 500 nm of very dilute and well-dispersed MWNTs/PBI (mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) dispersions against their concentrations. The inset of Fig. 2c shows that there is a good linear relationship (with R = 0.9998) between the absorbance and concentration of MWNTs, the extinction coefficient thus obtained was 0.05447 L mg−1 cm−1, which was slightly higher than previously reported value for MWNTs in DMAc.51
1) dispersions against their concentrations. The inset of Fig. 2c shows that there is a good linear relationship (with R = 0.9998) between the absorbance and concentration of MWNTs, the extinction coefficient thus obtained was 0.05447 L mg−1 cm−1, which was slightly higher than previously reported value for MWNTs in DMAc.51
Using ε for MWNTs/PBI dispersion, the concentrations of MWNTs can be determined, as shown in Fig. 2d–f. When fixed the concentration of MWNTs as 1 mg mL−1, while varied the dosage of PBI, we found the optimal concentration of PBI was 1 mg mL−1. With this concentration of PBI, the concentration of MWNTs in resultant dispersion was 0.247 mg mL−1. Further increasing of PBI resulted in lower concentration of dispersed MWNTs, which might be due to the higher viscosity. When fixed the PBI concentration as 1 mg mL−1, the highest concentration of dispersed MWNTs achieved was 1.610 mg mL−1 with the initial MWNT concentration of 9 mg mL−1. Further increasing of initial concentration of MWNTs resulted in decrease in concentrations of dispersed MWNTs. Notably, when the initial concentration of MWNT reached 15 mg mL−1, almost no MWNTs remained in dispersion. This might be due to the fact that too high concentration of MWNTs in solution would result in no free space to separate individual tubes from agglomerates. In addition, the effect of centrifugation force was also evaluated, the maximum concentration of MWNTs decreased from 1.610 to 1.309 mg mL−1 as the centrifugation force increased to 3819 g, and further decreased to 0.897 mg mL−1 as the centrifugation force increased to 8594 g. However, despite the different concentrations achieved by different centrifugation force, all dispersions were stable even for weeks, demonstrating well-dispersed MWNTs could be obtained at all the tested centrifugation force.
To better get the dispersion state of MWNTs and PBI-dispersed MWNTs in DMAc, we characterized the dispersions obtained at different centrifugation force by TEM, as shown in Fig. 3. It was found that MWNTs formed large aggregates (Fig. 3a), which was not disentangled simply by sonication in solvent. Some impurities, which appeared as black dots, could also be found in Fig. 3a. While most PBI dispersed MWNTs appeared as individual tubes due to the non-covalent binding of PBI on the sidewalls and the removal of non-disentangled MWNT aggregations (Fig. 3c, e, and g) by centrifugation. At higher magnification, the MWNT surface was smooth (Fig. 3b), while there were some amorphous lumps, which we attributed to the attached polymers that contributed to the stability of MWNTs in dispersions on surfaces of PBI dispersed MWNTs. Moreover, it was found that the attached polymers increased with increasing of centrifugation force (Fig. 3d, f, and h), suggesting MWNTs with less attached PBI were removed at higher centrifugation force. Some short tubes could also be found in Fig. 3e and g, demonstrating shorter tubes were more stable against centrifugation, which could be expected as heavier objects always settled faster than lighter ones. Based on the TEM characterization, we assumed that the centrifugation force should affect the final performance of buckypaper because of the different amount of adsorbed polymers and different morphologies of MWNTs in dispersions. Therefore, in the next step, we have prepared three buckypapers from dispersions obtained at centrifugation force of 954, 3819, and 8594 g, which were named MBP-1, MBP-2, and MBP-3, respectively. Buckypaper made from unmodified MWNT (MBP) was also tested for comparison. It should be noted that the mass of MWNTs in each papers was set to be 30 mg, and the thicknesses of MBP, MBP-1, MBP-2, and MBP-3 were 74, 59, 62, and 63 μm, respectively.
3.2. Fabrication and characterization of buckypapers
Buckypapers were prepared by traditional paper-making process. From the photographs of buckypapers (Fig. 4), it can be seen that MBP was brittle that fragmented upon drying because of the remained agglomerates and the relatively weak intertube interactions. While all the MWNTs/PBI papers were flexible without disruption. SEM images with low magnification (Fig. 4a–d) showed the surfaces of papers were relatively smooth because of the suction forces generated by the vacuum filtration. Moreover, the surfaces become more compact in MWNTs/PBI papers because of the presence of polymers. High magnification images (Fig. 4e–h) clearly demonstrated the differences among these papers. The junctions between individual tubes seemed more seamless with increased centrifugation force, the diameters of individual tubes also become larger from MBP to MBP-3. All of the phenomena were attributed to the thicker polymer coatings around the tubes, which were consistent with the TEM observations of MWNTs/PBI with different centrifugation force. To confirm this point, we carried out elemental analysis on the buckypapers, the results were listed in Table 1, from which we could calculate the amount of PBI in each paper. The weight ratios of elemental N in samples were used to estimate the weight percents of PBI in buckypapers. The nitrogen in pure PBI was founded to be 18.68 wt%, which was slightly lower than theoretical value of 19.72 wt%, the presence of oxygen in PBI was probably due to some impurities. As a result, the calculated amounts of PBI in MBP-1, MBP-2, and MBP-3 were 9.53 wt%, 13.33 wt%, and 15.74 wt%, respectively, which were in agreement with our assumption, that is, with increased centrifugation force, MWNTs with more coated polymers were retained in dispersions. Such findings might be helpful to prepare MWNT papers with tailored properties. Moreover, from high magnification SEM images, we also noted that more ends of MWNTs could be found in MBP-2 (Fig. 4g) as compared to MBP-1 (Fig. 4f), and the ends of MWNTs were even more in MBP-3 (Fig. 4h), indicating more short tubes were existed in samples prepared from dispersions obtained by higher centrifugation force. We have also carried out SEM characterization on the bottom side of the buckypapers, it was found that the images of bottom side of buckypapers were almost the same as those of the top side (images not shown). Fig. 4i–l showed the cross-sections of buckypapers. However, the cross-sections of buckypapers seemed not as uniform as the surfaces, which might be due to the brittle fracture of the cross-sections in contrast to suction-flattened paper surfaces. In addition, the tubes in MBP-1, MBP-2, and MBP-3 stacked more tightly than those in MBP, and the diameters of tubes in MBP-1, MBP-2, and MBP-3 appeared slightly larger than those in MBP, which were consistent with the observations from paper surfaces (Fig. 4e–h).| Sample | C(wt%) | H(wt%) | N(wt%) | O(wt%) |
|---|---|---|---|---|
| MBP | 97.72 | 0.58 | — | 1.7 |
| MBP-1 | 95.28 | 0.98 | 1.78 | 1.66 |
| MBP-2 | 94.77 | 1.09 | 2.49 | 1.65 |
| MBP-3 | 94.02 | 0.89 | 2.94 | 2.15 |
| PBI | 74.64 | 4.85 | 18.68 | 1.83 |
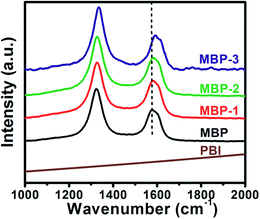
Raman spectra were then employed to characterize the buckypapers, as shown in Fig. 5, from which we can see that the PBI showed no peak in the spectrum, while the disorder mode (D-band) and the tangential mode (G-band) of MWNTs could be found in all buckypapers, and the intensity ratios of D-band to G-band (ID/IG), which were usually used as an indication for structural integration of graphitic carbons, were 1.559, 1.418, 1.448, and 1.495 for MBP, MBP-1, MBP-2 and MBP-3, respectively. The decrease of ID/IG from MBP to MBP-1 was attributed to the removal of amorphous carbons or impurities in raw MWNTs, and the increase of ID/IG from MBP-1 to MBP-3 was due to the settlement of longer MWNTs because of higher centrifugation force, thus left more open ends of MWNTs in the papers. Nevertheless, this comparison showed our non-covalent process preserved the integration of graphitic walls of MWNTs to a large extent. Moreover, we found the position of G-band gradually shift from 1577 cm−1 in MBP to 1592 cm−1 in MBP-3, which we attributed to the charge transfer from the CNTs to the conjugated polymers, demonstrating again the interactions between MWNT and PBI.51,52
A major advantage for using high-performance aromatic polymer to prepare buckypaper is the preservation of the high thermal stability of CNTs. Therefore, we have investigate the thermal stabilities of buckypapers in air condition. Fig. 6 presents the TGA and derivative thermogravimetry (DTG) curves of PBI and buckypapers. It was found that there was about 6.8% weight loss below 200 °C in PBI, which was ascribed to the elimination of bounded water in PBI. The maximum weight loss temperature (Tmax) of PBI appeared at about 586.4 °C, which was in consistent with previously reported values for PBI in air,50 demonstrating its high thermal stability. The raw MBP also showed good thermal stability with almost no weight loss below 500 °C. From DTG curve, the Tmax can be found to be 600.5 °C. To our surprise, the incorporation of PBI even improved the thermal stability of MBP. The Tmax for MBP-1, MBP-2, and MBP-3 were 611.6, 621.7, and 612.5 °C, respectively. We thought several reasons can be accounted for this observation: (1) the removal of less stable amorphous carbons or impurities by the non-covalent and centrifugation process; (2) as we have washed away the loosely attached polymers during the paper-making process, the strong interactions between MWNT and tightly adhered PBI could lower the decomposition rate of both MWNTs and polymers. In fact, maintaining or improving the thermal stability of buckypapers by polymer has rarely been reported in literatures, which was very important for the usage of buckypapers in high-temperature applications.
Then the mechanical and electrical properties of buckypapers were evaluated, the resulted values of modulus, strength, ultimate strain, and electrical conductivity of each papers were compared in Fig. 7 and listed in Table 2. It should be noted that as the MBP was too brittle to be handled in tensile test, so the mechanical properties of MBP were not included. From the comparison, it can be seen that MBP-2 shows the best mechanical properties with modulus, strength, and ultimate strain of 0.81 GPa, 8.1 MPa, and 3.2%, respectively, which were higher than some buckypapers made from oxidized MWNTs53 or small molecule cross-linked MWNTs,54,55 demonstrating the effective reinforcement of PBI to MWNT papers. Moreover, it was found that the fabrication process has also influenced the final properties of buckypapers. For example, due to the removal of less functionalized MWNTs in MBP-2, the mechanical properties of MBP-2 were higher than MBP-1. While too high centrifugation force would remove longer MWNTs as in the case of MBP-3, so the mechanical properties of MBP-3 were also lower than MBP-2.
 | ||
| Fig. 7 Comparisons of the (a) modulus, (b) strength, (c) ultimate strain, and (d) electrical conductivity of buckypapers. | ||
| Sample | Young's modulus (GPa) | Tensile strength (MPa) | Ultimate strain (%) | Tmax (°C) | Conductivity (S cm−1) |
|---|---|---|---|---|---|
| MBP | — | — | — | 600.5 | 30.0 ± 1.5 |
| MBP-1 | 0.64 ± 0.05 | 7.5 ± 1.2 | 3.1 ± 0.2 | 611.6 | 8.14 ± 1.81 |
| MBP-2 | 0.81 ± 0.03 | 8.1 ± 1.1 | 3.2 ± 0.1 | 621.7 | 0.21 ± 0.01 |
| MBP-3 | 0.68 ± 0.06 | 7.3 ± 0.9 | 2.7 ± 0.3 | 612.5 | 0.18 ± 0.03 |
However, in contrast to the improved thermal and mechanical properties, the electrical conductivities of buckypapers were deteriorated by the introduce of polymers. The conductivity decreased from 30.0 S cm−1 of MBP to 8.14 S cm−1 of MBP-1, and then dramatically decreased to 0.21 and 0.18 S cm−1 in MBP-2 and MBP-3. It is noted that in the study of Chan-Park et al.,51 the conductivity of MWNTs/polyimide composites could reach 38.8 S cm−1 with 30 wt% MWNTs. However, the conductivities of our composite papers were much lower even with more than 85 wt% MWNTs. Besides the intrinsic lower conductivity of our MWNTs, the major reason was thought to be the efficient wrapping of MWNTs by PBI, which hindered the electron transport among MWNTs.
3.3. Fabrication and characterization of Ag/buckypaper hybrids
As the imidazole group could coordinate metal centre through its amino heteroatom,44–46 it is thought that MNPs could be easily deposited onto our PBI reinforced buckypapers. So we next investigated the formation and properties Ag/buckypaper hybrids. MBP-2 was used as model substrate because of its better mechanical properties, PBI and MBP were also used as a reference. All hybrids were prepared by adsorption-reduction process. Fig. 8 shows the corresponding Ag/PBI, Ag/MBP and Ag/MBP-2. When immersed the substrates in AgNO3 solution for 2 h and then reduced, AgNPs were uniformly deposited on the surface of PBI (Fig. 8a and b), the sizes of most AgNPs were 20–50 nm with some occasionally appeared larger particles (less than 100 nm), demonstrating the coordination ability of PBI to silver ions and AgNPs. However, irregular large particles with sizes of several hundreds nanometers were attached on the surface of MBP (Fig. 8c and d), which might be due to the lack of interaction between MWNTs and AgNPs. In contrast, AgNPs with much smaller sizes were evenly deposited on MBP-2 (Fig. 8e and f), demonstrating the positive effect of PBI on the formation of uniform Ag/buckypaper hybrids. Energy Dispersive Spectroscopy (EDS) showed that about 1.14 wt% Ag were presented in this sample. Increasing the immersion time of MBP-2 in AgNO3 solution to 24 h further increased the deposited AgNPs, as demonstrated in Fig. 8g and h, EDS suggested about 4.43 wt% AnNPs were deposited, demonstrating the controlled deposition of Ag on MBP-2. Due to the higher loading of AgNPs on Ag/MBP-2 with immersion time of 24 h, we would carry out further characterizations on this sample.Fig. 9a shows an enlarged SEM image of Ag/MBP-2, from which we can calculate the size distribution of AgNPs. The statistics of sizes from a large number (∼180 pieces) of particles are presented in Fig. 9b. Based on the size statistical analysis, it is found that the diameter of most AgNPs (∼87.3%) are smaller than 50 nm, and about 72.8% of AgNPs were in the region of 35–50 nm, suggesting the uniform size distribution. The average size were found to be even smaller than AgNPs deposited on polydopamine-coated graphene paper (∼80 nm),51 demonstrating again the strong interaction between Ag ions and PBI. Fig. 9c shows the XRD pattern of Ag/MBP-2, the peak at 2θ = 25.9° were attributed to the (002) planes of MWNTs. The smaller peaks at 2θ = 38.0°, 44.0°, 64.6°, and 77.3° were assigned to the (111), (200), (220), and (311) planes of face-centered cubic (fcc) silver, which were identical to previously reported values for crystalline AgNPs.56,57
 | ||
| Fig. 9 SEM image of Ag/MBP-2 with high magnification. (b) Size distribution of AgNPs in Ag/MBP-2 based on SEM images. (c) XRD pattern of Ag/MBP-2. | ||
The mechanical and electrical properties of Ag/MBP-2 were also evaluated. The modulus, strength, and ultimate strain of Ag/MBP-2 were found to be 0.51 GPa, 6.41 MPa, and 2.43%, respectively, which were slightly lower than those of MBP-2. However, the electrical conductivity of MBP-2 were dramatically increased from 0.21 to 24.2 S cm−1 after deposition of AgNPs, suggesting the reconstruction of conductive network in Ag/MBP-2.
3.4. Catalysis applications of Ag/buckypaper hybrids
AgNPs have been frequently used as catalyst since their high reactivity and selectivity. However, due to the problem of aggregation in small AgNPs during catalysis, and the difficulty in recovery of catalyst, AgNPs were usually immobilized on suitable solid substrate to maintain their high activity and facilitate their recycling. Due to the stability of both MWNTs and PBI in harsh chemical environment, it was expected that our Ag/MBP-2 could serve as reusable catalyst. Here we have chosen the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) as a model reaction. The initial concentration of NaBH4 was set to be much higher than 4-NP to make the reaction follow pseudo-first-order kinetics.58,59 The reduction process was monitored by UV-vis spectra, as shown in Fig. 10a. It can be seen that the intensity of characterized peak at 400 nm for 4-nitrophenolate ions gradually disappeared as the reduction processed. Meanwhile, a new peak at 295 nm, which was attributed to 4-AP, was progressively established, demonstrating the reduction of 4-NP and formation of 4-AP. After 55 min reaction, the solution become colorless, indicating the complete conversion of 4-NP. Since the concentration of 4-NP in solution at given time is proportional to the absorbance, the rate constant (k) can be determined from the linear plot of ln(At/A0) against the reaction time, where At is the absorbance of solution at time (t), and A0 is the absorbance of the solution at reaction t = 0, Fig. 1b showed the k thus obtained is 0.0588 min−1. Considering the Ag/MBP-2 used in the reduction process was 1 mg, and the content of Ag in Ag/MBP-2 was 4.43 wt%, the true activity parameter (κ) of AgNPs on Ag/MBP-2, which represents the ratio of k to the mass of catalyst, was about 22.21 S−1 g−1. The relatively high κ of our AgNPs supported on MBP-2 as compared to some polymer-coated AgNPs60 or AgNPs supported on spherical substrate61,62 suggested that the PBI attached on MWNTs can effectively prevent AgNPs from aggregation and maintain their high catalytic activity.To investigate the reusability of the catalyst, we have carried out five successive catalysis process on the same catalyst. After one cycle of catalysis, the Ag/MBP-2 was taken out of the solution, washed with water and dried, and then for the next cycle of catalysis. The absorbance for each cycle was measured at the same time as the first cycle. The test results show that the catalyst still exhibit relatively high activity as the conversion was only slightly lowered to 86.3% after five times catalysis. SEM image of Ag/MBP-2 after five cycles of catalysis (Fig. 10d) showed that some large clusters with sizes up to micrometers were formed, which might be accounted for the lowered catalytic activity. However, it should be noted that the Ag/MBP-2 maintained its integration during the whole test process, as no cracks could be found from the image. Such results show that MBP-2 could act as reliable and easily reusable substrate for supporting AgNPs for catalysis application.
4. Conclusions
We reported the fabrication of MWNT buckypapers and their hybrids with AgNPs with the assist of PBI. PBI was a high-performance aromatic polymer that could effectively and homogeneously disperse MWNTs in organic solvent with concentrations as high as 1.6 mg mL−1, which greatly facilitated the process of MWNT-based macroscopic materials. With the help of PBI, free-standing and flexible papers with good mechanical properties can be prepared by facile filtration method in contrast to fragile paper prepared from pure MWNTs. The thermal stability of MWNT paper was also improved by the introduction of PBI due to the inherent excellent thermal stability of PBI and the strong interactions between MWNTs and PBI, which was very important for use of buckypapers in high-temperature applications. In contrast, the electrical conductivities of MWNTs/PBI papers were lower than MWNT paper due to the wrapping of PBI on MWNTs. Moreover, the centrifugation force applied to obtain homogeneous dispersion of MWNTs was found to be an important parameter for the final performance of thus-obtained papers, as the centrifugation force could influence the attached amount of polymers and the geometrical morphologies of the dispersed MWNTs. Because of the coordination effect of PBI to the metal ions, AgNPs with relatively uniform sizes in the region of 35–50 nm were then homogenously deposited on MWNTs/PBI papers. The deposited AgNPs increased the conductivity of MWNTs/PBI paper by several orders of magnification. Catalysis experiments also demonstrated the good catalytic activity and recyclability of our Ag/buckypaper hybrids. All results demonstrated the great potential of PBI as an effective dispersant for fabrication of high-performance MWNT buckypapers and multifunctional Ag/buckypapers hybrids.Acknowledgements
The authors thank the National Nature Science Foundation of China (no. 51203019), the National 973 Project of China (no. 2011CB606103), the National 863 Project of China (no. 2012AA03A212), and the Fundamental Research Funds for the Central Universities (no. 14D110613) for the support.References
- Z. Spitalsky, T. Dimitrios, P. Konstantinos and G. Costas, Prog. Polym. Sci., 2010, 35, 357–401 CrossRef CAS PubMed.
- J. N. Coleman, U. Khan and Y. K. Gun'ko, Adv. Mater., 2006, 18, 689–706 CrossRef CAS PubMed.
- M. Moniruzzaman and K. I. Winey, Macromolecules, 2006, 39, 5194–5205 CrossRef CAS.
- L. Q. Liu, W. J. Ma and Z. Zhang, Small, 2011, 7, 1504–1520 CrossRef CAS PubMed.
- H. D. Espinosa, T. Filleter and M. Naraghi, Adv. Mater., 2012, 24, 2805–2823 CrossRef CAS PubMed.
- H. Qian, E. S. Greenhalgh, M. S. P. Shaffer and A. Bismarck, J. Mater. Chem., 2010, 20, 4751–4762 RSC.
- J. W. Zhang, D. Z. Jiang and H. X. Peng, RSC Adv., 2013, 3, 15290–15297 RSC.
- S. Roy, V. Jain, R. Bajpai, P. Ghosh, A. S. Pente, B. P. Singh and D. S. Misra, J. Phys. Chem. C, 2012, 116, 19025–19031 CAS.
- K. Evanoff, J. Benson, M. Schauer, I. Kovalenko, D. Lashmore, W. J. Ready and G. Yushin, ACS Nano, 2012, 6, 9837–9845 CrossRef CAS PubMed.
- J. W. Zhang, D. Z. Jiang, H. X. Peng and F. X. Qin, Carbon, 2013, 63, 125–132 CrossRef CAS PubMed.
- J. Liu, A. G. Rinzler, H. Dai, J. H. Hafner, R. K. Bradley, P. J. Boul, A. Lu, T. Iverson, K. Shelimov, C. B. Huffman, F. R. -Macias, Y.-S. Shon, T. R. Lee, D. T. Colbert and R. E. Smalley, Science, 1999, 280, 1253–1256 CrossRef.
- S. M. Cooper, H. F. Chuang, M. Cinke, B. A. Cruden and M. Meyyappan, Nano Lett., 2003, 3, 189–192 CrossRef CAS.
- I. W. P. Chen, Chem. Commun., 2013, 49, 2753–2755 RSC.
- G. H. Chen, D. N. Futaba, S. Sakurai, M. Yumura and K. Hata, Carbon, 2014, 67, 318–325 CrossRef CAS PubMed.
- I. W. P. Chen, R. Liang, H. Zhao, B. Wang and C. Zhang, Nanotechnology, 2011, 22, 485708 CrossRef PubMed.
- Q. Cao and J. A. Rogers, Adv. Mater., 2009, 21, 29–53 CrossRef CAS PubMed.
- L. Liu, W. Ma and Z. Zhang, Small, 2011, 7, 1504–1520 CrossRef CAS PubMed.
- D. Tuncel, Nanoscale, 2011, 3, 3545–3554 RSC.
- Z. X. Song, J. J. Dai, S. Zhao, Y. Zhou, F. Su, J. Cui and Y. H. Yan, RSC Adv., 2014, 4, 2327–2338 RSC.
- N. R. Tummala, B. H. Morrow, D. E. Resasco and A. Striolo, ACS Nano, 2010, 4, 7193–7204 CrossRef CAS PubMed.
- L. Vaisman, H. D. Wagner and G. Marom, Adv. Colloid Interface Sci., 2006, 128–130, 37–46 CrossRef CAS PubMed.
- Q. L. Liu, M. Li, Z. Z. Wang, Y. Z. Gu, Y. X. Li and Z. G. Zhang, Composites, Part A, 2013, 55, 102–109 CrossRef CAS PubMed.
- A. Satti, A. Perret, J. E. McCarthy and Y. K. Gun'ko, J. Mater. Chem., 2010, 20, 7941–7943 RSC.
- J. Boge, L. J. Sweetman, M. Panhuis and S. F. Ralph, J. Mater. Chem., 2009, 19, 9131–9140 RSC.
- A. Aldalbahi and M. Panhuis, Carbon, 2012, 50, 1197–1208 CrossRef CAS PubMed.
- J. Li, Y. Gao, W. Ma, L. Liu, Z. Zhang, Z. Niu, Y. Ren, X. Zhang, Q. Zeng, H. Dong, D. Zhao, L. Cai, W. Zhoua and S. Xie, Nanoscale, 2011, 3, 3731–3736 RSC.
- Z. Wang, Z. Liang, B. Wang, C. Zhang and L. Kramer, Composites, Part A, 2004, 35, 1225–1232 CrossRef PubMed.
- S. Wang, Z. Liang, G. Pham, Y.-B. Park, B. Wang, C. Zhang, L. Kramer and P. Funchess, Nanotechnology, 2007, 18, 095708 CrossRef.
- G. T. Pham, Y.-B. Park, S. Wang, Z. Liang, B. Wang, C. Zhang, P. Funchess and L. Kramer, Nanotechnology, 2008, 19, 325705 CrossRef PubMed.
- A. M. Díez-Pascual, J. Guan, B. Simard and M. A. Gómez-Fatou, Composites, Part A, 2012, 43, 997–1006 CrossRef PubMed.
- A. M. Díez-Pascual, J. Guan, B. Simard and M. A. Gómez-Fatou, Composites, Part A, 2012, 43, 1007–1015 CrossRef PubMed.
- M. T. Byrne, C. A. Hanley and Y. K. Gun'ko, J. Mater. Chem., 2010, 20, 2949–2951 RSC.
- C. F. McAndrew and M. Baxendale, Nanotechnology, 2013, 24, 305202 CrossRef PubMed.
- D. N. Ventura, S. Li, C. A. Baker, C. J. Breshike, A. L. Spann, G. F. Strouse, H. W. Kroto and S. F. A. Acquah, Carbon, 2012, 50, 2672–2674 CrossRef CAS PubMed.
- H. T. Wang, Z. X. Dong and C. Z. Na, ACS Sustainable Chem. Eng., 2013, 1, 746–752 CAS.
- M. P. Sk, C. K. Jana and A. Chattopadhyay, Chem. Commun., 2013, 49, 8235–8237 RSC.
- Y. P. Huang, H. Ma, S. G. Wang, M. W. Shen, R. Guo, X. Y. Cao, M. F. Zhu and X. Y. Shi, ACS Appl. Mater. Interfaces, 2012, 4, 3054–3061 CAS.
- Z. M. Wang, C. L. Xu, G. Q. Gao and X. Li, RSC Adv., 2014, 4, 13644–13651 RSC.
- Y. Wang, Z. X. Shi, J. H. Fang, H. J. Xu and J. Yin, Carbon, 2011, 49, 1199–1207 CrossRef CAS PubMed.
- Y. Wang, J. R. Yu, L. Chen, Z. M. Hu, Z. X. Shi and J. Zhu, RSC Adv., 2013, 3, 20353–20362 RSC.
- K. Matsumoto, T. Fujigaya, K. Sasaki and N. Nakashima, J. Mater. Chem., 2011, 21, 1187–1190 RSC.
- M. Okamoto, T. Fujigaya and N. Nakashima, Small, 2009, 5, 735–740 CrossRef CAS PubMed.
- T. Fujigaya and N. Nakashima, Adv. Mater., 2013, 25, 1666–1681 CrossRef CAS PubMed.
- A. A. D’Archivio, L. Galantini, A. Biffis, K. Jeǐábek and B. Corain, Chem.–Eur. J., 2000, 6, 794–799 CrossRef.
- B. K. Balan, V. S. Kale, P. P. Aher, M. V. Shelke, V. K. Pillai and S. Kurungot, Chem. Commun., 2010, 46, 5590–5592 RSC.
- B. K. Balan, H. D. Chaudhari, U. K. Kharul and S. Kurungot, RSC Adv., 2013, 3, 2428–2436 RSC.
- M. Lv, S. Su, Y. He, Q. Huang, W. B. Hu, D. Li, C. H. Fan and S. T. Lee, Adv. Mater., 2010, 22, 5463–5467 CrossRef CAS PubMed.
- S. Wunder, F. Polzer, Y. Lu, Y. Mei and M. Ballauff, J. Phys. Chem. C, 2010, 114, 8814–8820 CAS.
- M. M. Zhu, C. J. Wang, D. H. Meng and G. W. Diao, J. Mater. Chem. A, 2013, 1, 2118–2125 CAS.
- M. Okamoto, T. Fujigaya and N. Nakashima, Adv. Funct. Mater., 2008, 18, 1776–1782 CrossRef CAS PubMed.
- W. Yuan, J. F. Che and M. B. C. Park, Chem. Mater., 2011, 23, 4149–4157 CrossRef CAS.
- W. Yuan, W. F. Li, Y. G. Mu and M. B. C. Park, ACS Appl. Mater. Interfaces, 2011, 3, 1702–1712 CAS.
- Z. Špitalský, C. Aggelopoulos, G. Tsoukleri, C. Tsakiroglou, J. Parthenios, S. Georga, C. Krontiras, D. Tasis, K. Papagelis and C. Galiotis, Mater. Sci. Eng., B, 2009, 165, 135–138 CrossRef PubMed.
- S. Steiner, S. Busato and P. Ermanni, Carbon, 2012, 50, 1713–1719 CrossRef CAS PubMed.
- D. N. Ventura, R. A. Stone, K. S. Chen, H. H. Hariri, K. A. Riddle, T. J. Fellers, C. F. Strouse, H. W. Kroto and S. F. A. Acquah, Carbon, 2010, 48, 987–994 CrossRef CAS PubMed.
- S. K. Li, Y. X. Yan, J. L. Wang and S. H. Yu, Nanoscale, 2013, 5, 12616–12623 RSC.
- H. Zhao, H. Fu, T. Zhao, L. Wang and T. Tan, J. Colloid Interface Sci., 2012, 375, 30–34 CrossRef CAS PubMed.
- H. S. Shin and S. Huh, ACS Appl. Mater. Interfaces, 2012, 4, 6324–6331 CAS.
- B. Liu, J. C. Wang, S. X. Sun, X. M. Wang, M. Zhao, W. Zhang, H. Zhang and X. L. Yang, RSC Adv., 2013, 3, 18506–18518 RSC.
- M. H. Rashid and T. K. Mandal, J. Phys. Chem. C, 2007, 111, 16750–16760 CAS.
- Y. Chi, Q. Yuan, Y. J. Li, J. C. Tu, L. Zhao, N. Li and X. T. Li, J. Colloid Interface Sci., 2012, 383, 96–102 CrossRef CAS PubMed.
- S. C. Tang, S. Vongehr and X. K. Meng, J. Phys. Chem. C, 2010, 114, 977–982 CAS.
| This journal is © The Royal Society of Chemistry 2014 |