Open Access Article
Open Access ArticleCopper-catalyzed nucleophilic trifluoromethylation of propargylic halides†
Yoshihiro
Miyake
,
Shin-ichi
Ota
,
Masashi
Shibata
,
Kazunari
Nakajima
and
Yoshiaki
Nishibayashi
*
Institute of Engineering Innovation, School of Engineering, The University of Tokyo, Yayoi, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: ynishiba@sogo.t.u-tokyo.ac.jp; Fax: +81 3-5841-1175; Tel: +81 3-5841-1175
First published on 4th July 2013
Abstract
Reactions of propargylic halides with trifluoromethyltrimethylsilane in the presence of a catalytic amount of copper(I) thiophene-2-carboxylate (CuTC) have been found to give the corresponding trifluoromethylated products in good to high yields with a high selectivity.
Trifluoromethylation of organic compounds is one of the most important methodologies in organic synthesis1 because a wide range of organic molecules bearing a trifluoromethyl (CF3) group have attracted considerable attention as highly promising skeletons in the field of pharmaceuticals and materials.2 In this context, the development of methods for the formation of C(sp2)–1 and C(sp3)–CF33–6 bonds has been expected and extensively studied for the last several years. Recently, we have also succeeded in the development of copper-catalyzed nucleophilic trifluoromethylation of allylic halides, where the introduction of a CF3 group occurred at the α-position of the carbon–halogen bond with complete regioselectivity (Scheme 1a).7,8
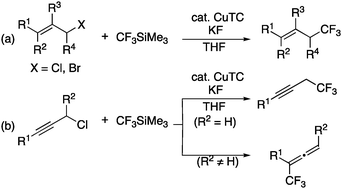 | ||
| Scheme 1 (a) Copper-catalyzed nucleophilic trifluoromethylation of allylic halides. (b) Copper-catalyzed nucleophilic trifluoromethylation of propargylic halides (this work). | ||
Transition metal-catalyzed propargylic substitution reactions of propargylic alcohol derivatives and halides are expected to be simple methods for constructing carbon–carbon and carbon–heteroatom bonds at the propargylic position.9 We have continuously studied ruthenium-10 and copper-catalyzed11 propargylic substitution reactions of propargylic alcohols and derivatives with a variety of nucleophiles to give the corresponding propargylic substituted products in good to high yields with complete selectivity. In sharp contrast, successful examples of nucleophilic trifluoromethylation of propargylic halides using transition metal complexes are quite limited.8b,12 In these reactions, stoichiometric amounts of copper metal or copper salts are required to obtain the trifluoromethylated products in good yields.12b,c To the best of our knowledge, there is no report on catalytic trifluoromethylation of propargylic halides. As an extension of our study, we have now envisaged copper(I)-catalyzed nucleophilic trifluoromethylation of propargylic halides with trifluoromethyltrimethylsilane (Ruppert–Prakash reagent; CF3SiMe3).13 In fact, we have succeeded in the introduction of a CF3 group at the propargylic- (R2 = H) and allenylic- (R2 ≠ H) positions from reactions of primary and secondary propargylic halides, respectively (Scheme 1b). Preliminary results are described herein.
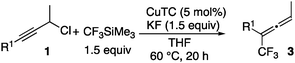
Treatment of 3-phenylpropargyl chloride (1a) with 3.0 equiv. of CF3SiMe3 in the presence of a catalytic amount of copper(I) thiophene-2-carboxylate (CuTC) (5 mol%) and a stoichiometric amount of potassium fluoride (KF) (3.0 equiv.) in tetrahydrofuran (THF) at 60 °C for 20 h gave 1,1,1-trifluoro-4-phenylbut-3-yne (2a) in 72% yield with complete regioselectivity, where no formation of 1,1,1-trifluoro-2-phenylbuta-2,3-diene (3a) was observed at all (Table 1, entry 1). The choice of leaving groups is one of the most important factors to promote this trifluoromethylation effectively. In fact, when 3-phenylpropargyl bromide was used in place of 1a, a mixture of 2a and 3a was obtained in 35% and 16% yields, respectively (Table 1, entry 2). Other substrates such as 3-phenylpropargyl iodide, mesylate (OMs = OSO2Me), and alcohol were ineffective (Table 1, entries 3–5). Use of a smaller amount of CF3SiMe3 afforded 2a effectively. In fact, the reaction of 1a with 1.5 equiv. of CF3SiMe3 took place smoothly to give 2a in 78% yield (Table 1, entry 6). Unfortunately, when reactions of 3-phenylpropargyl acetate and trifluoroacetate were carried out, only a small amount of 2a was obtained in both cases (4% and 3%, respectively). Separately, we confirmed that no formation of 2a was observed in the absence of CuTC or KF.
|
|
||||
|---|---|---|---|---|
| Entry | X | CF3SiMe3 and KF (equiv.) | Yield of 2ab (%) | Yield of 3ab (%) |
| a All reactions of the phenylpropargyl substrate (0.50 mmol) with CF3SiMe3 were carried out in the presence of CuTC (0.025 mmol) and KF in THF (3 mL) at 60 °C for 20 h. b Determined by 1H NMR. c Isolated yield. | ||||
| 1 | Cl (1a) | 3.0 | 72 | 0 |
| 2 | Br | 3.0 | 35 | 16 |
| 3 | I | 3.0 | 48 | 8 |
| 4 | OMs | 3.0 | 47 | 2 |
| 5 | OH | 3.0 | 7 | 0 |
| 6 | Cl (1a) | 1.5 | 78 (73)c | 1 |
Propargylic trifluoromethylation of a variety of 3-arylpropargyl chlorides in the presence of a catalytic amount of CuTC proceeded smoothly to give the corresponding propargylic trifluoromethylated products 2 in good to high yields. Typical results are shown in Table 2. The introduction of a substituent such as methyl, chloro, or methoxy at the para-position in the benzene ring of 1a did not affect the yields of 2 much (Table 2, entries 1–3). The reaction of p-trifluoromethylphenylpropargyl chloride (1e) for 48 h gave 2e in 75% yield although a larger amount (10 mol%) of CuTC was required (Table 2, entry 4). Reactions of 3-tolylpropargyl chlorides (1f and 1g) and 3-naphthylpropargyl chlorides (1h and 1i) also took place to give the corresponding propargylic trifluoromethylated products (2f–2i) in good to high yields (Table 2, entries 5–8). Propargylic chlorides bearing no conjugated aromatic rings (1j and 1k) were also applicable to this reaction system, giving the corresponding propargylic trifluoromethylated products (2j–2k) in high yields (Table 2, entries 9 and 10).
|
|
||
|---|---|---|
| Entry | Propargylic chloride (1) | Yield of 2b (%) |
| a All reactions of 1 (0.50 mmol) with CF3SiMe3 (0.75 mmol) were carried out in the presence of 5 mol% of CuTC (0.025 mmol) in THF (3 mL) at 60 °C for 20 h. b Isolated yield. c 10 mol% of CuTC was used. d For 48 h. | ||
| 1 | R1 = p-MeC6H4 (1b) | 83 (2b) |
| 2 | R1 = p-ClC6H4 (1c) | 71 (2c) |
| 3 | R1 = p-MeOC6H4 (1d) | 79 (2d) |
| 4c,d | R1 = p-CF3C6H4 (1e) | 75 (2e) |
| 5 | R1 = o-MeC6H4 (1f) | 84 (2f) |
| 6 | R1 = m-MeC6H4 (1g) | 62 (2g) |
| 7 | R1 = 1-naphthyl (1h) | 86 (2h) |
| 8 | R1 = 2-naphthyl (1i) | 72 (2i) |
| 9 | R1 = PhCH2 (1j) | 80 (2j) |
| 10 | R1 = PhCH2CH2 (1k) | 93 (2k) |
Recently, Szabó's group has reported the copper-mediated trifluoromethylation of propargylic halides.12c In their reaction system, the reaction of 1l with a stoichiometric amount of Cu(CF3)(PPh3)3 at room temperature gave the corresponding trifluoromethylated allene 3l in good yield, while the formation of the propargylic trifluoromethylated product 2l was observed when the reaction was carried out at 50 °C (Scheme 2; Szabó's system).12c In sharp contrast to the result obtained by Szabó's group, only 2l was obtained from the reaction of 1l in the presence of 5 mol% of CuTC even at room temperature (Scheme 2; our system). These results indicate that our catalytic reaction proceeds via a different pathway from that of Szabó's system.
 | ||
| Scheme 2 Copper-mediated and catalyzed propargylic trifluoromethylation of 1l. | ||
Next, nucleophilic trifluoromethylation of a variety of secondary propargylic chlorides in the presence of a catalytic amount of CuTC was investigated. Typical results are shown in Table 3. When we carried out the reaction of 2-chloro-4-phenylbut-3-yne (1m) with 1.5 equiv. of CF3SiMe3, the corresponding trifluoromethylated allene (3m) was obtained in 89% isolated yield (Table 3, entry 1). The introduction of a substituent such as methyl and chloro groups at the para-position in the benzene ring of 1m did not affect the yields of 3 much (Table 3, entries 2 and 3). The reactions of 2-chloro-4-(4-methoxyphenyl)but-3-yne (1p) at lower temperature (40 °C) gave the good result (Table 3, entry 4). Secondary propargylic chloride bearing no conjugated aromatic ring (1q) was also applicable to this reaction system, giving the corresponding trifluoromethylated allene (3q) in 71% yield (Table 3, entry 5). Only a small amount (8%) of the corresponding trifluoromethylated allene was obtained from the reaction of 1-chloro-1-phenylprop-2-yne under the same reaction conditions.
|
|
||
|---|---|---|
| Entry | Propargylic chloride (1) | Yield of 3b (%) |
| a All reactions of 1 (0.50 mmol) with CF3SiMe3 (0.75 mmol) were carried out in the presence of 5 mol% of CuTC (0.025 mmol) in THF (3 mL) at 60 °C for 20 h. b Isolated yield. c At 40 °C for 48 h. | ||
| 1 | R1 = Ph (1m) | 89 (3m) |
| 2 | R1 = p-MeC6H4 (1n) | 79 (3n) |
| 3 | R1 = p-ClC6H4 (1o) | 81 (3o) |
| 4c | R1 = p-MeOC6H4 (1p) | 77 (3p) |
| 5 | R1 = PhCH2CH2 (1q) | 71 (3q) |
In order to obtain information on the reaction pathway, we investigated the reaction of an optically active secondary propargylic chloride. Treatment of (R)-1m (94% ee) with 1.5 equiv. of CF3SiMe3 under the same reaction conditions afforded 3m in 83% yield with a complete loss of optical purity (Scheme 3). This result indicates that our catalytic reaction proceeds not via an anti-SN2′ pathway14 but via other pathways involving cationic propargyl/allenyl–copper complexes as reactive intermediates.7,9c
 | ||
| Scheme 3 Copper-catalyzed trifluoromethylation of (R)-1m with trifluoromethyltrimethylsilane. | ||
In summary, we have found the copper-catalyzed nucleophilic trifluoromethylation of propargylic chlorides with trifluoromethyltrimethylsilane. In our system, reactions of primary propargylic chlorides (1) afford the corresponding propargylic trifluoromethylated products (2), while the trifluoromethylated allenes (3) can be obtained from reactions of secondary propargylic chlorides. This is the first successful example of catalytic trifluoromethylation of propargylic halides. We believe that the method described here provides an efficient strategy for the synthesis of CF3-containing compounds at the propargylic and allenylic positions, which are useful building blocks in pharmaceuticals.12c,15 Further work is currently in progress to apply this strategy to the enantioselective reactions and to clarify the precise reaction mechanism.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Advanced Molecular Transformations by Organocatalyst” from the Ministry of Education, Culture, Sports, Science and Technology, Japan and the Funding Program for Next Generation World-Leading Researchers (GR025). We thank Dr Shingo Ito and Prof. Dr Kyoko Nozaki at The University of Tokyo for measurement of 19F NMR.
Notes and references
- For recent reviews on trifluoromethylation, see: (a) J.-A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1 CrossRef CAS; (b) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS; (c) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef CAS; (d) T. Besset, C. Schneider and D. Cahard, Angew. Chem., Int. Ed., 2012, 51, 5048 CrossRef CAS; (e) A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8950 CrossRef CAS.
- (a) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC.
- For reviews on trifluoromethylation of carbonyl compounds, see: (a) G. K. S. Prakash and F. Wang, in Organic Chemistry – Breakthroughs and Perspectives, ed. K. Ding and L.-X. Dai, Wiley-VCH, Weinheim, 2012, ch. 12, pp. 413–476 Search PubMed; (b) N. Shibata, S. Mizuta and H. Kawai, Tetrahedron: Asymmetry, 2008, 19, 2633 CrossRef CAS.
- For examples of enantioselective α-trifluoromethylation of aldehydes, see: (a) D. A. Nagib, M. E. Scott and D. W. C. MacMillan, J. Am. Chem. Soc., 2009, 131, 10875 CrossRef CAS; (b) A. E. Allen and D. W. C. MacMillan, J. Am. Chem. Soc., 2010, 132, 4986 CrossRef CAS.
- (a) A. T. Parsons and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 9120 CrossRef CAS; (b) J. Xu, Y. Fu, D.-F. Luo, Y.-Y. Jiang, B. Xiao, Z.-J. Liu, T.-J. Gong and L. Liu, J. Am. Chem. Soc., 2011, 133, 15300 CrossRef CAS; (c) X. Wang, Y. Ye, S. Zhang, J. Feng, Y. Xu, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2011, 133, 16410 CrossRef CAS; (d) R. Shimizu, H. Egami, Y. Hamashima and M. Sodeoka, Angew. Chem., Int. Ed., 2012, 51, 4577 CrossRef CAS; (e) L. Chu and F.-L. Qing, Org. Lett., 2012, 14, 2106 CrossRef CAS.
- For recent examples, see: (a) P. G. Janson, I. Ghoneim, N. O. IIchenko and K. J. Szabó, Org. Lett., 2012, 14, 2882 CrossRef CAS; (b) R. Zhu and S. L. Buchwald, J. Am. Chem. Soc., 2012, 134, 12462 CrossRef CAS; (c) Y. Li and A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8221 CrossRef CAS; (d) Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2012, 51, 9567 CrossRef CAS.
- Y. Miyake, S. Ota and Y. Nishibayashi, Chem.–Eur. J., 2012, 18, 13255 CrossRef CAS.
- For examples of nucleophilic trifluoromethylation of allylic halides using a stoichiometric amount of copper salts, see: (a) Y. Kobayashi, K. Yamamoto and I. Kumadaki, Tetrahedron Lett., 1979, 20, 4071 CrossRef; (b) J.-P. Bouillon, C. Maliverney, R. Merényi and H. G. Viehe, J. Chem. Soc., Perkin Trans. 1, 1991, 2147 RSC; (c) H. Urata and T. Fuchikami, Tetrahedron Lett., 1991, 32, 91 CrossRef CAS; (d) D.-B. Su, J.-X. Duan and Q.-Y. Chen, Tetrahedron Lett., 1991, 32, 7689 CrossRef CAS; (e) Q.-Y. Chen and J.-X. Duan, J. Chem. Soc., Chem. Commun., 1993, 1389 RSC; (f) J. Kim and J. M. Shreeve, Org. Biomol. Chem., 2004, 2, 2728 RSC.
- For recent reviews on propargylic substitution reactions, see: (a) G. W. Kabalka and M. L. Yao, Curr. Org. Synth., 2008, 5, 28 CrossRef CAS; (b) N. Ljungdahl and N. Kann, Angew. Chem., Int. Ed., 2009, 48, 642 CrossRef CAS; (c) Y. Miyake, S. Uemura and Y. Nishibayashi, ChemCatChem, 2009, 1, 342 CrossRef CAS; (d) R. J. Detz, H. Hiemstra and J. H. van Maarseveen, Eur. J. Org. Chem., 2009, 6263 CrossRef CAS; (e) C.-H. Ding and X.-L. Hou, Chem. Rev., 2011, 111, 1914 CrossRef CAS; (f) Y. Nishibayashi, Synthesis, 2012, 489 CrossRef CAS.
- For examples of ruthenium-catalyzed reactions, see: (a) K. Fukamizu, Y. Miyake and Y. Nishibayashi, J. Am. Chem. Soc., 2008, 130, 10498 CrossRef CAS; (b) M. Ikeda, Y. Miyake and Y. Nishibayashi, Angew. Chem., Int. Ed., 2010, 49, 7289 CrossRef CAS; (c) M. Ikeda, Y. Miyake and Y. Nishibayashi, Chem.–Eur. J., 2012, 18, 3321 CrossRef CAS and references therein.
- For examples of copper-catalyzed reactions, see: (a) G. Hattori, H. Matsuzawa, Y. Miyake and Y. Nishibayashi, Angew. Chem., Int. Ed., 2008, 47, 3781 CrossRef CAS; (b) G. Hattori, K. Sakata, H. Matsuzawa, Y. Tanabe, Y. Miyake and Y. Nishibayashi, J. Am. Chem. Soc., 2010, 132, 10592 CrossRef CAS; (c) A. Yoshida, G. Hattori, Y. Miyake and Y. Nishibayashi, Org. Lett., 2011, 13, 2460 CrossRef CAS and references therein.
- (a) D. J. Burton, G. A. Hartgraves and J. Hsu, Tetrahedron Lett., 1990, 31, 3699 CrossRef CAS; (b) H. Kawai, T. Furukawa, Y. Nomura, E. Tokunaga and N. Shibata, Org. Lett., 2011, 13, 3596 CrossRef CAS; (c) T. S. N. Zhao and K. J. Szabó, Org. Lett., 2012, 14, 3966 CrossRef CAS.
- (a) G. K. S. Prakash and A. K. Yudin, Chem. Rev., 1997, 97, 757 CrossRef CAS; (b) R. P. Singh and J. M. Shreeve, Tetrahedron, 2000, 56, 7613 CrossRef CAS.
- For recent examples of copper-catalyzed reactions, see: (a) C. Zhong, Y. Sasaki, H. Ito and M. Sawamura, Chem. Commun., 2009, 5850 RSC; (b) H. Ohmiya, U. Yokobori, Y. Makida and M. Sawamura, Org. Lett., 2011, 13, 6312 CrossRef CAS; (c) M. Yang, N. Yokokawa, H. Ohmiya and M. Sawamura, Org. Lett., 2012, 14, 816 CrossRef CAS.
- Y. Matsuya, D. Ihara, M. Fukuchi, D. Honma, K. Itoh, A. Tabuchi, H. Nemoto and M. Tsuda, Bioorg. Med. Chem., 2012, 20, 2564 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3cc44434a |
| This journal is © The Royal Society of Chemistry 2013 |