In vitro and in vivo cytocompatibility of electrospun nanofiber scaffolds for tissue engineering applications
N. Goonoo,
A. Bhaw-Luximon and
D. Jhurry*
ANDI Centre of Excellence for Biomedical and Biomaterials Research, University of Mauritius, MSIRI Building, Réduit, Mauritius. E-mail: djhurry@uom.ac.mu
First published on 4th July 2014
Abstract
The use of polymeric-based nanofibers has gained more and more attention during the past decade in the biomedical and pharmaceutical fields and as a result, nanotoxicology research is inevitable to satisfy the requirements of regulating agencies such as FDA as well as biosafety needs. Recent advances have witnessed the emergence of an increasing number of nanosized materials. While the number of potential applications related to the use of electrospun nanofibers continues to increase, studies to characterize their effects after exposure and to address their potential cytocompatibility are few in comparison. A comprehensive understanding of nano-bio and physico-chemical interactions is necessary from the early stage of nanomaterial conception to prevent pitfalls of materials failure at preclinical and clinical stages. This review presents a summary of both in vitro and in vivo cytocompatibility data currently available on synthetic and natural polymer-based electrospun nanofibers under investigation for tissue engineering applications. Cellular response dependence on cell type and nature of scaffold is also addressed.
Introduction
Over the past few years, electrospinning has grown from a small niche process to a widely used fiber fabrication technique. Major applications of electrospun fibers include tissue engineering, controlled drug delivery, sensing, separations, filtration, catalysis and nanowires.1Several excellent reviews have been published on electrospinning and the use of electrospun nanofibers.2–6 Huang et al.5 stressed on developments related to electrospun polymer nanofibers including processing, structure and property characterization, applications as well as modelling and simulations. Xie et al.7 focused on the attributes of electrospun nanofibers which make them suitable for a range of biomedical applications including drug delivery and tissue engineering. Indeed, the high porosity, large surface area and the possibility of functionalization of nanofibers through encapsulation or attachment of bioactive species allow them to serve as ideal scaffolds, mimicking the extracellular matrix (ECM) of the target tissue. Furthermore, nanofibers have been highlighted as promising candidates for bone, cartilage, vascular, neural and bladder tissue engineering applications.8 The potential risk and toxicity of nanomaterial synthesis as well as its use related to human health were also identified as an important future area of research.8
Recently, much attention has been given to cytocompatibility testing of electrospun nanofibers. Nanofiber matrices support cell attachment and proliferation, and at the same time maintain cell phenotypes. In a comprehensive review by Nisbet et al.,9 cellular interactions with electrospun scaffolds, with particular focus on neural, bone, cartilage, and vascular tissue regeneration were addressed. Aspects of scaffold design, including architectural properties, surface functionalization and materials selection were also highlighted. The use of nanostructures in the creation of the next generation of biomaterials with well-defined nanotopography capable of eliciting the desired cellular and tissue response has been reviewed by Yim et al.10 Cytocompatibility experiments conducted on nanofibers were briefly discussed by Ma et al.11 In an exhaustive review on the design, fabrication and use of PCL scaffolds for tissue engineering applications, Cipitria et al. summarized the knowledge about factors affecting cellular responses such as mesh morphology, topography, chemistry and mechanical properties.12
A number of clinical applications for electrospun fibers are currently being considered. Preliminary studies have demonstrated that a range of electrospun nanofibers showed no toxicity towards living cells, no inflammatory response or loss of cell integrity, as well no cellular damage. Cells and scaffolds are the two major elements for successful tissue engineering.13 Due to the unique capabilities of stem cells such as self-renewal and multi-lineage differentiation, the combination of stem cells and nanofibrous scaffolds have become the focal point of many investigations.13,14 The latest review paper on biocompatibility and cellular response of nanofibers dates back to 2011 whereby in vitro and in vivo studies on electrospun nanofibrous scaffolds have been presented, however limited to PCL and PCL blend mats.12 The authors stressed on the physical and chemical characterization of electrospun mats and on thorough reporting of experimental parameters regarding cytocompatibility studies for a better comparison among laboratories.
As nanofibers for tissue engineering applications move towards the use of blends of natural and synthetic polymers, a review of the status of cytocompatibility and cytotoxicity studies on a range of polymers belonging to these categories would serve the community of researchers and engineers. After a preliminary presentation of in vitro cytotoxicity assays including microscopy and spectrophotometric measurements, in vivo evaluation of biomaterials correlated with factors affecting cellular responses of electrospun nanofibers such as surface roughness, fiber alignment, fiber composition are here highlighted. Cellular response based on cell type and nature of scaffold is also here reviewed.
General background: biocompatibility and cellular response
Tissue engineered polymeric-based scaffolds are an integral part of future regenerative efforts and as reported in our recent review, biocompatibility, biodegradability and mechanical performance of the polymers highly impact on the scaffolds.15 Cells sense and respond to the physical properties of the matrix by converting mechanical cues into intracellular chemical signals, which in turn, control gene expression, protein production and phenotypic behavior. According to the International Standard ISO 10993 (Biological Evaluation of Medical Devices), all materials used in humans are subjected to in vitro and in vivo biocompatibility tests to verify response and behavior of cells interacting with them. A material is said to be biocompatible when the latter interacts with the body without inducing unacceptable toxic, immunogenic, thrombogenic and carcinogenic responses, and any other side effects. The complex and dynamic cell–ECM communication takes place through both integrin and non-integrin membrane bound receptors (Fig. 1). Fibronectin and integrins play crucial roles in a variety of morphogenetic processes, in which they mediate cell adhesion, migration and signal transduction.17 Integrins cluster in specific cell–matrix adhesions to provide dynamic links between extracellular and intracellular environments by bi-directional signalling and by organizing the ECM and intracellular cytoskeletal and signalling molecules.18,19 Initially, cell adhesion to the ECM is induced by multiple, low affinity charge and hydrophobic interactions. The spreading phase of cell adhesion is induced by integrins on the cell surface which bind to specific small peptide fragment sequences on the ECM. This allows cell attachment to the ECM through focal adhesions and promotes direct communication between the two. | ||
| Fig. 1 Cell–ECM communication (SEM and fluorescence image adapted from Biomater. Sci.16 | ||
The different ECM components like laminin, fibronectin, and collagen type I interact differently with cell behavior patterns: attachment dynamics such as adhesion kinetics and force, formation of focal adhesion complexes, morphology, proliferation, and intercellular communication. Schlie-Wolter et al. carried a detailed in vitro comparison of fibroblasts, endothelial cells, osteoblasts, smooth muscle cells, and chondrocytes which showed significant differences in their cell responses to the ECM: cell behavior follows a cell specific ligand priority ranking, which was independent of the cell type origin. Fibroblasts responded best to fibronectin, chondrocytes best to collagen I, the other cell types best to laminin.20 This knowledge is essential for optimization of tissue–biomaterial interfaces in all tissue engineering applications and gives insight into tissue-specific cell guidance.20
Biocompatibility is a term that encompasses cytocompatibility and cytotoxicity.21 Cytocompatibility involves testing of the material in contact with cells. Cytotoxicity, on the other hand, deals mainly with the substances that leach out of the materials for instance, degradation products. Cytotoxicity testing relies more on biochemical tests, while cytocompatibility is evaluated through cell morphological changes.
Evaluating the biocompatibility of materials has been a complex task. This complexity arises from the fact that materials have various intended uses, with body contact ranging from transient skin contact to contact with blood to permanent implantation. Biocompatibility is generally demonstrated by testing materials, and their leachable chemicals, using toxicological principles. The biomaterials should not—either directly or through the release of their constituents—produce adverse local or systemic effects, be carcinogenic, or produce adverse reproductive and developmental effects. Therefore, evaluation of any material intended for human use requires data from systematic testing to ensure that the benefits provided by the final product will exceed any potential risks posed by device materials.
A number of factors need to be addressed when evaluating the biocompatibility of a material. Firstly, biocompatibility is highly anatomically dependent which means that the reactions to a particular material vary from one location to another.22,23 Often, drug/gene eluting vascular grafts are fabricated to enhance vascular cell attachment. In such cases, it is crucial to consider the drug release aspect. For instance, a material may not cause any tissue injury at all but nonetheless kill the animal, either from drug release24 or from some unforeseen side effects such as intravascular coagulation,25 embolic events,26 chelation of ions vital to homeostasis, etc. It is therefore important to consider that the drug itself can have important effects on the biocompatibility especially for formulations involving a stationary depot. Porosity and surface modification of polymers are important features that need to be taken into consideration in promoting biocompatibility of a scaffold.27 Since scaffolds are specifically designed to interact with cells, it is important to ensure that these enhancements do not cause any adverse effects. It is also crucial to consider biodegradation of scaffolds and the cellular responses induced by the degraded products. For instance, in the case of nanoparticles, it has been reported that biodegradation leads to intracellular changes such as disruption of organelle integrity or gene alterations.28
Generally, the biocompatibility of a material is evaluated through both in vitro and in vivo phases. In vitro studies provide a rough assessment of the ability of relevant cell types to survive in the presence of a material. This can be achieved using a number of tests such as the MTT assay, measures of DNA synthesis and cell proliferation, and dye-based cell membrane integrity tests.29 It may be useful to assess the effects of both direct contact with cells and indirect exposure to diffusible components (residual solvents or monomers, breakdown products, drugs, acid etc.). Even though, cell-based models used in vitro accurately reflect their counterparts inside the body, they do not take the rest of the body into account. Hence, in vivo studies are equally important. A material may not be directly cytotoxic to particular cells, but may yet induce a reaction that is destructive at other locations. An accurate and precise in vitro cytotoxicity assay can decrease the number of animal studies required to develop a new medical device or implanted biomaterial.30 At the same time, they should be sufficiently rapid to allow screening of large numbers of potential biomaterials.
Cytotoxicity or biocompatibility is usually decided by the natural property of the material. Polyesters (polycaprolactone (PCL), poly(lactides) (PLAs)), poly(ester–ethers) (polydioxanone (PDX)) and their copolymers are the most widely used biocompatible scaffolds. Poly(lactide-co-glycolide) (PLGA) is one of the most commonly known FDA-approved materials with excellent biocompatibility. However, when fabricated into electrospun mats, they degrade faster due to higher surface area to volume ratio31 and the degradation products affect cellular responses. Indeed, better scaffold mineralization was observed for PDX containing 50% HA compared to the corresponding PLGA scaffolds in ionic simulated body fluid (i-SBF) and revised simulated body fluid (r-SBF) as shown by Madurantakam et al.32 This clearly shows that the acidic degradation products of PLGA inhibited mineral growth on the scaffolds. The creation of an ECM analogue is extremely challenging, yet possible, may be through the use of natural polymers since they possess the signalling capabilities normally required by cells.33 The primary goal is to minimize the risk of rejection or failure by regulating the response such that it promotes healing.34,35
Bio-testing of biomaterials
Biocompatibility tests generally include two levels: (i) biosafety testing and (ii) bio-functional testing.36 In biosafety tests, the materials are tested for their toxicity to cultured cells, haemolysis or allergic responses, or whether they induce heritable genetic alterations or tissue necrosis after animal implantation. The second level testing focuses on the specific functions of a biomaterial, in which the responses of all the cell and tissue types in contact with the material are investigated using both in vitro and in vivo methods. Advantages of in vitro tests include low costs, quick turnover and high throughput screening. In vivo tests, on the other hand, provides multi-system interactions. In addition, it is costly, has low turnover (weeks to months), low throughput, and has animal use concerns.37Cytotoxicity assays for nano-biomaterials
Williams38 defined the biocompatibility of a scaffold as follows: “The biocompatibility of a scaffold or matrix for a tissue engineering product refers to the ability to perform as a substrate that will support the appropriate cellular activity, including the facilitation of molecular and mechanical signalling systems in order to optimize tissue regeneration, without eliciting any undesirable local or systemic responses in the eventual host”. For many years, cell culture methods have been used to understand how a potential biomaterial will react in the body. Several major cell types are used for in vitro testing, including phagocytic, neural, hepatic, epithelial, endothelial, red blood cells and various cancer cell lines. The specific cell line selected for in vitro assay is intended to model a response likely observed or sensitized by particles in vivo.39 Cell cultures are sensitive to changes in their environment such as changes in temperature, pH and nutrient and waste concentrations as well as the concentration of the potentially toxic agent being tested.28 Therefore, it is crucial to control the experimental conditions to ensure that the measured cell death corresponds to the toxicity of the added electrospun nanofibers versus the unstable culturing conditions. Three cell culture assays are usually used to evaluate biocompatibility including direct contact, agar diffusion and elution as described in standards published by ASTM, ISO, and BSI.40 They are all morphological assays which mean that the outcome is measured by the observations of changes in cell morphology. L-929 mouse fibroblast cells are the most extensively used cells in biomaterials evaluation because they are easy to maintain and produce results with high correlation with animal bioassays. Furthermore, fibroblasts are one of the first cells to invade the wound healing area and a major cell in tissue attachment to biomaterials.Microscopy
One simple cytotoxicity test involves visual inspection of the cells with bright-field microscopy for changes in cellular or nuclear morphology. Usually, the cells are stained with a fluorescent dye such as 4′,6-diamidino-2-phenylindole (DAPI) or Hematoxylin and Eosin stain (H&E).41–43 DAPI is a fluorescent stain that binds strongly to A–T rich regions in DNA. For example, DAPI and H&E staining of fibroblasts cultured on electrospun 80/20 elastin–collagen scaffold showed cell infiltration of about 150 μm throughout the scaffold.42 However, the majority of cytotoxicity assays used for electrospun nanofibers measure cell death via colorimetric methods. These colorimetric methods can be further categorized into tests that measure plasma membrane integrity and mitochondrial activity.The LIVE–DEAD viability test is another assay measuring the number of damaged cells.44,45 Cells are stained with calcein acetoxymethyl (calcein AM) and ethidium homodimer and viewed under a microscope. Calcein AM is an electrically neutral esterified molecule which can easily penetrate cells through diffusion. It is then converted to calcein, a green fluorescent molecule by intracellular esterases inside cells. Damaged or dead cells, on the other hand, are stained by ethidium homodimer, a membrane impermeable molecule and fluoresce red when the dye binds to nucleic acids. Calcein AM and ethidium homodimer emit distinct fluorescence signatures at 515 nm and 635 nm respectively when excited at 495 nm.46 Fig. 2 shows the micrographs of live–dead staining of osteoblasts on electrospun PHBV/silk/n-HA.
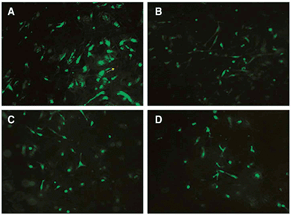 | ||
| Fig. 2 Microscopic micrographs of live–dead staining after 1 day (A and C) and 3 days (B and D): A – control P0 (1 day), B – control P0 (3 days), C – P5 (1 day), D – P5 (3 days). Live cells are stained green and dead/damaged cells are stained red (original magnification 10×) (yellow arrow indicates the dead cells visualized in red colour). Reprinted with permission from,45 E. I. Paşcu, J. Stokes and G. B. McGuinness, Mater. Sci. Eng., C 2013, 33, 4905. © 2013, Elsevier. | ||
Metabolic activity tests
Exposure to certain cytotoxic agents can compromise the cell membrane, which allows cellular contents to leak out.28 Quantitative viability tests based on this include the neutral red47 and trypan blue assays. Neutral red or toluylene red, is a weak cationic dye that can cross the plasma membrane by diffusion. This dye tends to accumulate in lysosomes within the cell. If the cell membrane is altered, the uptake of neutral red is decreased and can leak out, allowing for discernment between live and dead cells. Cytotoxicity can be quantified by taking spectrophotometric measurements of the neutral red uptake at 540 nm. Intensity of the red colour obtained is proportional to the viability of the cell population and inversely proportional to the cytotoxicity of scaffolds.Resazurin or alamar blue is another commonly used colorimetric assay where the non-fluorescent alamar blue dye is reduced to a pink fluorescent dye by cell metabolic activity mainly by acting as an electron acceptor for enzymes such as NADP and FADH during oxygen consumption.48,49
Other tests
Cell adhesion is an important factor when evaluating the integration of implanted biomaterials. The Actin Cytoskeleton & Focal Adhesion Staining Kit (Millipore, USA) can be used to investigate the cytoskeletal organization and focal adhesion.63Inflammation is also a possible adverse effect of exposure to electrospun nanofibers. Commonly tested pro-inflammatory cytokines or protein signals of inflammatory response include IL-1β, IL-6, and TNF-α plus the chemokine IL-8.64,65 These cytokines are detected using enzyme-linked immunosorbant assay (ELISA) and can be quantified by measuring the absorbance from either alkaline phosphatase or strepavidinhorseradish peroxidase labelled antibodies at 405 or 620 nm, respectively.66
Flow cytometric analysis is used to evaluate antigen expression of cells cultured on electrospun mats.41 Results from the study reported by Baiguera et al. showed that decellularized brain extracellular matrix (dBECM)–gelatin mats induced a significant (p < 0.05) decrease in CD54 expression and the higher GFAP expression, suggesting a more effective differentiation potential towards neural (glial) pathway.41
In vivo evaluation of tissue responses to biomaterials
The in vivo assessment of the compatibility of biomaterials with tissue is a critical element of the development and implementation of implants for human use. While in vitro systems yield important fundamental information about certain elements of cellular and molecular interactions with biomaterials, they cannot replace in vivo evaluations. In vivo testing of a biomaterial often involves sterilization of the material followed by implantation in an animal model. In vivo tests listed under the ISO 10993 guidelines include the following: part 3 – tests for genotoxicity, carcinogenicity and reproductive toxicity, part 4 – selection of tests for interaction with blood, part 6 – tests for local effects after implantation, part 10 – tests for irritation and sensitization, and part 11 – tests for systemic toxicity.67All materials undergo tissue responses when implanted into living tissues.68 Fundamental aspects of tissue responses to materials include injury, inflammatory and wound healing responses, foreign body reactions, and fibrous encapsulation of the biomaterial. Studies of the tissue response to implants require a methodology capable of measurements at the molecular, cellular and tissue level. This complex sequence of biological events cannot be simulated in vitro, thus, explaining the need for an appropriate model for in vivo evaluation of tissue compatibility and device efficacy.
Factors affecting cytocompatibility of electrospun nanofibers
Major material properties that may influence the host response may be divided into characteristics of the bulk material and those of the surface.69 These include the following: bulk material composition, micro/nano-structure, morphology, crystallinity, elastic constants, water content, hydrophobic–hydrophilic balance, macro/micro/nano-porosity, surface chemical composition, surface molecular mobility, surface topography, surface energy, surface electrical/electronic properties, degradation profile, degradation product and toxicity, additives, catalysts, contaminants and their toxicity.Interaction of cells and nanoscaffolds
The complex and dynamic cell–ECM communication takes place through both integrin and non-integrin membrane bound receptors70 (Fig. 3). Initially, cell adhesion to the ECM is induced by multiple, low affinity charge and hydrophobic interactions. The spreading phase of cell adhesion is induced by integrins on the cell surface which bind to specific small peptide fragment sequences on the ECM. This allows cell attachment to the ECM through focal adhesions and promotes direct communication between the two. Integrin binding is specific and reversible. It has been shown that cells behave differently when cultured in 3D compared to traditional 2D cultures. Also, they adopt more in vivo like morphologies.71 The mechanical signalling of cells is altered when cultured in 3D, compared to those in 2D, thereby influencing cell–receptor litigation, intercellular signalling and cellular migration.72,73 The diffusion and adhesion of proteins, growth factors and enzymes, which ensures cell viability and functions, are influenced by the 3D environment.73 | ||
| Fig. 3 Diagram depicting integrins favoring cell–ECM interaction. Reprinted with permission from,70 S. A. Sell, P. S. Wolfe, K. Garg, J. M. McCool, I. A. Rodriguez and G. L. Bowlin, Polymers, 2010, 2(4), 522. | ||
Numerous physico-chemical features of the scaffolds such as fiber diameter, pore size, surface patterning, topography, hydrophilicity, alignment and roughness, are reported to play important roles in cell attachment, proliferation as well as differentiation.74–86
Pelipenko et al.78 showed that keratinocytes attached more strongly to the electrospun PVA nanofibers, compared to PVA film. The high surface area of the nanofibers enabled the attachment of a large number of cells, physical entrapment of the cells in the nanofibrillar network as well as multiple focal adhesion points on different nanofibers. Furthermore, in a study by Wang et al., a genipin cross-linked chitosan/nano-HA composite framework (GCFH) was fabricated and compared with genipin cross-linked chitosan framework (GCF).87 GCFH enhanced the osteogenic differentiation of rat MSCs in comparison to GCF after incubation in an osteogenic medium for 7 days. It was postulated that the scaffold's chemical property and nanotopography favoured stem cell proliferation and differentiation in GCFH samples.
 | ||
| Fig. 4 AFM images of (a) PU–PEG2000 (Ra = 20.10 ± 7.87) (b) PU–PEGmix (Ra = 39.79 ± 10.48). Adapted with permission from,88 T. W. Chung, D. Z. Liu, S. Y. Wang and S. S. Wang, Biomaterials, 2003, 24(25), 4655–4661. © 2003, Elsevier. | ||
PU–PEGmix: different molecular weights or chain lengths of polyethylene glycol (PEG) were mixed and then grafted to a polyurethane (PU) surface.
PU–PEG2000: PEG with a molecular weight of 2000 grafted to PU.
 | ||
| Fig. 5 Laser scanning confocal microscopy (LSCM) micrographs of immunostained α-actin filaments in MG63 cells after 1 day of culture. Cell actin (green) and nuclei (red) were stained in cells cultured on (a) TCP; (b) random; (c) parallel-aligned; (d) hyperparallel-aligned scaffolds. Reprinted with permission from,79 B. Wang, Q. Cai, S. Zhang, X. Yang and X. Deng, J. Mech. Behav. Biomed. Mater, 2011, 4(4), 600. © 2011, Elsevier. | ||
Cellular response and suitability of electrospun scaffolds
Aforementioned features such as fiber diameter, surface roughness, fiber alignment and wettability influence cell morphology, proliferation and migration. In addition, cell type and nature of polymer also affect the performance of electrospun scaffolds, as summarised in Table 1. For example, better osteoblast growth, with a higher aspect ratio (contact guidance) was noted on larger diameter PDLA fiber compared to smaller diameter ones.83 On the other hand, this effect was reversed for osteoblasts cultured on electrospun PCL mats. In fact, higher ALP activity was observed on microfibers compared to nanofibers.84 Although PDLA is a poly(ester), its thermal properties are very different to PCL. PDLA is an amorphous polymer while PCL is semi-crystalline. A study by Asran et al.,101 demonstrated that increasing PVA content in electrospun PVA/PHB mats (enhanced wettability) resulted in a decrease in number of viable fibroblasts and enhanced adhesion and proliferation of keratinocytes. This was explained by the strong cell–cell adhesions of keratinocytes which is absent in fibroblasts.103 Hence, cellular response depends on cell type and nature of scaffold and it is inappropriate to draw general conclusions.| Cell type (organ) | Polymer | Fiber diameter (FD)/pore size (PS) | Factor influencing cell growth | Cellular effect | Ref. |
|---|---|---|---|---|---|
| Chondrocytes (cartilage) | PLA | • Fiber alignment | • Cells exhibited a more elongated morphology in oriented PLLA scaffolds compared to non-oriented ones | Areis et al.100 | |
| • Crystallinity | • Higher crystallinity suppresses cell proliferation and the cells produce higher amounts of ECM | ||||
| Chitosan | FD: 300, 500 and 1030 nm | Fiber diameter | • Number of live chondrocytes on smaller diameter mat was higher compared to the larger diameter one | Noriega et al.81 | |
| Fibroblasts (skin) | PCL | PS: 6,8, 20 μm | Pore size | • Optimal pore size for proliferation of HDFs appears to be greater than 6 μm, but less than 20 μm | Lowery et al.82 |
| Keratinocytes (skin) | PVA | FD: 321.8 ± 89.3 nm, PS: 2560 ± 1260 nm | • Contact angle | • Poor cell adhesion on PVA film (CA = 63.1°) compared to electrospun fibers (CA = 54.2°) | Pelipenko et al.78 |
| • Thickness of scaffold | • Thicker scaffolds caused the cells to adopt a more rounded morphology | ||||
| • Pore size | • Cells grown on the electrospun scaffold were practically immobile in contrast to those on the glass cover slip | ||||
| • Fiber orientation | • Cell growth directed by the orientation of nanofibers, and guide the cell migration and elongation | ||||
| • 3D v/s 2D environment | • Higher metabolic activity on electrospun mat compared to glass slide | ||||
| Fibroblasts and keratinocytes (skin) | PHB | FD pure PHB: 680 nm | Contact angle pure PHB: 70° | Decreased number of viable fibroblasts and enhanced adhesion and proliferation of keratinocytes with increasing PVA content | Asran et al.101 |
| PVA/PHB | FD 50/50 PVA/PHB: 615 nm | 50/50 PVA/PHB: 41° | |||
| Chitin | FD nanofibers: 163 nm | Fiber diameter | Higher cell attachment and spreading of cells on nanofibers compared to microfibers | Noh et al.102 | |
| FD microfibers: 8.77 μm | |||||
| Osteoblasts (bone) | Gelatin | FD: 110 ± 40 nm and 600 ± 110 nm | Fiber diameter | • Poor migration of MG63 cells in small diameter scaffold (maximum depth of 18 μm) compared to larger diameter scaffold (maximum depth of 50 μm) | Sisson et al.85 |
| • MG63 cell differentiation favored on small diameter scaffolds compared to large diameter ones at days 3 and 7, but the ALP levels were the same for both scaffold types by day 14 | |||||
| PLA | FD random fibers: 450 nm | Fiber alignment | • Cells had irregular polygonal forms on random mats while those on aligned nanofibers exhibited shuttle-like shapes | Wang et al.79 | |
| FD aligned fibers: 275 nm | |||||
| PS random fibers: 2.2 μm | • Lowest and highest ALP activity observed random and aligned mats respectively | ||||
| PS aligned fibers: 1.0 μm | • Higher cell infiltration in random mats | ||||
| PDLA | FD: 0.14, 2.1 μm | Fiber diameter | Cells on 2.1 μm diameter fibers exhibited a higher aspect ratio (contact guidance) compared to the 0.14 μm fibers | Badami et al.83 | |
| PCL | FD: 930 nm and 5.0 μm | Fiber diameter | Higher ALP activity observed on microfiber compared to nanofibers | Li et al.84 |
Cytocompatibility tests on electrospun nanofibers
In this section, results of few cytocompatibility studies carried out on electrospun PDX, PCL, PLA, chitosan, collagen and their blends will be summarized. Regardless of the ultimate purpose of the electrospun scaffold for in vitro investigations (cell viability, proliferation, differentiation or migration), cell and scaffold handling should be thoroughly described for repeatability purposes.12 In most articles reporting biological assays, cell type and origin, type of culture medium and passage number are often included. However, crucial details such as scaffold sterilization methods, seeding method etc. are often omitted. Scaffold sterilization methods include ethylene oxide, UV radiation or soaking in ethanol. It is important to analyse the scaffold morphology after sterilization since some sterilization techniques may cause degradation.99,104,105 Although the initial cell seeding density is often reported, the initial seeding volume and subsequent incubation time for cell attachment is not always mentioned.99 The method employed to immobilize the scaffold i.e. whether it is in direct contact with a substrate underneath or it is suspended between two rings, also affects the results.Polyester-based nanofibers
Electrospun PCL-based nanofibers
PCL is an aliphatic linear biocompatible and bioresorbable polyester, with a glass transition temperature of 62 °C and a melting point of 55–60 °C.99 Due to its semi-crystalline and hydrophobic nature, it exhibits a very slow degradation rate (2–4 years depending on the starting molecular weight) and has mechanical properties suitable for a variety of applications.106–108 It has been approved by the Food and Drug Administration (FDA) and has been clinically used as a slow release drug delivery device and suture material since the 1980s (i.e. Capronor®, SynBiosys®, Monocryl® suture). A major disadvantage of PCL, however, is its hydrophobic nature, which results in lack of cell attachment and uncontrolled biological interactions with the material.109Yoshimoto et al. used mesenchymal stem cells (MSCs) derived from the bone marrow of neonatal rats for in vitro culture studies for up to 4 weeks on electrospun PCL nanofibers.110 Cells penetrated in the cell–polymer constructs after 1 week. SEM revealed that the surfaces of the cell–scaffold constructs were covered with cell multilayers at 4 weeks. Furthermore, mineralization and type I collagen were observed at 4 weeks. In another study, Li et al.111 seeded hMSCs onto PCL nanofibrous scaffolds. The cells were induced to differentiate along adipogenic, chondrogenic, or osteogenic lineages by culturing in specific differentiation media. Histological and SEM observations, gene expression analysis and immunohistochemical detection of lineage-specific marker molecules confirmed the formation of 3-D constructs containing cells differentiated into the specified cell types.
Blending and copolymerization have been used to overcome this problem.112–116
Table 2 summarizes results of in vitro cell culture studies carried out on electrospun PCL and PCL-blend nanofibers with natural polymers such as collagen, gelatin, HA, chitin, fucoidan, and synthetic polymers such as PEG. Table 3 gives a summary of in vivo studies on electrospun PCL and PCL based nanofibers.
| Cell type | Seeding density/cells per cm2 | Sterilization method | Results | Time (cell viability) | Average fiber diameter/nm | Ref. |
|---|---|---|---|---|---|---|
| PCL | ||||||
| Fetal bovine chondrocytes (FBCs) | 1 × 104 | UV radiation | MTS assay revealed a 21-fold increase in cell growth over 21 days when the cultures were maintained in serum-containing medium compared to TCPS | 21 days | 700 | Li et al.117 |
| 4 × 105 | ||||||
| Neonatal Lewis rat cardiomyocytes | 2.5 × 106 | 70% ethanol | Started beating after 3 days; good attachment of cardiomyocytes; expressed cardiac-specific proteins such as α-myosin heavy chain, connexin43 and cardiac troponin I | 14 days | — | Shin et al.118 |
| Neonatal Lewis rat cardiomyocytes | 2.5 × 106 | — | Good attachment of cultured cardiomyocytes, and strong beating was observed | 14 days | 100–5000 | Ishii et al.119 |
| Human bone marrow MSCs | 4 × 105 | UV radiation | MSCs cultured in NFSs in the presence of TGF-β1 differentiated to a chondrocytic phenotype, as evidenced by chondrocyte-specific gene expression and synthesis of cartilage-associated extracellular matrix (ECM) proteins. The level of chondrogenesis observed in MSCs seeded within scaffolds was comparable to that observed for MSCs maintained as cell aggregates or pellets | 21 days | 500–900 | Li et al.120 |
| Human MSCs | 2.5 × 105 | UV radiation | With increasing, rotating speed, cell morphology changed from pyramidal to more elongated cells, with actin aligned in the direction of the fibers | 28 days | 700 | Li et al.121 |
| Rat brain derived neural stem cells | 5 × 105 cells per mL | 70% ethanol | Stem cells primarily differentiated into oligodendrocytes, showing the ability of PCL to direct differentiation of cells into a specific lineage | 7 days | 750 | Nisbet et al.122 |
| Human dermal fibroblasts | 5 × 105 cells per mL | — | LIVE–DEAD staining of scaffolds at 14 days revealed primarily live cells, with little evidence of dead cells. Cell counts close to 6 × 106 cells per scaffold after 21 days | 21 days | 730–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 530 |
Lowery et al.82 |
| PCL–collagen | ||||||
| Human coronary smooth muscle cells | 4 × 104 | Ethanol | SMCs migrated towards inside the nanofibrous matrices and formed smooth muscle tissue | 3 days | 300–600 | Venugopal et al.123 |
| Human dermal fibroblasts | 1 × 104 | Ethanol | Cell proliferation was lower on PCL nanofibrous membrane compared to PCL–collagen membrane | 6 days | 250–275 | Venugopal et al.124 |
| Human bone marrow derived MSCs | 3 × 104 | Oxygen plasma | Supported cell attachment in a way similar to traditionally used TCPS | 6 weeks | — | Srouji et al.125 |
| Human skeletal muscle cells (hSkMCs) | 4 × 104 | Ethylene oxide | Electrospun PCL–collagen nanofibers guided and oriented skeletal muscle cells into organized structures. Unidirectional oriented nanofibers significantly induced muscle cell alignment and myotube formation as compared to randomly oriented nanofibers | 7 days | Random: 334 | Choi et al.126 |
| Aligned: 296 | ||||||
| Human dermal fibroblasts | 1 × 105 cells per mL | — | High cell adhesion (88.2%) and rapid cell spreading with spindle-like morphology was observed on the nanofiber surface | 1 day | 455 | Yang et al.127 |
| (1) Human aortic smooth muscle cells (external surface) | (1) 5 × 104 cells per mL | Ethylene oxide | (1) Cells infiltrated the scaffold | 4 weeks | 270–4450 | Ju et al.128 |
| (2) Human aortic endothelial cells (onto lumen) | (2) 4 × 104 cells per mL | (2) Cells adhered and spread over multiple fibers | ||||
| PCL–gelatin | ||||||
| Rat bone marrow stromal cells (BMSCs) | 2 × 104 | UV radiation | Cells could not only favorably attach and grow well on the surface of these scaffolds, but were also able to migrate inside the scaffold up to 114 μm within 1 week of culture | 1 week | 10–1000 | Zhang et al.129 |
| Human coronary artery endothelial cells | 2.5 μM mL−1, 200 μL per well | 75% ethanol | Immunostaining micrographs showed that the cells were able to maintain the expression of three characteristic markers: platelet-endothelial cell adhesion molecule 1 (PECAM-1), intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) | 4 days | 200–1000 | Ma et al.130 |
| Human dermal fibroblasts | 1 × 104 cells per well | UV radiation and 70% ethanol | Successful HDF proliferation and adherence on scaffold; however, not much significant fibroblast infiltration within the scaffold structure | 7 days | 470 | Chong et al.131 |
| L 929 mouse fibroblasts | 1 × 105 cells per well | 70% ethanol | Proliferation of L929 cells was as good as on TCPS. Morphology of cells was fibroblastic | 7 days | 488–663 | Tigli et al.132 |
| L 929 mouse fibroblasts | 2 × 105 cells per mL | UV radiation | PCL–gelatin composite nanofibrous scaffold showed the percentage cell viability of 96.8%, 97.1% and 98.4% at 24, 48 and 72 h, respectively, whereas the PCL nanofibrous scaffold showed reduced cell viability of 93.5%, 93.8% and 94.5% at 24, 48 and 72 h respectively, under similar experimental conditions | 3 days | 291–1173 | Gautam et al.133 |
| Mouse embryonic stem cells | 5 × 103 cells per mm2 | — | Cell growth and attachment were supported | 3 days | 570 | Lim et al.134 |
| PCL–HA | ||||||
| Murine embryonic stem cells | 8 × 104 | 70% ethanol | Cells proliferated and maintained pluripotency markers at essentially the same rate as cells growing on standard tissue culture plates with no detectable signs of cytotoxicity, despite a lower cell adhesion at the beginning of culture | 3 days | 1500 | Bianco et al.135 |
| Bone marrow mesenchymal stem cells | 1 × 105 cells per well | — | Supported the growth of mesenchymal stem cells without compromising their osteogenic differentiation capability for up to 21 days. The ALP activity of cells in PCL/50% HA scaffold was 2.7 and 1.8 times higher than in PCL and in PCL/25% HA scaffolds at day 21 respectively | 21 days | 340 | Chen et al.136 |
| PCL–chitin | ||||||
| Human dermal fibroblasts | 1 × 105 cells per well | Ethanol | Cells not only spread on the surface of scaffold, but also penetrated and migrated inside the scaffold | 14 days | 200–400 | Ji et al.137 |
| PCL–fucoidan | ||||||
| Osteoblast-like cells (MG63) | 1 × 105 | — | Cells were distributed more widely and were agglomerated on PCL–fucoidan mats compared to pure PCL mats. Total protein content, ALP activity and calcium mineralization were higher with PCL–fucoidan mats than with pure PCL mats | 10 days | 500 | Lee et al.138 |
| Animal model | Organ/tissue | Results | Ref. |
|---|---|---|---|
| PCL | |||
| Rat | Omenta | After 4 weeks of in vitro culture, the scaffolds were implanted. Cells and ECM formation were observed throughout the constructs. In addition, mineralization and type I collagen were also detected after 4 weeks | Shin et al.139 |
| Abdominal aorta | Good patency, endothelization, and cell ingrowth were observed following the 12 week implantation period | Nottelet et al.140 | |
| Abdominal aorta | Digital subtraction angiography revealed no stenosis in the PCL group but stenotic lesions in 1 graft at 18 weeks (40%). Macrophage and fibroblast ingrowth with ECM formation and neoangiogenesis were better in the PCL group compared to ePTFE. After 12 weeks, foci of chondroid metaplasia located in the neointima of PCL grafts were observed in all samples | Pektok et al.141 | |
| Cranial bone | In vivo results after 6 months exhibited osseous tissue integration within the implant and mineralized bone restoration of the calvarium | Piskin et al.142 | |
| Caudate putamen of brain | Neurites infiltrated the implants, providing evidence of scaffold-neural integration | Nisbet et al.143 | |
| Skin | Thicknesses of fibrous capsule on fibrous scaffolds were 7.55 ± 0.54 micron for aligned fibers and 4.13 ± 0.31 micron for random fibers, which were significantly thinner than that of film implants 37.7 ± 0.25 micron (p < 0.001) | Cao et al.144 | |
| Sciatic nerve | Microscopic examination of the regenerating tissue revealed dense, parallel arrays of myelinated and non-myelinated axons, 7 weeks post-implantation | Jha et al.145 | |
| Swine | Cartilage | After 6 months, the MSC-seeded PCL constructs regenerated hyaline cartilage-like tissue and restored a smooth cartilage surface. No evidence of immune reaction to the regenerated cartilage was observed, possibly related to the immunosuppressive activities of MSCs | Li et al.146 |
| PCL–collagen | |||
| Rabbit | Corpus cavernosa | After 3 months, PCL–collagen scaffolds used in conjunction with autologous smooth muscle cells resulted in better integration with host tissue when compared to cell free scaffolds. On a cellular level pre-seeded scaffolds showed a minimized foreign body reaction | Chen et al.147 |
| Aortoiliac artery | Scaffolds were able to retain their structural integrity over 1 month of implantation as demonstrated by serial ultrasonography. At retrieval, scaffolds continued to maintain biomechanical strength that was comparable to native artery | Tillman et al.148 | |
| Mice | Skin | Good integration with the surrounding tissues and neovascularization | Srouji et al.125 |
| PCL-b-PEG-b-PCL | |||
| Rabbit | Calvarial bone | New bone formed originally from the margin of host bone and then grew towards the center of defects. At 20th week, the defects of treatment group were covered with the new solid cortical bone | Fu et al.149 |
Electrospun PLA-based nanofibers
Poly(lactic acid) (PLA) is the most extensively studied and utilized biodegradable and renewable thermoplastic bio-based polyester. Early studies have investigated the use of PLA as bone plates and screws.150 A 5 year in vitro and in vivo study of the biodegradation of polylactide plates showed that the foreign-body reaction was mainly mild and the osteotomies were well united.151More recent applications involve the use of electrospun PLA as tissue engineering scaffolds,152–155 in drug delivery applications,156 as suitable bio-absorbable membranes,157 and for suture application.158 Briefly, braided PLA nanofibers coated with chitosan could tie wounded tissues for a complete healing without any breakage, had no cellular toxicity and could promote cell growth.158 The chitosan-coated PLA sutures showed better histological compatibility than a silk suture in the in vivo study.
Table 4 summarizes in vitro cytocompatibility studies carried out on electrospun PLA or PLA based nanofibers with HA, tri-calcium phosphate, gelatin and PCL. A summary of in vivo results are shown in Table 5.
| Cell type | Seeding density/cells per cm2 | Sterilization method | Results | Time (cell viability) | Average fiber diameter/nm | Ref. |
|---|---|---|---|---|---|---|
| PLA | ||||||
| Pre-osteoblast cells (MC3T3-E1) | — | Ethanol | The bone mineralized protein-2 (BMP-2) and osteocalcin (OCN) expressions from cells on the melt electrospun PLA fibers were 6-fold and 1.8-fold greater than those on the solution electrospun fibers respectively, due to the solvent-free condition. In addition, melt electrospun fibers provide a significantly higher cell-viability, approximately 2-fold greater than solution electrospun fibers | 7 days | 1500 | Lee et al.159 |
| PDLA | ||||||
| Human embryonic stem cells (hESC) | 1 × 105 | — | Attached cells started to migrate and maturate within 3 days in culture. hESC derived neurons grow and mature well on 3D PDLA scaffolds | 7 days | 800–5000 | Ylä-Outinen et al.160 |
| PLGA | ||||||
| Human mesenchymal stem cells (hMSCs) | 2 × 106 cells per mL | — | The majority of hMSCs was viable and proliferating in PLGA nanofiber scaffolds up to the tested 14 days | 14 days | 760 | Xin et al.161 |
| PLA–HA | ||||||
| Rat osteosarcoma ROS17/2.8 cells | 2 × 104 | — | All the HA–PLLA nanofibrous scaffolds studied exhibited good biocompatibility and cell signaling properties. Compared with a PLLA scaffold, the HA–PLLA scaffolds demonstrated better support for cell attachment, proliferation and differentiation | 10 days | 300 | Peng et al.162 |
| Human fetal osteoblasts (hFOB) | 10 × 103 | — | hFob cells were found to proliferate better on PLLA–HA scaffolds than on PLLA scaffolds. Mineralization increased from day 10 to day 20 and was found to be higher on PLLA–HA scaffolds than on PLLA scaffolds | 20 days | 845 | Prabhakaran et al.163 |
| Osteoblast cell (MG-63) | — | — | Cell adhesion and growth on the PLLA–HA hybrid mats were far better than those on the pure PLLA mats | 5 days | 313 | Sui et al.164 |
| PLA–tricalcium phosphate | ||||||
| Human adipose-derived stem cells (hASCs) | 2 × 104 | — | Human ASCs were able to adhere, proliferate and osteogenically differentiate on all scaffold combinations | 18 days | 503–1267 | McCullen et al.165 |
| PLA–gelatin | ||||||
| Dermal fibroblast cell lines | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
UV radiation | Gelatin–PLLA nanofibrous mat showed excellent in vitro biocompatibility | 4 days | 77.3–147.7 | Gu et al.166 |
| Primary human chondrocytes | 2.5 × 105 cells per mL | — | Cells grown on the scaffolds exhibit good proliferation and increased values of the differentiation parameters, especially for intermediate PLLA/GEL compositions | 14 days | PLLA fiber diameter = 560 nm; gelatin fiber diameter = 500 nm | Torricelli et al.167 |
| PLA–PCL | ||||||
| Human adipose-derived stem cells (hASCs) | 5 × 105 cells per mL | — | Scaffolds supported hASCs well. However, the 1/1 wt ratio PLA–PCL showed better properties and cellular responses in all assessments | 7 days | 682–1250 | Chen et al.168 |
| P(LA–CL) | ||||||
| Smooth muscle cells and endothelial cells | 1–5 × 105 | Ethanol | Both SMC and EC adhered and spread on the nanofiber surface, they interacted and integrated well with the surrounding fibers | 7 days | 450–600 | Mo et al.169 |
| PLGA–HA | ||||||
| Neonatal mouse calvaria-derived MC3T3-E1 osteoblasts | 4 × 104 cells per well | — | Cells cultured on the PLGA/5HAp scaffolds showed significantly higher ALP activity than that on the control PLGA scaffolds by a factor of 60% at 7 days | 14 days | 267 | Lao et al.170 |
| Animal model | Organ/tissue | Results | Ref. |
|---|---|---|---|
| PLA | |||
| Rat | Calvarial bone | Bone regeneration was significantly increased in vivo and after 12 weeks osteocalcin, BMP-2 and Smad5 were all significantly higher in the PLLA/BMP-2 group than in all other groups. No histological foreign body reaction was noted with either PLLA or PLLA/BMP-2 implants | Schofer et al.171 |
| PLA–PCL | |||
| Rat | Dorsal pocket | At 1 week after implantation, the scaffolds were surrounded by a large number of CD68+ macrophagocytes. The number of macrophagocytes decreased with time, and then vanished at 4 weeks, which meant the inflammation reaction ended | Chen et al.168 |
Poly(ester–ether)-based nanofibers
Electrospun PDX nanofibers
PDX is a semi-crystalline (55% crystalline fraction), biodegradable polyester that was originally developed for use as a degradable suture (Ethicon, Inc., a Johnson and Johnson Company).33 Electrospinning of PDX was first reported by Boland et al.172 in 2005. The compatibility, degradation rate, and mechanical properties of PDX are of interest in the design of tissue engineering scaffolds. Since then, many papers have been published regarding the use of electrospun PDX blend nanofibers for potential biomedical applications.173–177 Recently, Kalfa et al. used an electrospun PDX valved patch to replace the right ventricular outflow tract (RVOT) in a growing lamb model.178 Compared with control polytetrafluoroethylene (PTFE)-pericardial patches, tissue-engineered RVOT were neither stenotic nor aneurysmal and displayed a growth potential, with less fibrosis, less calcifications and no thrombus. The PDX scaffold was completely degraded within 8 months and replaced by a viable, three-layered, endothelialized tissue and an extracellular matrix with elastic fibers similar to that of native tissue. Hakimi et al. evaluated the suitability of PDS sutures for the construction of a patch by measuring cell survival, proliferation and migration of human tendon-derived fibroblasts.179 The degradable PDSII showed good interaction with human tendon-derived fibroblasts in vitro, but relatively poor cell adhesion. Cytocompatibility studies carried out on electrospun PDX demonstrated that tendon derived cells grew very well for up to 21 days and that the degradation products which leached from the patch over 8 weeks were safe with only a minimal effect by the end of the experiment.180 Cells seeded on the electrospun patch showed good cell attachment, with no visible clumps of cells (Fig. 6). Cells appeared elongated along the electrospun fibers whilst forming numerous cell–cell contacts. | ||
| Fig. 6 The appearance of human supraspinatus derived cells attached to plastic (a and b), a polydioxanone “drop” (c and d), polydioxanone sutures (e and f) and the electrospun patch (g and h). Cells were stained with nuclei stain (blue, DAPI) and actin filaments (red, rhodamine–phalloidin stain, in images a, b, g, and h). Reprinted with permission with,180 O. Hakimi, R. Murphy, U. Stachewicz, S. Hislop and A. J. Carr, Eur. Cells Mater., 2012, 24, 344. | ||
Electrospun PDX/natural polymer blends
Preliminary cell culture studies on electrospun PDX/elastin blend nanofibers revealed that cells migrated into the fibrous networks of the blends, while the human dermal fibroblasts (HDFs) remained on the surface of the pure PDX scaffold after 24 hours.173 Histological examination further confirmed that HDFs penetrated the full thickness of the elastin-containing scaffolds, with no penetration in the case of the pure PDX scaffold after 7 days. PDX/collagen electrospun blends have also been fabricated.181 Similar results were obtained whereby preliminary in vitro cell culture with HDFs demonstrated favourable cellular interactions on PDX/collagen nanofibers, with prominent cell migration into the scaffolds compared to simple surface spreading with no penetration on pure PDX scaffolds.Natural polymer-based nanofibers
Electrospun collagen-based nanofibers
Collagen is a key component of tissue architecture, providing tensile strength and allowing cell–matrix and matrix–matrix interaction.182,33 Up to now, 28 different types of collagen have been identified, with types I, II, III, V and XI involved in forming fibrillar structures. All collagen molecules have a triple helix structure and have 4-hydroxyproline as distinctive marker. It is an attractive biomaterial for tissue engineering applications given its low antigenicity, low inflammatory and cytotoxic responses, high water affinity, good cell compatibility, availability of various methods of isolation from a variety of sources and biodegradability. Table 6 summarizes the different types of collagen and their body location.183–185| Types | Locations |
|---|---|
| Type I | Bone, skin, dentin, cornea, blood vessels, fibrocartilage and tendon |
| Type II | Cartilaginous tissues |
| Type III | Skin, ligaments, blood vessels and internal organs |
| Type IV | Basement membrane in various tissues |
| Type V | Blood vessel wall, synovium, corneal stoma, tendon, lung, bone, cartilage and skeletal muscle |
Electrospinning of collagen was first reported with the use of poly(ethylene oxide) (PEO) in 2001.186 Since then, many papers have been published on this aspect.181,187,188 Electrospun collagen mats lack mechanical and structural stability upon hydration. Cross-linking with glutaraldehyde vapours, formaldehyde and epoxy compounds has been considered to increase the strength of electrospun collagen mats. However, this leads to an enhanced risk of cytotoxicity and calcification when used in vivo.160 A new technique of imparting desirable mechanical properties and maintaining the nanofibrous structure, while preventing any cytotoxic effects, involves the use of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) in ethanol.160,189 Carbodiimides have been used to cross-link collagen in gels and in lyophilized native tissue specimens but had not been used for electrospun mats until recently.189 Another way to improve the mechanical properties is by blending collagen with synthetic polymers.190
Tables 7 and 8 summarize in vitro cell culture and in vivo studies on electrospun collagen and collagen-blend nanofibers.
| Cell type | Seeding density/cells per cm2 | Sterilization method | Results | Time (cell viability) | Average fiber diameter/nm | Ref. |
|---|---|---|---|---|---|---|
| Collagen | ||||||
| Aortic smooth muscle cells | 5 × 105 cells per mL | — | The scaffolds were densely populated with the smooth muscle cells within 7 days | 7 days | 390 | Matthews et al.191 |
| Chondrocytes | 5–10 × 106 | — | Cells were able to infiltrate the scaffold surface and interior | 7 days | Uncrosslinked fibers: 496 | Shields et al.192 |
| Crosslinked fibers: 1460 | ||||||
| Human keratinocytes | 1 × 105 | — | Relatively low cell adhesion was observed on uncoated collagen nanofibers, whereas collagen nanofibrous matrices treated with type I collagen or laminin were functionally active in responses in normal human keratinocytes | 7 days | 460 | Rho et al.193 |
| Rabbit conjunctiva fibroblast | 5 × 104 cells per well | Ethanol | Aligned collagen scaffold exhibited lower cell adhesion but higher cell proliferation compared to the random one | 7 days | 180, 250 | Zhong et al.194 |
| Human bone marrow-derived mesenchymal stem cells (BMSCs) | 3000 | UV radiation | Cells grown on larger diameter nanofibers (500–1000 nm) had significantly higher cell viability than the TCPS control. Type I collagen nanofibers supported the growth of BMSCs without compromising their osteogenic differentiation capability | 12 days | 50–1000 | Shih et al.195 |
| Collagen–glycosaminoglycan | ||||||
| Rabbit conjunctiva fibroblasts | 1 × 104 cells per well | UV radiation | Cell density on collagen–glycosaminoglycan scaffold was significantly greater than on collagen scaffold | 7 days | 260 | Zhong et al.196 |
| Collagen–PLGA | ||||||
| Human fibroblasts | 5 × 103 cells per well | — | A high level of cell adhesion was observed on the surface of the matrix at 1 week and in-depth cell growth was noted at 2 weeks | 2 weeks | 170–650 | Liu et al.197 |
| Collagen–HA | ||||||
| Pre-osteoblast cell line MC3T3-E1 | 1.5 × 104 | — | The MC3T3-E1 osteoblastic cells were shown to adhere and grow actively on the collagen–HA nanofibrous web. The alkaline phosphatase (ALP) activity expressed by the cells on the collagen – 20 wt% HA nanofiber was lower at day 7, but was higher at day 14 than that on the pure collagen nanofiber | 14 days | 70–170 | Song et al.198 |
| Osteoblasts | 2 × 104 | — | Osteoblasts cultured on both scaffolds and show insignificant level of proliferation but mineralization was significantly (p < 0.001) increased to 56% in Col–HA nanofibrous scaffolds compared to collagen | 6 days | 293 | Venugopal et al.199 |
| Collagen–chitosan | ||||||
| (1) Endothelial cells (EC) | (1) 4 × 103 | 75% radiation | Both EC and SMC spread well on the surface of the nanofiber and cells interacted and integrated well with the surrounding fibers. Cells also migrated and proliferated in certain patterns and formed a continuous monolayer | 14 days | 434–691 | Chen et al.200 |
| (2) Smooth muscle cells (SMC) | (2) 3 × 104 | |||||
| Animal model | Organ/tissue | Results | Ref. |
|---|---|---|---|
| Collagen | |||
| Mice | Skin | At 2 weeks after surgery, scaffolds were well-integrated into surrounding murine skin and possessed a uniformly dry epidermis. Fibroblasts and keratinocytes were well stratified on scaffolds with a continuous basal layer of keratinocytes. A layer of cornified keratinocytes was also forming | Powell et al.201 |
Electrospun elastin-based nanofibers
Elastin is a key structural protein found in the native ECM of connective tissues. It constitutes the walls of arteries and veins, ligaments, lung parenchyma, skin and intestines.202,203 Elastin is chemically inert, highly insoluble polymer, and is composed of several covalently cross-linked molecules of its precursor, tropoelastin, a 67 kDa soluble, non-glycosylated, highly hydrophobic protein.203,204 Elastin has become increasingly popular as a biomaterial for various tissue engineering applications, such as skin,205 heart valves,206 and elastic cartilage.207 When used as a scaffold in vivo, soluble elastin exhibits no signs of calcification, a problem with insoluble elastin scaffolds. Soluble elastin scaffolds have also been shown to exert positive biological effects on a variety of cell types, including increasing angiogenesis and elastic fiber synthesis.208 Nivison-Smith et al. electrospun tropoelastin from HFIP, followed by cross-linking to form synthetic elastin microfibrous scaffolds.209 Cells were found to attach and grow on the seeded surface of the cross-linked scaffolds, with no negative effects from the different cross-linking methods on cell morphology and proliferation.Tables 9 and 10 give a summary of in vitro and in vivo studies conducted on electrospun elastin and elastin blend nanofibers.
| Cell type | Seeding density/cells per cm2 | Sterilization method | Results | Time (cell viability) | Average fiber diameter/nm | Ref. |
|---|---|---|---|---|---|---|
| Elastin | ||||||
| (1) Human fibroblasts | (1) 2.5 × 104 | — | Good cell attachment and proliferation | 24 hours | 1800 | Nivison-Smith et al.209 |
| (2) Primary human umbilical vein endothelial cells (HUVECs) | (2) 5 × 104 | |||||
| (3) Human coronary artery smooth muscle cells (HCASMCs) | (3) 5 × 104 | |||||
| Human umbilical vein endothelial cells | 300 cells per mm2 | — | Tropoelastin enhanced endothelial cell attachment (threefold vs. control) and proliferation by 54.7 ± 1.1% (3 days vs. control) | 3 days | — | Wise et al.210 |
| Bone marrow derived endothelial outgrowth cells (BMEOCs) | 5 × 103 | — | Endothelial cell growth with typical endothelial cell cobblestone morphology was observed after 48 h in culture. Confocal images showed that cells attached well and spread on the electrospun fiber matrix | 2 days | 580 | McKenna et al.211 |
| Human embryonic palatal mesenchymal cells (HEPM) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per sample 000 cells per sample |
Ethanol | HE PM cells attached, spread, migrated, and proliferated to confluence equally well on collagenous scaffolds as on the scaffolds made of elastin | 6 days | 200–500 | Li et al.212 |
| Primary human dermal fibroblasts | 5 × 104 | — | Cells attached and proliferated to form monolayers spanning the entire scaffold surface | 14 days | 2.3 ± 0.5 μm prior to cross-linking and 3.4 ± 0.8 μm following GA cross-linking | Rnjak et al.213 |
| Primary human dermal fibroblasts | 5 × 104/cm2 | — | Low and high porosity scaffolds supported early attachment, spreading and proliferation of primary dermal fibroblasts, but only high porosity scaffolds supported active cell migration and infiltration into the scaffold | 35 days | 2.3 ± 0.5 μm when electrospun at 1 mL h−1 compared to 3.2 ± 1.0 μm at 3 mL h−1 | Rnjak-Kovacina et al.214 |
| Elastin–collagen | ||||||
| Dermal fibroblasts | 2 × 105 per scaffold | — | Enhanced proliferation and migration rates of dermal fibroblasts were observed | 8 days | 1100–6500 | Rnjak-Kovacina et al.215 |
| Elastin–PCL | ||||||
| Human umbilical vein endothelial cells (HUVECs) | 300 cells per mm2 | — | HUVECs attached to and spread on all of the scaffold surfaces by day 3 | 5 days | — | Wise et al.216 |
| PLGA–gelatin–elastin | ||||||
| H9c2 rat cardiac myoblasts and rat bone marrow stromal cells (BMSCs) | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per mL 000 per mL |
— | Myoblasts grew slightly better on the scaffolds than on TCPS, reaching confluence on the scaffold surfaces while simultaneously growing into the scaffolds. BMSCs penetrated into the center of scaffolds and began proliferating shortly after seeding | 8 days | 380 | Li et al.217 |
| Animal model | Organ/tissue | Results | Ref. |
|---|---|---|---|
| Elastin | |||
| Mouse | Skin | The scaffolds persisted for at least 6 weeks, with fibroblasts on the exterior and infiltrating, evidence of scaffold remodeling including de novo collagen synthesis and early stage angiogenesis | Rnjak-Kovacina et al.214 |
| Elastin–collagen | |||
| Mouse | Skin | The scaffolds generated a mild inflammatory response. However, this did not prevent dermal fibroblast infiltration, and de novo collagen deposition which was observed throughout the scaffolds over the 6 weeks study | Rnjak-Kovacina et al.215 |
| Elastin–PCL | |||
| Rabbit | Carotid artery | Animals recovered well from surgery and displayed normal characteristic behavior including mobility and feeding until the study end-point. After explantation at 1 month, the grafts showed no evidence of dilatation, anastomotic dehiscence or seroma | Wise et al.216 |
General trend in cellular/tissue response versus polymer/scaffold
Both in vitro and in vivo data show that cellular and tissue response are scaffold dependent and the latter's performance in general depends on mechanical performance and biocompatibility. For instance, PCL–collagen and PCL–chitin showed better fibroblast infiltration compared to pure PCL scaffold. In vivo studies using PCL scaffolds demonstrated good response with blood vessels, bone and neural cells. The combination of polyester–collagen also gave good response with blood vessels. Elastin, on the other hand, caused mild inflammatory skin reaction. PLA scaffolds favoured osteoblast adhesion, proliferation and growth both in vitro and in vivo. HA addition to both synthetic and natural polymers provided a favourable environment for bone cells.Preclinical/clinical applications of electrospun nanofibers
Several preclinical studies have been conducted on the use of electrospun fibers for tissue regeneration as summarized in Tables 3, 5, 8 and 10. For example, implantation of electrospun PCL scaffolds into the flexor digitorum profundus tendon of mice hindpaws gave promising results with minimal inflammatory reaction. In addition, cells infiltrated into the scaffold.218The Clinical Trials Website219 was used to check for applications of electrospun nanofibers that are currently being evaluated by a clinical trial. The clinical trials summarised in Table 11 are the result of a search using the terms: electrospinning, nanotechnology, scaffold, electrospun nanofibers. Recently, poly(lactide-co-glycolide) biodegradable scaffolds, seeded with neural stem cells have been proposed by InVivo Therapeutics to treat acute spinal cord injuries. The company received approval from FDA for the First Human Trial Using Biomaterials for Traumatic Spinal Cord Injury last year and the trial is now underway.220
| ClinicalTrials.gov identifier | Clinical trial name | Details | Tissue | Status/phase |
|---|---|---|---|---|
| NCT02138110 | Pilot Study of Clinical Safety of the PLGA Poly-L-Lysine Scaffold for the Treatment of Complete (ASIA A) Traumatic Acute Spinal Cord Injury | Poly(lactide-co-glycolide) biodegradable scaffolds seeded with neural stem cells | Spinal cord | Currently recruiting participants |
| NCT01849458 | BioFiber and BioFiber-CM Absorbable Biologic Scaffold for Soft Tissue Repair and Reinforcement Post-Market Surveillance Clinical Study | Matrix consisting of collagen and P4HB | Soft tissue repair | Ongoing |
| NCT01041885 | Prospective Feasibility, Non-randomized, Single Arm Multicentre, Multinational Interventional Clinical Investigation Using INSTRUCT Therapy for the Repair of Knee Cartilage Defects | Isolated primary chondrocytes with autologous bone marrow cells seeded onto a polymeric scaffold | Cartilage repair | Ongoing |
Few electrospun polymeric-based products have been commercialized. For instance, AVflo™, Nicast's CE certified polyurethane vascular access graft was the first one commercialized using electrospun nanofibers and is currently available on the EU market, several Asian countries and Israel.221 Another product commercialized by the same company is NovaMesh™ which is used in the treatment of ventral hernia.222 The latter consists of a smooth side and a nanofibrous side which is placed in contact with the tissue. Studies showed improved resistance to tissue adhesion on the visceral-facing surface, with excellent tissue ingrowth on the fascial surface. Several other nanofiber-based products such as NPmimetic, VISION and BIO-DISC are currently under development by Nicast. Furthermore, the CartiGro ACT technique (Stryker, Montreux, Switzerland) uses a collagen I/III based scaffold (Chondro-Gide; Geistlich Biomaterials, Switzerland) onto which cells (CellGenix – Freiburg, Germany) are cultured.223 This product is marketed for cartilage repair by Stryker in Austria and Germany. Animalclot™ (St. Teresa Medical Inc.,), an electrospun nanofibrous dextran matrix loaded with fibrin producing proteins such as thrombin and fibrinogen is being used in dogs and horses for traumatic bleeding.224 European regulatory approval for human use is anticipated in mid-2014 and in the United States in early 2016 (Fastclot® and Wrapclot®).224 Few more products are available for veterinary use. These include NanoCareV™ scaffolds for surface skin and wound care,225 NanoLigV™ scaffolds to replace and amend ligaments and tendons,226 NanoVesselIV™ synthetic blood vessel for enhancement of vein and artery formation227 and NanoBoneV™ bone replacement scaffolds for bone regeneration.228 Each product line is available in a variety of sizes to meet the needs of any animal. Several companies (Cytoweb, eSpin, NanofiberSOLUTIONS™, SNS Nano Fiber Technology, Engineered Fibers Technology) are producing electrospun nanofibrous mats for cell culture research or clinical applications. Mats fabricated from a wide range of polymers with varying molecular weights, copolymer composition, fiber diameter and fiber orientation are available.
Perspectives and conclusions
As the field of regenerative medicine experiences rapid growth, the development of polymeric-based nanofibers for tissue engineering applications attracts accrued interest. Despite enormous advancements, the best combination of material and nano features still remains unknown. Moreover, a number of hurdles need to be overcome in the translation of scaffolds from lab to clinic. To reach commercial stage, a number of stringent requirements imposed by regulating agencies have to be fulfilled. Another major issue concerns the scaling-up of the manufacturing process such that scaffolds may be fabricated in large quantities with low batch-to-batch variability. Despite the widespread use and the billion-dollar industry producing medical devices and implants, there is still a lack of fundamental understanding of the interlinked reactions that occur when an artificial material is exposed to cells. In that respect, we have summarized in this review the status of cytocompatibility and cytotoxicity studies of a range of electrospun nanofibers based on poly(esters), poly(ester–ether), natural polymers and blends of natural/synthetic polymers that are currently being investigated in different fields of tissue engineering. While the majority of in vitro tests have demonstrated that a range of electrospun nanofibers showed no toxicity and inflammatory response towards living cells, the optimized fiber diameter or inter-fiber diameter for cell growth and migration remains a challenge to be addressed. In vivo testing of electrospun nanofibers are very few in comparison to in vitro tests. In vivo evaluation of biomaterials correlated with factors affecting their cellular responses such as surface roughness, fiber alignment, fiber composition has been discussed. The use of electrospun nanofibers at clinical level is still embryonic as a number of issues such as animal use concerns, lack of reliable correlations between in vitro–in vivo experiments, analytical or technical limitations and high cost need to be addressed.Acknowledgements
We thank the Tertiary Education Commission (Mauritius) for awarding a PhD scholarship to N. Goonoo. We are grateful to the Mauritius Research Council (Mauritius) for supporting biomaterials and drug delivery research at the ANDI Centre of Excellence for Biomedical and Biomaterials Research (CBBR). We are also most indebted to our close collaborator, Prof. Gary Bowlin at the University of Memphis, for his continuous support.Notes and references
- M. T. Hunley and T. E. Long, Polym. Int., 2008, 57, 385 CrossRef CAS.
- J. Doshi and D. H. Reneker, J. Electrost., 1995, 35(2–3), 151 CrossRef CAS.
- J. Zeng, X. Xu, X. Chen, Q. Liang, X. Bian, L. Yang and X. Jing, J. Controlled Release, 2003, 92(3), 227 CrossRef CAS.
- T. J. Sill and H. A. Von Recum, Biomaterials, 2008, 29(13), 1989 CrossRef CAS PubMed.
- Z. M. Huang, Y. Z. Zhang, M. Kotaki and S. Ramakrishna, Compos. Sci. Technol., 2003, 63, 2223 CrossRef CAS.
- R. Gopal, S. Kaur, Z. Ma, C. Chan, S. Ramakrishna and T. Matsuura, J. Membr. Sci., 2006, 281, 581 CrossRef CAS PubMed.
- J. Xie, X. Li and Y. Xia, Macromol. Rapid Commun., 2008, 29(22), 1775 CrossRef CAS PubMed.
- L. Zhang and T. J. Webster, Nano Today, 2009, 4, 66 CrossRef CAS PubMed.
- D. R. Nisbet, J. S. Forsythe, W. Shen, D. I. Finkelstein and M. K. Horne, J. Biomater. Appl., 2009, 24(1), 7 CrossRef CAS PubMed.
- E. K. F. Yim and K. W. Leong, Nanomedicine: Nanotechnology, Biology and Medicine, 2005, 1(1), 10 CrossRef CAS PubMed.
- Z. Ma, M. Kotaki, R. Inai and S. Ramakrishna, Tissue Eng., 2005, 11(1/2), 101 CrossRef CAS PubMed.
- A. Cipitria, A. Skelton, T. R. Dargaville, P. D. Dalton and D. W. Hutmacher, J. Mater. Chem., 2011, 21, 9419 RSC.
- C. Zhao, A. Tan, G. Pastorin and H. K. Ho, Biotechnol. Adv., 2013, 31, 654 CrossRef CAS PubMed.
- S. Martino, F. D`Angelo, I. Armentano, J. M. Kenny and A. Orlacchio, Biotechnol. Adv., 2012, 30, 338 CrossRef CAS PubMed.
- N. Goonoo, A. Bhaw-Luximon, G. L. Bowlin and D. Jhurry, Polym. Int., 2013, 62(4), 523 CrossRef CAS.
- N. Goonoo, A. Bhaw-Luximon, I. A. Rodriguez, D. Wesner, H. Schönherr, G. L. Bowlin and D. Jhurry, Biomater. Sci., 2014, 2, 339 RSC.
- S. Miyamoto, B. Z. Katz, R. M. Lafrenie and K. M. Yamada, Ann. N. Y. Acad. Sci., 1998, 857, 119 CrossRef CAS PubMed.
- A. L. Berrier and K. M. Yamada, J. Cell. Physiol., 2007, 213(3), 565 CrossRef CAS PubMed.
- J. Halper and M. Kjaer, Adv. Exp. Med. Biol., 2014, 802, 31 CrossRef.
- S. Schlie-Wolter, A. Ngezahayo and B. N. Chichkov, Exp. Cell Res., 2013, 319(10), 1553 CrossRef CAS PubMed.
- Biodegradable Systems in Tissue Engineering & Regenerative Medicine, ed. R. L. Reis and J. San Roman, 2004 Search PubMed.
- D. S. Kohane, M. Lipp, R. C. Kinney, D. C. Anthony, D. N. Louis, N. Lotan and R. Langer, J. Biomed. Mater. Res., Part A, 2002, 59, 450 CAS.
- J. M. Anderson, Eur. J. Pharm. Biopharm., 1994, 40, 1 CAS.
- H. Epstein-Barash, I. Shichor, A. H. Kwon, S. Hall, M. W. Lawlor, R. Langer and D. S. Kohane, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 7125 CrossRef CAS PubMed.
- S. P. Hudson, R. F. Padera, R. Langer and D. S. Kohane, Biomaterials, 2008, 29, 4045 CrossRef CAS PubMed.
- D. S. Kohane, N. Plesnila, S. S. Thomas, D. Le, R. Langer and M. A. Moskowitz, Brain Res., 2002, 946, 206 CrossRef CAS.
- M. Goldberg, R. Langer and X. Jia, J. Biomater. Sci., Polym. Ed., 2007, 18, 241 CrossRef CAS PubMed.
- N. Lewinski, V. Colvin and R. Drezek, Small, 2008, 4(1), 26 CrossRef CAS PubMed.
- T. F. Slater, B. Sawyer and U. Straeuli, Biochim. Biophys. Acta, 1963, 77, 383 CrossRef CAS.
- S. K. Bhatia and A. B. Yetter, Cell Biol. Toxicol., 2008, 24, 315 CrossRef PubMed.
- H. K. Makadia and S. J. Siegel, Polymers, 2011, 3, 1377 CrossRef CAS PubMed.
- P. A. Madurantakam, I. A. Rodriguez, C. P. Cost, R. Viswanathan, D. G. Simpson, M. J. Beckman, P. C. Moon and G. L. Bowlin, Biomaterials, 2009, 30(29), 5456 CrossRef CAS PubMed.
- C. P. Barnes, S. A. Sell, E. D. Boland, D. G. Simpson and G. L. Bowlin, Adv. Drug Delivery Rev., 2007, 59, 1413 CrossRef CAS PubMed.
- M. S. Schoichet, Macromolecules, 2010, 43, 581 CrossRef.
- B. N. Brown, J. E. Valentin, A. M. Stewart-Akers, G. P. McCabe and S. F. Badylak, Biomaterials, 2009, 30, 1482 CrossRef CAS PubMed.
- M. Zhang, Biocompatibility of materials, in Biomaterials and Tissue Engineering, ed. D. Shi, Springers, Heidelberg, 2003, pp. 83–137 Search PubMed.
- An Introduction to Biomaterials, ed. J. O. Hollinger , 2001, 2nd edn, p. 64 Search PubMed.
- D. F. Williams, Biomaterials, 2008, 29, 2941 CrossRef CAS PubMed.
- C. F. Jones and D. W. Grainger, Adv. Drug Delivery Rev., 2009, 61, 438 CrossRef CAS PubMed.
- Biomaterials Science, An Introduction to Materials, in Medicine, ed. B. Ratner and A. Hoffman , 2012, ch. 5 Search PubMed.
- S. Baiguera, C. Del Claudio, E. Lucatelli, E. Kuevda, M. Boieri, B. Mazzanti, A. Bianco and P. Macchiarini, Biomaterials, 2014, 35, 1205 CrossRef CAS PubMed.
- J. Rnjak-Kovacina, S. G. Wise, Z. Li, P. K. M. Maitz, C. J. Young, Y. Wang and A. S. Weiss, Acta Biomater., 2012, 8, 3714 CrossRef CAS PubMed.
- J. Xue, B. Feng, R. Zheng, Y. Lu, G. Zhou, W. Liu, Y. Cao, Y. Zhang and W. J. Zhang, Biomaterials, 2013, 34, 2624 CrossRef CAS PubMed.
- S. G. Kumbar, S. P. Nukavarapu, R. James, L. S. Nair and C. T. Laurencin, Biomaterials, 2008, 29, 4100 CrossRef CAS PubMed.
- E. I. Paşcu, J. Stokes and G. B. McGuinness, Mater. Sci. Eng., C, 2013, 33, 4905 CrossRef PubMed.
- R. Moore, I. MacCoubrey and R. Haugland, J. Cell Biol., 1990, 111, 58A Search PubMed.
- S. J. Lee, J. J. Yoo, G. J. Lim, A. Atala and J. Stitzel, J. Biomed. Mater. Res., Part A, 2007, 83(4), 999 CrossRef PubMed.
- L. Li, G. Yang, J. Li, S. Ding and S. Zhou, Mater. Sci. Eng., C, 2014, 34, 252 CrossRef CAS PubMed.
- J. O'Brien, I. Wilson, T. Orton and F. Pognan, Eur. J. Biochem., 2000, 267, 5421 CrossRef.
- G. Haslam, D. Wyatt and P. A. Kitos, Cytotechnology, 2000, 32(1), 63 CrossRef CAS.
- P. Brun, F. Ghezzo, M. Roso, R. Danesin, G. Palù, A. Bagno, M. Modesti, I. Castagliuolo and M. Dettin, Acta Biomater., 2011, 7, 2526 CAS.
- J. J Chang, Y. H. Lee, M. H. Wu, M. C. Yang and C. T. Chien, Carbohydr. Polym., 2012, 88, 1304 CrossRef PubMed.
- R. Chen, C. Huang, Q. Ke, C. He, H. Wang and X. Mo, Colloids Surf., B, 2010, 79, 315 CrossRef CAS PubMed.
- S. H. Chen, Y. Chang, K. R. Lee and J. Y. Lai, J. Membr. Sci., 2014, 450, 224 CrossRef CAS PubMed.
- B. Liu, F. Xu, M. Y. Guo, S. F. Cheng, J. Wang and B. Zhang, Surf. Coat. Technol., 2013, 228, S568 CrossRef CAS PubMed.
- G. Ma, D. Fang, Y. Liu, X. Zhu and J. Nie, Carbohydr. Polym., 2012, 87(1), 737 CrossRef CAS PubMed.
- Z. X. Meng, H. F. Li, Z. Z. Sun, W. Zheng and Y. F. Zheng, Mater. Sci. Eng., C, 2013, 33, 699 CrossRef CAS PubMed.
- W. Cui, X. Zhu, Y. Yang, X. Li and Y. Jin, Mater. Sci. Eng., C, 2009, 29, 1869 CrossRef CAS PubMed.
- Y. Zhang, J. R. Venugopal, A. El-Turki, S. Ramakrishna, B. Su and C. T. Lim, Biomaterials, 2008, 29, 4314 CrossRef CAS PubMed.
- H. Chen, J. Huang, J. Yu, S. Liu and P. Gu, Int. J. Biol. Macromol., 2011, 48, 13 CrossRef PubMed.
- G. Malich, B. Markovic and C. Winder, Toxicology, 1997, 124, 179 CrossRef CAS.
- H. Tominaga, M. Ishiyama, F. Ohseto, K. Sasamoto, T. Hanamoto, K. Suzuki and M. Watanabe, Anal. Commun., 1999, 36, 47 RSC.
- Y. M. Ju, J. S. Choi, A. Atala, J. J. Yoo and S. J. Lee, Biomaterials, 2010, 31, 4313 CrossRef CAS PubMed.
- K. Ley, Physiology of inflammation, Oxford University Press, New York, 2001 Search PubMed.
- C. Dinarello, Chest, 2000, 188, 503 CrossRef.
- N. Favre, G. Bordmann and W. Rudin, J. Immunol. Methods, 1997, 204, 57 CrossRef CAS.
- Cellular Response to Biomaterials, ed. L. Di Silvio , 2008 Search PubMed.
- J. M. Anderson, Annu. Rev. Mater. Res., 2001, 31, 81 CrossRef CAS.
- D. F. Williams, Biomaterials, 2008, 29, 2941 CrossRef CAS PubMed.
- S. A. Sell, P. S. Wolfe, K. Garg, J. M. McCool, I. A. Rodriguez and G. L. Bowlin, Polymers, 2010, 2(4), 522 CrossRef CAS PubMed.
- D. R. Nisbet, S. Pattanawong, N. E. Ritchie, W. Shen, D. I. Finkelstein, M. K. Horne and J. S. Forsythe, J. Neural Eng., 2007, 4, 35 CrossRef CAS PubMed.
- B. Knight, C. Laukaitis, N. Akhtar, N. A. Hotchin, M. Edlund and A. R. Horwitz, Curr. Biol., 2000, 10, 576 CrossRef CAS.
- L. G. Griffith and M. A. Swartz, Nat. Rev. Mol. Cell Biol., 2006, 7, 211 CrossRef CAS PubMed.
- C. Zhao, A. Tan, G. Pastorin and H. K. Ho, Biotechnol. Adv., 2013, 31, 654 CrossRef CAS PubMed.
- J. Mitra, G. Tripathi, A. Sharma and B. Basu, RSC Adv., 2013, 3, 11073 RSC.
- C. Xu, F. Yang, S. Wang and S. Ramakrishna, J. Biomed. Mater. Res., Part A, 2004, 71A(1), 154 CrossRef CAS PubMed.
- C. H. Kim, M. S. Khil, H. Y. Kim, H. U. Lee and K. Y. Jahng, J. Biomed. Mater. Res., Part B, 2006, 78B(2), 283 CrossRef CAS PubMed.
- J. Pelipenko, P. Kocbek, B. Govedarica, R. Rošic, S. Baumgartner and J. Kristl, Eur. J. Pharm. Biopharm., 2013, 84(2), 401 CrossRef CAS PubMed.
- B. Wang, Q. Cai, S. Zhang, X. Yang and X. Deng, J. Mech. Behav. Biomed. Mater, 2011, 4(4), 600 CrossRef CAS PubMed.
- B. Veleirinho, F. V. Berti, P. F. Dias, M. Maraschin, R. M. Ribeiro-do-Valle and J. A. Lopes-da-Silva, Mater. Sci. Eng., C, 2013, 33(1), 37 CrossRef CAS PubMed.
- S. E. Noriega, G. I. Hasanova, M. J. Schneider, G. F. Larsen and A. Subramanian, Cells Tissues Organs, 2012, 195(3), 207 CrossRef CAS PubMed.
- J. L. Lowery, N. Datta and G. C. Rutledge, Biomaterials, 2010, 31(3), 491 CrossRef CAS PubMed.
- A. S. Badami, M. R. Kreke, M. S. Thompson, J. S. Riffle and A. S. Goldstein, Biomaterials, 2006, 27(4), 596 CrossRef CAS PubMed.
- T. T. Li, k. Ebert, J. Vogel and T. Groth, Progress in Biomaterials, 2013, 2, 13 CrossRef.
- K. Sisson, C. Zhang, M. C. Farach- Carson, D. Bruce Chase and J. F. Rabolt, J. Biomed. Mater. Res., Part A, 2010, 94A(4), 1312 CAS.
- M. Chen, P. K. Patra, S. B. Warner and S. Bhowmick, Tissue Eng., 2007, 13(3), 579 CrossRef CAS PubMed.
- G. Wang, L. Zheng, H. Zhao, J. Miao, C. Sun, N. Ren, J. Wang, H. Liu and X. Tao, Tissue Eng., Part A, 2011b, 17, 1341 Search PubMed.
- T. W. Chung, D. Z. Liu, S. Y. Wang and S. S. Wang, Biomaterials, 2003, 24(25), 4655 CrossRef CAS.
- N. Hallab, K. Bundy, K. O'Connor, R. Clark and R. L. Moses, Proceedings 14th Southern Biomedical Engineering Conference, Shreveport, LA, 1995, pp. 81–84 Search PubMed.
- M. Lampin, R. Warocquier-clerout, C. Legris, M. Degrange and M. F. Sigot-Luizard, J. Biomed. Mater. Res., 1997, 36, 99 CrossRef CAS.
- K. Kieswetter, Z. Schwartz, T. W. Hummert, D. L. Cocharn, J. Simpson, D. D. Dean and B. D. Boyan, J. Biomed. Mater. Res., 1996, 32, 55 CrossRef CAS.
- C. Bao, W. Chen, M. D. Weir, W. Thein-Han and H. H. Xu, Acta Biomater., 2011, 7, 4037 CrossRef CAS PubMed.
- Y. Wang, M. Yao, J. Zhou, W. Zheng, C. Zhou, D. Dong, Y. Liu, Z. Teng, Y. Jiang, G. Wei and X. Cui, Biomaterials, 2011, 32, 6737 CrossRef CAS PubMed.
- J. Ma, X. He and E. Jabbari, Ann. Biomed. Eng., 2011, 39, 14 CrossRef PubMed.
- B. Bakhshandeh, M. Soleimani, N. Ghaemi and I. Shabani, Acta Pharmacol. Sin., 2011, 32, 626 CrossRef CAS PubMed.
- E. Biazar, M. Heidari, A. Asefnezhad and N. Montazeri, Int. J. Nanomed., 2011, 6, 631 CrossRef CAS PubMed.
- C. H. Kim, M. S. Khil, H. Y. Kim, H. U. Lee and K. Y. Jahng, J. Biomed. Mater. Res., 2006, 78, 283 CrossRef PubMed.
- Y. Arima and H. Iwata, Biomaterials, 2007, 28, 3074 CrossRef CAS PubMed.
- H. Wang, Y. Feng, Z. Fang, W. Yuan and M. Khan, Mater. Sci. Eng., C, 2012, 32, 2306 CrossRef CAS PubMed.
- A. C. Areis, C. Ribeiro, V. Sencadas, N. Garcia-Giralt, A. Diez-Perez, J. L. Gomez-Ribelles and S. Lanceros-Mendez, Soft Matter, 2012, 8, 5818 RSC.
- A. S. Asran, K. Razghandi, N. Aggarwal, G. H. Michler and T. Groth, Biomacromolecules, 2010, 11(12), 3413 CrossRef CAS PubMed.
- H. K. Noh, S. W. Lee, J. M. Kim, J. E. Oh, K. H. Kim, C. P. Chung, S. C. Choi, W. H. Park and B. M. Min, Biomaterials, 2006, 27(21), 3934 CrossRef CAS PubMed.
- Q. Ren, C. Kari, M. R. D. Quadros, R. Burd, P. McCue, A. P. Dicker and U. Rodeck, Cancer Res., 2006, 66, 5209 CrossRef CAS PubMed.
- W. J. Li, R. Tuli, C. Okafor, A. Derfoul, K. G. Danielson, D. J. Hall and R. S. Tuan, Biomaterials, 2005, 26, 599 CrossRef CAS PubMed.
- H. M. Powell and S. T. Boyce, Tissue Eng., Part A, 2009, 15(8), 2177 CrossRef CAS PubMed.
- I. Engelberg and J. Kohn, Biomaterials, 1991, 12, 292 CrossRef CAS.
- J. C. Middleton and A. J. Tipton, Biomaterials, 2000, 21(23), 2335 CrossRef CAS.
- L. S. Nair and C. T. Laurencin, Prog. Polym. Sci., 2007, 32, 762 CrossRef CAS PubMed.
- E. S. Place, J. H. George, C. K. Williams and M. M. Stevens, Chem. Soc. Rev., 2009, 38, 1139 RSC.
- H. Yoshimoto, Y. M. Shin, H. Terai and J. P. Vacanti, Biomaterials, 2003, 24, 2077 CrossRef CAS.
- W. J. Li, R. Tuli, X. Huang, P. Laquerriere and R. S. Tuan, Biomaterials, 2005, 26, 5158 CrossRef CAS PubMed.
- Z. C. C. Chen, A. K. Ekaputra, K. Gauthaman, P. G. Adaikan, H. Yu and D. W. Hutmacher, J. Biomater. Sci., Polym. Ed., 2008, 19(5), 693 CrossRef CAS PubMed.
- J. Venugopal, T. Y. L. L. Ma and S. Ramakrishna, Cell Biol. Int., 2005, 29, 861 CrossRef CAS PubMed.
- Y. C. Lim, J. Johnson, Z. Fei, Y. Wu, D. F. Farson, J. J. Lannutti, H. W. Choi and L. J. Lee, Biotechnol. Bioeng., 2011, 108(1), 116 CrossRef CAS PubMed.
- X. Yang, F. Yang, X. F. Walboomers, Z. Bian, M. Fan and J. A. Jansen, J. Biomed. Mater. Res., Part A, 2010, 93(1), 247 Search PubMed.
- G. Liao, K. Jiang, S. Jiang and H. Xia, J. Macromol. Sci., Part A: Pure Appl.Chem., 1116, 47, 11 Search PubMed.
- W. J. Li, K. G. Danielson, P. G. Alexander and R. S. Tuan, J. Biomed. Mater. Res., 2003, 67(4), 1105 CrossRef PubMed.
- M. Shin, O. Ishii, T. Sueda and J. P. Vacanti, Biomaterials, 2004, 25(17), 3717 CrossRef CAS PubMed.
- O. Ishii, M. Shin, T. Sueda and J. P. Vacanti, J. Thorac. Cardiovasc. Surg., 2005, 130(5), 1358 CrossRef PubMed.
- W. J. Li, R. Tuli, C. Okafor, A. Derfoul, K. G. Danielson, D. J. Hall and R. S. Tuan, Biomaterials, 2005, 26, 599 CrossRef CAS PubMed.
- W. J. Li, R. L. Mauck, J. A. Cooper, X. Yuan and R. S. Tuan, J. Biomech., 2007, 40, 1686 CrossRef PubMed.
- D. R. Nisbet, L. M. Y. Yu, T. Zahir, J. S. Forsythe and M. S. Shoichet, J. Biomater. Sci., Polym. Ed., 2008, 19(5), 623 CrossRef CAS PubMed.
- J. Venugopal, T. Y. L. L. Ma and S. Ramakrishna, Cell Biol. Int., 2005, 29, 861 CrossRef CAS PubMed.
- J. R. Venugopal, Y. Zhang and S. Ramakrishna, Artif. Organs, 2006, 30(6), 440 CrossRef CAS PubMed.
- S. Srouji, T. Kizhner, E. Suss-Tobi, E. Livne and E. Zussman, J. Mater. Sci.: Mater. Med., 2008, 19, 1249 CrossRef CAS PubMed.
- J. S. Choi, S. J. Lee, G. J. Christ, A. Atala and J. J. Yoo, Biomaterials, 2008, 29(19), 2899 CrossRef CAS PubMed.
- X. Yang, J. D. Shah and H. Wang, Tissue Eng., Part A, 2009, 15(4), 945 CrossRef CAS PubMed.
- Y. M. Ju, J. S. Choi, A. Atala, J. J. Yoo and S. J. Lee, Biomaterials, 2010, 31(15), 4313 CrossRef CAS PubMed.
- Y. Zhang, H. Ouyang, C. T. Lim, S. Ramakrishna and Z. M. Huang, J. Biomed. Mater. Res., 2005, 72(1), 156 CrossRef PubMed.
- Z. Ma, W. He, T. Yong and S. Ramakrishna, Tissue Eng., 2005, 11(7/8), 1149 CrossRef CAS PubMed.
- E. J. Chong, T. T. Phan, I. J. Lim, Y. Z. Zhang, B. H. Bay, S. Ramakrishna and C. T. Lim, Acta Biomater., 2007, 3, 321 CrossRef CAS PubMed.
- R. S. Tigli, N. M. Kazaroğlu, I. S. B. Mav and I. O. M. Gümüşderel, J. Biomater. Sci., Polym. Ed., 2011, 22(1–3), 207 CrossRef CAS PubMed.
- S. Gautam, A. K. Dinda and N. C. Mishra, Mater. Sci. Eng., C, 2013, 33, 1228 CrossRef CAS PubMed.
- Y. C. Lim, J. Johnson, Z. Fei, Y. Wu, D. F. Farson, J. J. Lannutti, H. W. Choi and L. J. Lee, Biotechnol. Bioeng., 2011, 108(1), 116 CrossRef CAS PubMed.
- A. Bianco, E. Di Federico, I. Moscatelli, A. Camaioni, I. Armentano, L. Campagnolo, M. Dottori, J. M. Kenny, G. Siracusa and G. Gusmano, Mater. Sci. Eng., C, 2009, 29, 2063 CrossRef CAS PubMed.
- J. P. Chen and Y. S. Chang, Colloids Surf., B, 2011, 86, 169 CrossRef CAS PubMed.
- Y. Ji, K. Liang, X. Shen and G. L. Bowlin, Carbohydr. Polym., 2014, 101, 68 CrossRef CAS PubMed.
- J. S. Lee, G. H. Jin, M. G. Yeo, C. H. Jang, H. Lee and G. H. Kim, Carbohydr. Polym., 2012, 90, 181 CrossRef CAS PubMed.
- M. Shin, H. Yoshimoto and J. P. Vacanti, Tissue Eng., 2004, 10(1/2), 33 CrossRef CAS PubMed.
- B. Nottelet, E. Pektok, D. Mandracchia, J. C. Tille, B. Walpoth, R. Gurny and M. Möller, J. Biomed. Mater. Res., Part A, 2009, 89(4), 865 CrossRef CAS PubMed.
- E. Pektok, B. Nottelet, J. C. Tille, R. Gurny, A. Kalangos, M. Moeller and B. H. Walpoth, Circulation, 2008, 118(24), 2563 CrossRef CAS PubMed.
- E. Piskin, I. A. Işoğlu, N. Bölgen, I. Vargel, S. Griffiths, T. Cavuşoğlu, P. Korkusuz, E. Güzel and S. Cartmell, J. Biomed. Mater. Res., Part A, 2009, 90(4), 1137 CrossRef PubMed.
- D. R. Nisbet, A. E. Rodda, M. K. Horne, J. S. Forsythe and D. I. Finkelstein, Biomaterials, 2009, 30(27), 4573 CrossRef CAS PubMed.
- H. Q. Cao, K. McHugh, S. Y. Chew and J. M. Anderson, J. Biomed. Mater. Res., Part A, 2010, 93(3), 1151 Search PubMed.
- B. S. Jha, R. J. Colello, J. R. Bowman, S. A. Sell, K. D. Lee, J. W. Bigbee, G. L. Bowlin, W. N. Chow, B. E. Mathern and D. G. Simpson, Acta Biomater., 2011, 7(1), 203 CrossRef CAS PubMed.
- W. J. Li, H. Chiang, T. F. Kuo, H. S. Lee, C. C. Jiang and R. S. Tuan, J. Tissue Eng. Regener. Med., 2009, 3, 1 CrossRef CAS PubMed.
- Z. C. Chen, A. K. Ekaputra, K. Gauthaman, P. G. Adaikan, H. Yu and D. W. Hutmacher, J. Biomater. Sci., Polym. Ed., 2008, 19(5), 693 CrossRef CAS PubMed.
- B. W. Tillman, S. K. Yazdani, S. J. Lee, R. L. Geary, A. Atala and J. J. Yoo, Biomaterials, 2009, 30(4), 583 CrossRef CAS PubMed.
- S. Fu, P. Ni, B. Wang, B. Chu, J. Peng, L. Zheng, X. Zhao, F. Luo, Y. Wei and Z. Qian, Biomaterials, 2012, 33, 8363 CrossRef CAS PubMed.
- J. E. Bergsma, W. C. de Bruijn, F. R. Rozema, R. R. Bos and G. Boering, Biomaterials, 1995, 16(1), 25 CrossRef CAS.
- R. Suuronen, T. Pohjonen, J. Hietanen and C. Lindqvist, J. Oral Maxillofac. Surg., 1998, 56(5), 604 CrossRef CAS.
- Z. Ma, C. Gao, Y. Gong and J. Shen, Biomaterials, 2005, 26, 1253 CrossRef CAS PubMed.
- F. Yang, R. Murugan, S. Ramakrishna, X. Wang, Y. Ma and S. Wang, Biomaterials, 2004, 25, 1891 CrossRef CAS PubMed.
- H. C. Liu, I. Lee, J. H. Wang, S. H. Yang and T. H. Young, Biomaterials, 2004, 25, 4047 CrossRef CAS PubMed.
- J. P. Chen and C. H. Su, Acta Biomater., 2011, 7, 234 CrossRef CAS PubMed.
- C. He, Z. Huang, X. Han, L. Liu, H. Zhang and L. Chen, J. Macromol. Sci., Phys., 2006, 45, 515 CrossRef CAS.
- X. Zong, K. Kim, D. Fang, S. Ran, B. Hsiao and B. Chu, Polymer, 2002, 43, 4403 CrossRef CAS.
- W. Hu and Z. M. Huang, Polym. Int., 2010, 59, 92 CrossRef CAS.
- H. Lee, S. Ahn, H. Choi, D. Cho and G. Kim, J. Mater. Chem. B, 2013, 1, 3670 RSC.
- L. Ylä-Outinen, C. Mariani, H. Skottman, R. Suuronen, A. Harlin and S. Narkilahti, Open Tissue Eng. Regener. Med. J., 2010, 3, 1 CrossRef.
- X. Xin, M. Hussain and J. J. Mao, Biomaterials, 2007, 28, 316 CrossRef CAS PubMed.
- F. Peng, X. Yu and M. Wei, Acta Biomater., 2011, 7(6), 2585–2592 CrossRef CAS PubMed.
- M. P. Prabhakaran, J. Venugopal and S. Ramakrishna, Acta Biomater., 2009, 5, 2884 CrossRef CAS PubMed.
- G. Sui, X. Yang, F. Mei, X. Hu, G. Chen, X. Deng and S. Ryu, J. Biomed. Mater. Res., Part A, 2007, 82(2), 445 CrossRef PubMed.
- S. D. McCullen, Y. Zhu, S. H. Bernacki, R. J. Narayan, B. Pourdeyhimi, R. E. Gorga and E. G. Loboa, Biomed. Mater., 2009, 4, 035002, DOI:10.1088/1748-6041/4/3/035002.
- S. Y. Gu, Z. M. Wang, J. Ren and C. Y. Zhang, Mater. Sci. Eng., C, 2009, 29, 1822 CrossRef CAS PubMed.
- P. Torricelli, M. Gioffre, A. Fiorani, S. Panzavolta, C. Gualandi, M. Fini, M. L. Focarete and A. Bigi, Mater. Sci. Eng., C, 2014, 36, 130 CrossRef CAS PubMed.
- L. Chen, Y. Bai, G. Liao, E. Peng, B. Wu, Y. Wang, X. Zeng and X. Xie, PLoS One, 2013, 8(8), 71265 Search PubMed.
- X. M. Mo, C. Y. Xu, M. Kotaki and S. Ramakrishna, Biomaterials, 2004, 25(10), 1883 CrossRef CAS PubMed.
- L. Lao, Y. Wang, Y. Zhu, Y. Zhang and C. Gao, J. Mater. Sci.: Mater. Med., 2011, 22(8), 1873 CrossRef CAS PubMed.
- M. D. Schofer, P. P. Roessler, J. Schaefer, C. Theisen, S. Schlimme, J. T. Heverhagen, M. Voelker, R. Dersch, S. Agarwal, S. Fuchs-Winkelmann and J. R. Paletta, PLoS One, 2011, 6(9), e25462 CAS.
- E. D. Boland, B. D. Coleman, C. P. Barnes, D. G. Simpson, G. E. Wnek and G. L. Bowlin, Acta Biomater., 2005, 1, 115 CrossRef PubMed.
- S. A. Sell, M. J. McClure, C. P. Barnes, D. C. Knapp, B. H. Walpoth, D. G. Simpson and G. L. Bowlin, Biomed. Mater., 2006, 1, 72 CrossRef CAS PubMed.
- M. J. McClure, S. A. Sell, D. G. Simpson and G. L. Bowlin, J. Eng. Fibers Fabr., 2009, 4(2), 18 CAS.
- M. J. McClure, S. A. Sell, C. P Barnes, W. C. Bowen and G. L. Bowlin, J. Eng. Fibers Fabr., 2008, 3(1), 1 CAS.
- I. A. Rodriguez, P. A. Madurantakam, J. M. McCool, S. A. Sell, H. Yang, P. C. Moon and G. L. Bowlin, Int. J. Biomater., 2012, 159484 Search PubMed.
- M. J. Smith, K. L. White, D. C. Smith and G. L. Bowlin, Biomaterials, 2009, 30(2), 149 CrossRef CAS PubMed.
- D. Kalfa, A. Bel, A. Chen-Tournoux, A. Della Martina, P. Rochereau, C. Coz, V. Bellamy, M. Bensalah, V. Vanneaux, S. Lecourt, E. Mousseaux, P. Bruneval, J. Larghero and P. Menasché, Biomaterials, 2010, 31, 4056 CrossRef CAS PubMed.
- O. Hakimi, S. Chaudhury, R. Murphy and A. Carr, J. Biomed. Mater. Res., Part B, 2012, 100(3), 685 CrossRef PubMed.
- O. Hakimi, R. Murphy, U. Stachewicz, S. Hislop and A. J. Carr, Eur. Cells Mater., 2012, 24, 344 CAS.
- C. P. Barnes, S. A. Sell, D. C. Knapp, B. H. Walpoth, D. D. Brand and G. L. Bowlin, Int. J. Electrospun. Nanofibers. Appl., 2007, 1, 73 Search PubMed.
- S. A. Sell, P. S. Wolfe, K. Garg, J. M. McCool, I. A. Rodriguez and G. L. Bowlin, Polymers, 2010, 2, 522 CrossRef CAS PubMed.
- M. C. Farach-Carson, R. C. Wagner and K. L. Kiick, Extracellular matrix: Structure, function, and applications to tissue engineering, in Tissue Engineering, ed. J. P. Fisher, A. G. Mikos and J. D. Bronzino, CRC Press, Boca Raton, FL, USA, 2007, pp. 3-1-3-22 Search PubMed.
- S. Sell, C. Barnes, M. Smith, M. McClure, P. Madurantakam, J. Grant, M. McManus and G. Bowlin, Polym. Int., 2007, 56, 1349 CrossRef CAS.
- S. A. Sell, M. J. McClure, K. Garg, P. S. Wolfe and G. L. Bowlin, Adv. Drug Delivery Rev., 2009, 61, 1007 CrossRef CAS PubMed.
- L. Huang, K. Nagapudi, R. P. Apkarian and E. L. Chaikof, J. Biomater. Sci., Polym. Ed., 2001, 12, 979 CrossRef CAS PubMed.
- J. A. Matthews, G. E. Wnek, D. G. Simpson and G. L. Bowlin, Biomacromolecules, 2002, 3, 232 CrossRef CAS PubMed.
- J. A. Matthews, E. D. Boland, G. E. Wnek, D. G. Simpson and G. L. Bowlin, J. Bioact. Compat. Polym., 2003, 18, 125 CrossRef CAS PubMed.
- C. P. Barnes, C. W. Pemble, D. D. Brand, D. G. Simpson and G. L. Bowlin, Tissue Eng., 2007, 13, 1593 CrossRef CAS PubMed.
- S. A. Sell, M. J. McClure, K. Garg, P. S. Wolfe and G. L. Bowlin, Adv. Drug Delivery Rev., 2009, 61, 1007 CrossRef CAS PubMed.
- J. A. Matthews, G. E. Wnek, D. G. Simpson and G. L. Bowlin, Biomacromolecules, 2002, 3(2), 232 CrossRef CAS PubMed.
- K. J. Shields, M. J. Beckman, G. L. Bowlin and J. S. Wayne, Tissue Eng., 2004, 10(9–10), 1510 CrossRef CAS PubMed.
- K. S. Rho, L. Jeong, G. Lee, B. M. Seo, Y. J. Park, S. D. Hong, S. Roh, J. J. Cho, W. H. Park and B. M. Min, Biomaterials, 2006, 27(8), 1452 CrossRef CAS PubMed.
- S. Zhong, W. E. Teo, X. Zhu, R. W. Beuerman, S. Ramakrishna and L. Y. Yung, J. Biomed. Mater. Res., Part A, 2006, 79A(3), 456 CrossRef CAS PubMed.
- Y. R. Shih, C. N. Chen, S. W. Tsai, Y. J. Wang and O. K. Lee, Stem Cells, 2006, 24(11), 2391 CrossRef CAS PubMed.
- S. P. Zhong, W. E. Teo, X. Zhu, R. Beuerman, S. Ramakrishna, L. Yue and L. Yung, Mater. Sci. Eng., C, 2007, 27, 262 CrossRef CAS PubMed.
- S. J. Liu, Y. C. Kau, C. Y. Chou, J. K. Chen, R. C. Wu and W. L. Yeh, J. Membr. Sci., 2010, 355, 53 CrossRef CAS PubMed.
- J. H. Song, H. E. Kim and H. W. Kim, J. Mater. Sci.: Mater. Med., 2008, 19, 2925 CrossRef CAS PubMed.
- J. Venugopal, S. Low, A. T. Choon, T. S. Sampath Kumar and S. Ramakrishna, J. Mater. Sci.: Mater. Med., 2008, 19(5), 2039 CrossRef CAS PubMed.
- Z. G. Chen, P. W. Wang, B. Wei, X. M. Mo and F. Z. Cui, Acta Biomater., 2010, 6, 372 CrossRef CAS PubMed.
- H. M. Powell, D. M. Supp and S. T. Boyce, Biomaterials, 2008, 29, 834 CrossRef CAS PubMed.
- C. P. Barnes, S. A. Sell, E. D. Boland, D. G. Simpson and G. L. Bowlin, Adv. Drug Delivery Rev., 2007, 59, 1413 CrossRef CAS PubMed.
- U. R. Rodgers and A. S. Weiss, Pathol. Biol., 2005, 53, 390 CrossRef CAS PubMed.
- L. Debelle and A. M. Tamburro, Int. J. Biochem. Cell Biol., 1999, 31, 261 CrossRef CAS.
- E. N. Lamme, R. T. van Leeuwen, A. Jonker, J. van Marle and E. Middelkoop, J. Invest. Dermatol., 1998, 111, 989 CrossRef CAS PubMed.
- S. Neuenschwander and S. P. Hoerstrup, Transplant Immunol., 2004, 12, 359 CrossRef CAS PubMed.
- J. W. Xu, T. S. Johnson, P. M. Motarjem, G. M. Peretti, M. A. Randolph and M. J. Yaremchuk, Plast. Reconstr. Surg., 2005, 115, 1633 CAS.
- W. F. Daamen, J. H. Veerkamp, J. C. M. van Hest and T. H. van Kuppevelt, Biomaterials, 2007, 28, 4378 CrossRef CAS PubMed.
- L. Nivison-Smith, J. Rnjak and A. S. Weiss, Acta Biomater., 2010, 6, 354 CrossRef CAS PubMed.
- S. G. Wise, M. J. Byrom, A. Waterhouse, P. G. Bannon, A. S. Weiss and M. K. Ng, Acta Biomater., 2011, 7(1), 295 CrossRef CAS PubMed.
- K. A. McKenna, M. T. Hinds, R. C. Sarao, P. C. Wu, C. L. Maslen, R. W. Glanville, D. Babcock and K. W. Gregory, Acta Biomater., 2012, 8(1), 225 CrossRef CAS PubMed.
- M. Li, M. J. Mondrinos, M. R. Gandhi, F. K. Ko, A. S. Weiss and P. I. Lelkes, Biomaterials, 2005, 26(30), 5999 CrossRef CAS PubMed.
- J. Rnjak, Z. Li, P. K. Maitz, S. G. Wise and A. S. Weiss, Biomaterials, 2009, 30(32), 6469 CrossRef CAS PubMed.
- J. Rnjak-Kovacina, S. G. Wise, Z. Li, P. K. Maitz, C. J. Young, Y. Wang and A. S. Weiss, Biomaterials, 2011, 32, 6729 CrossRef CAS PubMed.
- J. Rnjak-Kovacina, S. G. Wise, Z. Li, P. K. Maitz, C. J. Young, Y. Wang and A. S. Weiss, Acta Biomater., 2012, 8, 3714 CrossRef CAS PubMed.
- S. G. Wise, M. J. Byrom, A. Waterhouse, P. G. Bannon, A. S. Weiss and M. K. Ng, Acta Biomater., 2011, 7, 295 CrossRef CAS PubMed.
- M. Li, M. J. Mondrinos, X. Chen, M. R. Gandhi, F. K. Ko and P. I. Lelkes, J. Biomed. Mater. Res., Part A, 2006, 79(4), 963 CrossRef PubMed.
- L. A. Bosworth, Conference Papers in Science, 2014, 304974, DOI:10.1155/2014/304974.
- ClinicalTrials Homepage, available online at http://www.clinicaltrials.gov/, accessed on 25 May 2014.
- InVivo Therapeutics Homepage, available online at http://www.invivotherapeutics.com/2013/04/invivo-therapeutics-receives-approval-from-fda-for-first-human-trial-using-biomaterials-for-traumatic-spinal-cord-injury/, accessed on 22 May 2014.
- Available online at http://www.nicast.com/index.aspx?id=2930, accessed on 22 May 2014.
- Nicast Homepage, available online at http://www.nicast.com/index.aspx?id=2959, accessed on 25 May 2014.
- M. Stuart, start-up, 2009, 14(8), 1, available online at http://www.nuortho.com/assets/pdf/News_CartilageRepair0909su.pdf, accessed on 25 May 2014.
- St Teresa Medical Inc. Homepage, available online at http://www.stteresamedical.com/about_us.php, accessed on 25 May 2014.
- Nanofiber Veterinary, available online at http://nanofiberveterinary.com/nanocarev.html, accessed on 25 May 2014.
- Nanofiber Veterinary, available online at http://nanofiberveterinary.com/nanoligv.html, accessed on 25 May 2014.
- Nanofiber Veterinary, available online at http://nanofiberveterinary.com/nanovesselv.html, accessed on 25 May 2014.
- Nanofiber Veterinary, available online at http://nanofiberveterinary.com/nanobonev.html, accessed on 25 May 2014.
| This journal is © The Royal Society of Chemistry 2014 |
