A viscosity-responsive mitochondria-targeting probe for rapid imaging of fatty liver disease†
Jiamin Liu‡
a,
Hui Zhou‡ *a,
Deyi Lib,
Haotong Yina,
Yi Zhoua,
Yuquan Jia,
Yujing Zhanga,
Xinyue Zhanga,
Ben Wanga,
Chao Yin
*a,
Deyi Lib,
Haotong Yina,
Yi Zhoua,
Yuquan Jia,
Yujing Zhanga,
Xinyue Zhanga,
Ben Wanga,
Chao Yin *a and
Quli Fan
*a and
Quli Fan a
a
aState Key Laboratory of Flexible Electronics (LoFE), Institute of Advanced Materials (IAM), Jiangsu Key Laboratory of Smart Biomaterials and Theranostic Technology, Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), School of Materials Science and Engineering, Nanjing University of Posts and Telecommunications, Nanjing, 210023, China. E-mail: iamhzhou@njupt.edu.cn; iamcyin@njupt.edu.cn
bFujian Haixi Pharmaceuticals Co., Ltd, Fuzhou, 350028, China
First published on 15th April 2025
Abstract
Fatty liver disease (FLD) is a leading cause of chronic liver disease worldwide, yet current diagnostic methods remain limited by low sensitivity, poor accuracy, and prolonged detection times. Recent studies have linked liver viscosity, particularly mitochondrial viscosity variations, to the progression of FLD, highlighting the need for a rapid and noninvasive viscosity-sensitive imaging tool. Herein, we present a viscosity-responsive fluorescent probe ZLCN, designed for rapid real-time imaging of fatty liver disease. ZLCN integrates an acrylonitrile rotor for viscosity sensing and a pyridine moiety for selective mitochondrial localization, enabling precise detection of viscosity alterations at the subcellular level. The probe exhibits strong fluorescence in high-viscosity environments due to restricted intramolecular rotation. ZLCN exhibits excellent viscosity responsiveness, effectively distinguishing normal and cancerous liver cells based on viscosity differences in vitro. Furthermore, it differentiates AML12 cells with varying viscosity levels, demonstrating its capability to monitor viscosity changes. In fatty liver models, ZLCN could produce intense fluorescence signals in fatty liver tissues and enabled rapid viscosity detection within 30 minutes, demonstrating a significant improvement over conventional imaging technique. These findings establish ZLCN as a promising tool for real-time mitochondrial viscosity monitoring, offering new avenues for early diagnosis and therapeutic assessment of viscosity-related liver diseases.
Introduction
Fatty liver disease (FLD), characterized by excessive lipid accumulation in hepatocytes, has emerged as a major global health concern due to its increasing prevalence and potential progression to severe conditions such as cirrhosis, hepatocellular carcinoma, and cardiovascular diseases.1,2 Despite the urgent need for effective diagnostic tools, current imaging techniques such as ultrasonography (US) and computed tomography (CT) often suffer from low sensitivity and accuracy, particularly in cases of mild steatosis.3–5 As a result, developing a more reliable and efficient approach for identifying fatty liver related disease is critical, with the potential to address the limitations of existing diagnostic technologies. Viscosity is a key property in biological systems, and abnormal viscosity is closely linked to various diseases and dysfunctions.6 Specifically, viscosity changes play a significant role in the development of fatty liver disease.7–10 Excessive accumulation of fatty acids disrupts lipid homeostasis, induces inflammatory cytokine release, and leads to fatty liver. Additionally, lipid buildup alters the cellular microenvironment, including viscosity. Therefore, developing an accurate method for monitoring viscosity is crucial for the early identification and effective treatment of fatty liver disease.Fluorescence imaging, with its non-invasive approach, high sensitivity, real-time capability and ease of operation, has become a valuable tool for disease diagnosis.11–18 In particular, viscosity-responsive fluorescent probes have been developed for detecting viscosity alterations in neurodegenerative diseases (e.g., Alzheimer's disease),19–21 cancer,22–24 inflammation25–28 and fatty liver.7,29–34 Although there are reports on the use of NIR probes for monitoring viscosity in fatty liver disease, several significant challenges persist. For example, some viscosity-responsive probes lack the ability to target specific subcellular compartments, while others have small Stokes shifts, which fail to effectively reduce background fluorescence. Furthermore, the rapid detection of fatty liver in living organisms remains a relatively rare achievement. Therefore, it is of great significance and urgency to develop a viscosity probe with high performance for the imaging of fatty liver with rapid speed.
To address these challenges, we designed and synthesized ZLCN, a viscosity-responsive and mitochondria-targeting fluorescent probe (Scheme 1). In its molecular structure, acrylonitrile functions as the rotor, and in highly viscous environments, its intramolecular rotation is significantly restricted, resulting in a strong fluorescence emission. The pyridine moieties serve as mitochondrial-targeting groups. Through protonation, they form quaternized pyridinium cations, which facilitate selective accumulation in mitochondria by interacting with the negatively charged mitochondrial membranes. The viscosity-sensitive behavior of ZLCN is confirmed by its significant fluorescence response in the presence of glycerol, a common viscosity-enhancing agent. The probe exhibits excellent viscosity responsiveness, and its fluorescence intensity is strongly correlated with viscosity, enabling the detection of viscosity variations in various cellular and organ environments. The probe's ability to detect viscosity in living cells, particularly in liver cells, is also demonstrated, with ZLCN distinguishing between normal and cancerous cells based on their viscosity differences. More importantly, ZLCN successfully detects viscosity changes in fatty liver models, both at the cellular and organ levels, offering rapid and accurate imaging capabilities. Given the increasing prevalence of fatty liver disease and the need for early intervention, ZLCN presents a promising tool for both diagnostics and therapeutic evaluations. By enabling the rapid detection of viscosity changes in the liver, ZLCN could contribute significantly to improving the clinical management of fatty liver disease, providing a valuable platform for future research and clinical applications.
 | ||
| Scheme 1 Schematic representation of the structure, viscosity response mechanism, and in vivo imaging capabilities of the mitochondria-targeted fluorescent probe ZLCN. | ||
Results and discussion
Design and synthesis
ZLCN was synthesized through the coupling of thiophene-modified triphenylamine and 2-(4-(pyridin-4-yl)phenyl)acetonitrile units, which were linked by a double carbon–carbon (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) bond, creating a classic “donor–π–acceptor (D–π–A)” structure (Fig. S1, ESI†). The C
C) bond, creating a classic “donor–π–acceptor (D–π–A)” structure (Fig. S1, ESI†). The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond can function as a molecular motor, allowing the probe to sense viscosity, while the pyridine group can be protonated to facilitate mitochondrial targeting. ZLCN itself remained largely in a fluorescence “off” state due to the free rotation of the bond between the thiophene and cyano group, governed by a twisting intramolecular charge transfer (TICT) mechanism. In a higher-viscosity environment, the designed optical probe is expected to exhibit the fluorescence “on” signal as the free rotation of the vinyl bond becomes restricted. Therefore, ZLCN serves as a powerful tool for measuring viscosity in cellular and organ-level environments. The structure of ZLCN was characterized by proton nuclear magnetic resonance (1H NMR) spectroscopy and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, as shown in Fig. S2–S5 (ESI†).
C bond can function as a molecular motor, allowing the probe to sense viscosity, while the pyridine group can be protonated to facilitate mitochondrial targeting. ZLCN itself remained largely in a fluorescence “off” state due to the free rotation of the bond between the thiophene and cyano group, governed by a twisting intramolecular charge transfer (TICT) mechanism. In a higher-viscosity environment, the designed optical probe is expected to exhibit the fluorescence “on” signal as the free rotation of the vinyl bond becomes restricted. Therefore, ZLCN serves as a powerful tool for measuring viscosity in cellular and organ-level environments. The structure of ZLCN was characterized by proton nuclear magnetic resonance (1H NMR) spectroscopy and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, as shown in Fig. S2–S5 (ESI†).
Optical sensing properties of compounds
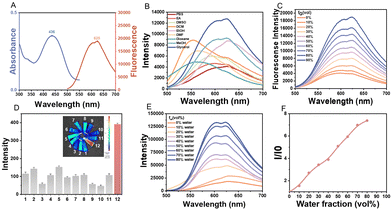
Afterward, the optical properties of ZLCN were investigated. It exhibited the maximum absorption wavelength at around 436 nm and the maximum emission wavelength at around 625 nm (Fig. 1A). Then, ZLCN's emission was tested in various solvents including phosphate buffered saline (PBS), ethyl acetate (EA), dimethyl sulfoxide (DMSO), dichloromethane (DCM), dimethylformamide (DMF), dioxane, methanol (MeOH), ethanol (EtOH) and glycerol. It was interestingly observed that the maximum emission wavelength of the fluorescence spectrum of the probe ZLCN was red-shifted with the change in solvent polarity (as the polarity increased), indicating that the probe is potentially polarity-sensitive as shown in Fig. 1B.35 Obviously, the fluorescent intensity of ZLCN in glycerol was significantly higher than that in other solvents. The spectroscopic response of ZLCN to varying viscosities was investigated in ethanol/glycerol mixtures with different volume ratios. As shown in Fig. 1C and Fig. S6 (ESI†), both the absorbance and fluorescence intensity of ZLCN increased with the proportion of glycerol, exhibiting a good linear relationship between fluorescence intensity and glycerol fractions (Fig. S7, ESI†), thereby demonstrating its viscosity responsiveness. To further evaluate the specificity of ZLCN for viscosity, its fluorescent intensity was also measured in the presence of various substances, including Na+, Cu2+, Fe2+, NO2−, SO32−, Cys, HClO, O2−, ONOO−, H2O2, NH4+, and glycerol. It was found that ZLCN exhibited the strongest fluorescent intensity in the presence of glycerol compared to the other substances (Fig. 1D). Meanwhile, its pH sensitivity was investigated. The absorbance and emission spectra of ZLCN did not show significant changes in the pH range of 4–6, indicating that its viscosity response was not affected by pH variations under these conditions (Fig. S8, ESI†). It has been reported that triphenylamine (TPA) groups are one of the fundamental aggregation-induced emission (AIE) motifs, and therefore, the dye ZLCN was examined for its AIE characteristics. To assess the AIE behavior of ZLCN, its emission spectrum was recorded in a water/DMSO mixture with different water fractions (fw), and the corresponding relative fluorescent intensity (I/I0) was evaluated (Fig. 1E and F). As the water ratio increased, its fluorescence intensity progressively strengthened, exhibiting the typical AIE characteristics. Moreover, the stability of ZLCN was evaluated under physiological conditions. ZLCN exhibited a slight decrease in fluorescence intensity in Dulbecco's Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) (Fig. S9, ESI†), whereas ICG showed significant degradation, indicating the superior photostability of ZLCN in biological environments.The biocompatibility of the probe is a critical factor in determining its suitability for biological applications. At first, the cytotoxicity of ZLCN was evaluated in several cell lines, including AML12, NIH-3T3 and human umbilical vein endothelial cells (HUVECs), using the methyl thiazolyl tetrazolium (MTT) assay. The results showed that cell viability remained above 80% after 24 hours of co-incubation at varying concentrations (0–160 μM) (Fig. 2A and Fig. S10, ESI†), indicating its good biocompatibility. These results indicate that ZLCN exhibits low cytotoxicity and favorable biocompatibility. Subsequently, its subcellular localization was examined. As mitochondria are characterized by a double membrane structure and a negatively charged membrane potential, the protonated ZLCN probe is anticipated to efficiently traverse the mitochondrial membrane and selectively accumulate within the mitochondrial matrix. Colocalization studies were then conducted in AML12 cells using the mitochondria-targeting fluorescent probe MitoTracker Green (MTG). The red fluorescence signal of ZLCN exhibited strong colocalization with the green fluorescence of MTG, with a Pearson's correlation coefficient (PC) of 0.94, confirming its selective accumulation in the mitochondria of living cells (Fig. 2B). Furthermore, fluorescence intensity profiles along the regions of interest (ROI) line showed a similar distribution pattern, further supporting mitochondrial localization (Fig. 2C).
We further investigated the capability of ZLCN to detect viscosity variations in cellular environments. Since previous studies have reported that tumor cells exhibit higher intracellular viscosity than normal cells, we employed 4T1 and Hep1-6 cancer cell lines as in vitro disease models to evaluate the diagnostic performance of ZLCN. To this end, we conducted confocal fluorescence imaging on normal liver cells (3T3 and AML12) and cancer cells (4T1 and Hep1-6), following a 6-hour incubation with ZLCN (60 μM) (Fig. 3A). Notably, the fluorescence intensity in cancer cells was significantly higher than that in normal cells (Fig. 3C), corroborating the previously reported elevated viscosity in tumor cells. Moreover, the fluorescence intensity in Hep1-6 cells was higher than that in AML12 cells, indicating that liver cancer cells possess greater intracellular viscosity compared to normal liver cells. Therefore, the ZLCN probe could also serve as a useful tool for distinguishing cancer cells from normal cells based on viscosity differences. Next, to assess the ability of ZLCN to detect viscosity changes in normal liver cells, we used the antifungal agent nystatin (Nys), which alters intracellular viscosity by disrupting the ion balance and inducing mitochondrial dysfunction. Compared with the control group, AML12 cells treated with Nys demonstrated elevated strong red fluorescence (Fig. 3B and D), and with the concentration of Nys increased, the red fluorescence also increased, suggesting that the viscosity of AML12 cells increased induced by Nys and the viscosity responsiveness of ZLCN. Moreover, flow cytometry was performed to compare the fluorescence intensities among different treatment groups. AML12 cells treated with Nys and incubated with ZLCN exhibited significantly higher fluorescence intensity (Fig. S11, ESI†). Taken together, these results suggested that the probe was capable of detecting viscosity changes at the subcellular level.
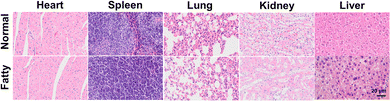
To better assess viscosity at the organ level in disease models, we created a fatty liver mouse model for visualizing changes in viscosity. Upon injection of the ZLCN probe (100 μL, 150 μM) into different mouse groups, the fluorescence signal in the fatty liver group became significantly more pronounced over time, indicating a much higher viscosity in the fatty liver compared to other organs (Fig. 4A). In addition, the ZLCN probe enabled rapid detection of fatty liver, with fluorescence signals appearing as early as 10 minutes post-injection and peaking at 30 minutes. Notably, the fluorescence intensity in the fatty liver group was 2.35 times stronger than that in the normal group. Compared to previous studies,9,36–38 this significantly reduces the detection time, facilitating early diagnosis and timely intervention, which could potentially improve clinical outcomes in patients with fatty liver disease. The mice from both groups were euthanized, and their organs, including the heart, liver, spleen, lungs, and kidneys, were harvested for analysis 60 minutes after the intravenous injection of ZLCN. As shown in Fig. 4B and Fig. S12 (ESI†), the liver in the fatty liver group displayed a strong fluorescence signal, while the livers of the normal group exhibited much weaker fluorescence. These findings suggest that the synthesized probe effectively detects the viscosity of fatty liver. Brightfield images showed that the livers in the normal group appeared dark red with well-defined edges. In contrast, the liver in the fatty liver group had a greasy surface. Furthermore, HE staining was performed on liver tissues from both groups, and the fatty liver tissue exhibited noticeable vacuoles and mild hepatocyte swelling (Fig. 5).
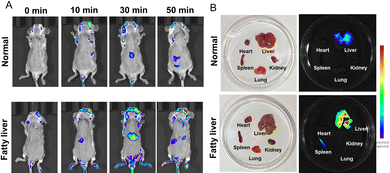 | ||
| Fig. 4 (A) In vivo fluorescence imaging of viscosity changes using ZLCN in normal and fatty liver mice. (B) Fluorescence intensity of ZLCN in different mouse models as shown in (A). | ||
Conclusions
In summary, a mitochondria-targeting fluorescent probe with viscosity responsiveness has been successfully developed. In low viscosity solutions, ZLCN remains non-fluorescent due to intramolecular rotation, but in high viscosity environments, this rotation is suppressed, resulting in strong fluorescence emission. The probe ZLCN exhibits excellent viscosity sensitivity, a large Stokes shift, and selective mitochondrial accumulation, enabling effective fluorescence imaging of viscosity alterations in both subcellular and organ-level fatty liver models. The strong correlation between fluorescence intensity and viscosity changes allows for the differentiation of normal and diseased liver tissues, providing a valuable tool for early diagnosis and disease monitoring. Furthermore, ZLCN demonstrates favourable biocompatibility and low cytotoxicity, making it highly suitable for biological applications. Given the increasing global prevalence of fatty liver disease, the development of such high-performance imaging probes holds significant promise for advancing diagnostic strategies and facilitating future therapeutic evaluations.Author contributions
Jiamin Liu: methodology, visualization, investigation and data curation. Hui Zhou: conceptualization, supervision, formal analysis, methodology, writing – review & editing and funding acquisition. Deyi Li: investigation. Haotong Yin: investigation. Yi Zhou: validation. Yuquan Ji: validation. Yujing Zhang: validation. Xinyue Zhang: investigation and visualization. Ben Wang: validation. Chao Yin: data curation, supervision, writing – review & editing and funding acquisition. Quli Fan: supervision and funding acquisition.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (22405132, 22475105, 52373142), the Project of Jiangsu Specially-Appointed Professor (RK030STP22003), the Natural Science Foundation of Jiangsu Province (BK20240655), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (23KJB150024), the Project of State Key Laboratory of Organic Electronics and Information Displays, Nanjing University of Posts and Telecommunications (GZR2023010036) and the Natural Science Research Start-up Foundation of Recruiting Talents of Nanjing University of Posts and Telecommunications (NY222066).Notes and references
- A. J. Sanyal, Nat. Rev. Gastroenterol. Hepatol., 2019, 16, 377–386 CrossRef PubMed.
- E. M. Brunt, V. W. Wong, V. Nobili, C. P. Day, S. Sookoian, J. J. Maher, E. Bugianesi, C. B. Sirlin, B. A. Neuschwander-Tetri and M. E. Rinella, Nat. Rev. Dis. Primers, 2015, 1, 15080 CrossRef PubMed.
- M. Noureddin, J. Lam, M. R. Peterson, M. Middleton, G. Hamilton, T. A. Le, R. Bettencourt, C. Changchien, D. A. Brenner, C. Sirlin and R. Loomba, Hepatology, 2013, 58, 1930–1940 CrossRef CAS PubMed.
- S. Lv, S. Jiang, S. Liu, Q. Dong, Y. Xin and S. Xuan, J. Clin. Transl. Hepatol., 2018, 6, 217–221 CrossRef PubMed.
- W. Huang, Y. Peng and L. Kang, View, 2024, 5, 20240010 CrossRef.
- X. Yang, D. Zhang, Y. Ye and Y. Zhao, Coord. Chem. Rev., 2022, 453, 214336 CrossRef CAS.
- Y. Liu, S. Feng, S. Gong and G. Feng, Anal. Chem., 2022, 94, 17439–17447 CrossRef CAS PubMed.
- S. Zeng, Y. Wang, C. Chen, H. Kim, X. Liu, M. Jiang, Y. Yu, Y. S. Kafuti, Q. Chen, J. Wang, X. Peng, H. Li and J. Yoon, Angew. Chem., Int. Ed., 2024, 63, e202316487 CrossRef CAS PubMed.
- X. Wang, X. Zhou, Z. Zhang, L. Shen, X. Yan, H. Xu, C. Redshaw and Q. L. Zhang, J. Mater. Chem. B, 2025, 13, 3677–3684 RSC.
- L. Hu, J. Yang, C. Zhang, J. Pan, S. Shen, L. Su, X. Shen, J. He and H. Wang, Sens. Actuators, B, 2024, 398, 134776 CrossRef CAS.
- O. S. Wolfbeis, Chem. Soc. Rev., 2015, 44, 4743–4768 RSC.
- J. Yao, M. Yang and Y. Duan, Chem. Rev., 2014, 114, 6130–6178 CrossRef CAS PubMed.
- H. Chen, H. Zhou, X. Zhang, Y. Ding, X. Zhang, Q. Xu, B. Wang, C. Yin and Q. Fan, Chem. Commun., 2024, 60, 9618–9621 RSC.
- B. Wang, H. Zhou, L. Chen, Y. Ding, X. Zhang, H. Chen, H. Liu, P. Li, Y. Chen, C. Yin and Q. Fan, Angew. Chem., Int. Ed., 2024, 63, e202408874 CrossRef CAS PubMed.
- T. Yan, C. Guo, C. Wang and K. Zhu, View, 2023, 4, 20220059 CrossRef.
- Y. Xu, C. Li, X. Ma, W. Tuo, L. Tu, X. Li, Y. Sun, P. J. Stang and Y. Sun, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2209904119 CrossRef CAS PubMed.
- Y. Xu, C. Li, S. Lu, Z. Wang, S. Liu, X. Yu, X. Li and Y. Sun, Nat. Commun., 2022, 13, 2009 CrossRef CAS PubMed.
- Y. Xu, C. Li, J. An, X. Ma, J. Yang, L. Luo, Y. Deng, J. S. Kim and Y. Sun, Sci. China: Chem., 2022, 66, 155–163 CrossRef.
- S. Li, P. Wang, W. Feng, Y. Xiang, K. Dou and Z. Liu, Chem. Commun., 2020, 56, 1050–1053 RSC.
- J. Yang, Y. Guo, M. Pistolozzi and J. Yan, Dyes Pigm., 2021, 193, 109466 CrossRef CAS.
- Y. Guo, H. Leng, Q. Chen, J. Su, W.-J. Shi, C. Xia, L. Zhang and J. Yan, Sens. Actuators, B, 2022, 372, 132648 CrossRef CAS.
- L. Yu, J. F. Zhang, M. Li, D. Jiang, Y. Zhou, P. Verwilst and J. S. Kim, Chem. Commun., 2020, 56, 6684–6687 RSC.
- L. Fan, Q. Zan, X. Wang, S. Wang, Y. Zhang, W. Dong, S. Shuang and C. Dong, Chin. J. Chem., 2021, 39, 1303–1309 CrossRef CAS.
- J. Yin, X. Kong and W. Lin, Anal. Chem., 2021, 93, 2072–2081 CrossRef CAS PubMed.
- Y. Liang, Y. Zhao, C. Lai, X. Zou and W. Lin, J. Mater. Chem. B, 2021, 9, 8067–8073 RSC.
- T. Liang, D. Zhang, W. Hu, C. Tian, L. Zeng, T. Wu, D. Lei, T. Qiang, X. Yang and X. Sun, Talanta, 2021, 235, 122719 CrossRef CAS PubMed.
- Y. Li, Z. Zhou, S. Chen, X. Pang, C. Wu, H. Li and Y. Zhang, Anal. Chim. Acta, 2023, 1250, 340967 CrossRef CAS PubMed.
- G. Q. Fu, Q. T. Liao, Z. Q. Wang, Z. K. Tan, G. J. Mao, B. Yang and C. Y. Li, Anal. Chim. Acta, 2022, 1226, 340192 CrossRef CAS PubMed.
- Y. Zhou, P. Li, X. Wang, C. Wu, N. Fan, X. Liu, L. Wu, W. Zhang, W. Zhang, Z. Liu and B. Tang, Chem. Sci., 2020, 11, 12149–12156 RSC.
- L. Niu, Q. Cao, T. Zhang, Y. Zhang, T. Liang and J. Wang, Talanta, 2023, 260, 124591 CrossRef CAS PubMed.
- L. Fan, Q. Zan, X. Wang, X. Yu, S. Wang, Y. Zhang, Q. Yang, W. Lu, S. Shuang and C. Dong, Chem. Eng. J., 2022, 449, 137762 CrossRef CAS.
- J. J. Chao, H. Zhang, Z. Q. Wang, Q. R. Liu, G. J. Mao, D. H. Chen and C. Y. Li, Anal. Chim. Acta, 2023, 1242, 340813 CrossRef CAS PubMed.
- Y. Shen, Q. Zhou, W. Li and L. Yuan, Chem. – Asian J., 2022, 17, e202200320 CrossRef CAS PubMed.
- Z. Zhan, Z. Wei, B. Ying, L. Chai, Q. Yu, X. Yu, L. Zhou, C. Wan, F. Li, J. Huang, P. Chen, W. Huang and W. Li, Sens. Actuators, B, 2022, 371, 132575 CrossRef CAS.
- W.-L. Cui, M.-H. Wang, X.-Q. Chen, Z.-H. Zhang, J. Qu and J.-Y. Wang, Dyes Pigm., 2022, 204, 110433 CrossRef CAS.
- X. Xu, X. Wang, G. Xu, X. Chen, T. Chen, C. Jin, Z. Duan, J. Song, T. Lv, Y. Zhou, W. Li, J. Yang and Z. Zhan, Sens. Actuators, B, 2025, 433, 137580 CrossRef CAS.
- J. Hao, S. Shi, Y. Zhang, X. Li, X. Ren, J. Gao, S. Liu, H. Zhang, J. Wu and B. Zhang, Sens. Actuators, B, 2025, 433, 137581 CrossRef CAS.
- J. Hao, X. Li, S. Shi, H. Zhang, H. Zhu, J. Wu, M. Gao and B. Zhang, Bioorg. Chem., 2025, 155, 108162 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tb00556f |
| ‡ J. Liu and H. Zhou contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |