Frontiers in fluorescence imaging: tools for the in situ sensing of disease biomarkers
Lei
Yang
 a,
Hongwei
Hou
*b and
Jinghong
Li
a,
Hongwei
Hou
*b and
Jinghong
Li
 *ab
*ab
aDepartment of Chemistry, Center for Bioanalytical Chemistry, Key Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology, Tsinghua University, Beijing 100084, China. E-mail: jhli@mail.tsinghua.edu.cn
bBeijing Life Science Academy, Beijing 102209, China. E-mail: houhw@ztri.com.cn
First published on 3rd December 2024
Abstract
Fluorescence imaging has been recognized as a powerful tool for the real-time detection and specific imaging of biomarkers within living systems, which is crucial for early diagnosis and treatment evaluation of major diseases. Over the years, significant advancements in this field have been achieved, particularly with the development of novel fluorescent probes and advanced imaging technologies such as NIR-II imaging, super-resolution imaging, and 3D imaging. These technologies have enabled deeper tissue penetration, higher image contrast, and more accurate detection of disease-related biomarkers. Despite these advancements, challenges such as improving probe specificity, enhancing imaging depth and resolution, and optimizing signal-to-noise ratios still remain. The emergence of artificial intelligence (AI) has injected new vitality into the designs and performances of fluorescent probes, offering new tools for more precise disease diagnosis. This review will not only discuss chemical modifications of classic fluorophores and in situ visualization of various biomarkers including metal ions, reactive species, and enzymes, but also share some breakthroughs in AI-driven fluorescence imaging, aiming to provide a comprehensive understanding of these advancements. Future prospects of fluorescence imaging for biomarkers including the potential impact of AI in this rapidly evolving field are also highlighted.
1. Introduction
In modern medicine and biological research, the real-time detection and specific imaging of biomarkers in vivo are essential for early diagnosis, monitoring disease progression, and evaluating treatment efficacy for major diseases.1–5 To date, technologies have emerged as one of the most powerful bioanalytical techniques for the in situ sensing and visualization of various biomarkers including pH, metal ions, reactive species or enzymes within living systems.6–9 Compared to classical imaging methods such as nuclear magnetic resonance imaging (NMRI), ultrasound (US), positron emission tomography (PET), and X-ray computed tomography (CT), fluorescence imaging offers unique advantages including high spatiotemporal resolution, non-invasiveness, and real-time detection.6–13 These characteristics have significantly enhanced its role in both fundamental research and clinical pathological detection.To meet the increasing demands of scientific research and biological applications, significant progress has been made in developing advanced microscopy technologies. Remarkable progress with the development of a variety of advanced microscopy technologies including single-molecule imaging, super-resolution imaging, multi-color imaging, second near-infrared (NIR-II) imaging, and 3D imaging for the precise targeting and in situ imaging of disease-related biomarkers has been achieved.14,15 These technologies are often paired with innovatively designed fluorescent probes based on classical fluorophores such as xanthene, cyanine, oxazine, boron-dipyrromethene (BODIPY), coumarin, and hemicyanine.14,16–18 The synergetic effect between advanced imaging technology and fluorescent probes has allowed for the illumination of biological processes at the molecular level, which is crucial for early detection, disease monitoring, and therapeutic interventions.19 Besides, electrochemiluminescence (ECL) and chemiluminescence (CL) microscopy have been equipped with fluorescence imaging to develop a dual-mode imaging method for single cell analysis,20,21 which indicated that lots of fabulous ECL/CL probes, such as ruthenium and luminol-based probes,22–25 can be applied to enhance the imaging quality of single cells or biomolecules with multi-signal output capability.
Despite significant advancements in fluorescence imaging, several challenges still hinder its broader application in clinical and research settings. A major challenge lies in the development of fluorescent probes with high selectivity and stability in complex biological environments.16,18 The design of probes that can specifically target disease biomarkers without being affected by other cellular components is critical for accurate imaging. Additionally, improving the resolution and depth of imaging, especially in thick tissues or whole organisms, is essential to capture detailed and comprehensive biological information. Another challenge lies in optimizing signal-to-noise ratios (SNR) in fluorescence imaging. Biological tissues often exhibit autofluorescence, which can interfere with the detection of the fluorescence signal from the probes. This necessitates the development of probes with unique spectral properties and advanced imaging techniques that can differentiate between signal and noise effectively. Moreover, the photostability of fluorescent probes is a crucial factor, as prolonged exposure to light can lead to photobleaching, reducing the accuracy and duration of imaging studies.
To date, various chemical approaches have been developed to optimize the optical properties of a wide variety of classic fluorescent molecules and nanoparticles with improved performance and great potential in high-resolution microscopy technologies.14,26–29 Chemically modified fluorescent probes are expected to conquer limitations such as short absorption and emission wavelengths, low brightness, poor photostability, small Stokes shift, and unsuitable permeability to achieve high-quality bioimaging applications in living systems.30 Due to the superior optical tissue penetration abilities, lower interference from biological autofluorescence, and higher in vivo imaging contrast, NIR-II fluorescent probes have enabled the visualization of these cellular components in early diagnosis and monitoring of progression of different diseases.1,17,29,31,32 For instance, the use of specific NIR-II fluorescent probes has facilitated the understanding of mitochondrial dysfunction and protein aggregation pathways, which are common hallmarks in these diseases.30,32 Long-wavelength NIR-II probes can penetrate deeper into tissues and face less interference from scattering and autofluorescence generated by biomolecules, which has revolutionized our understanding and approach to diagnosing and treating complex diseases like cancers, neurodegenerative diseases, and cardiovascular diseases.33–35
Recently, artificial intelligence (AI) has found success in diverse applications such as image recognition, speech processing, recommendation systems, and autonomous vehicles.36–38 AI involves training models on large datasets to recognize patterns and make predictions. AI-enhanced fluorescence imaging represents a promising field, and new AI algorithms have opened brand-new avenues for developing advanced fluorescence imaging techniques.28,29,39 These recently reported successful attempts in integrating AI with fluorescence microscopy have shown its great potential in revolutionizing the design of high-performance probes and biomarker detection for precise disease diagnosis.2,37,40–43
This review aims to provide a comprehensive overview of the latest advancements in fluorescence imaging, with particular focus on the development of novel fluorescent probes and their applications for in situ imaging of diverse biomarkers, including metal ions, reactive species, and enzymes. We will discuss the emerging sensing mechanisms that drive these innovations, and emphasize their significance in improving the specificity, resolution, and depth of imaging within biological systems. Additionally, we will explore the integration of different AI algorithms into fluorescence imaging, highlighting recent AI-driven novel strategy for probe designs and imaging techniques. By examining these developments, this review underscores the transformative potential of AI in advancing fluorescence imaging, paving the way for more precise, efficient, and insightful diagnostic tools in both research and clinical applications.
2. Optimization of the probes and sensing mechanism for fluorescence imaging
Fluorescence imaging technologies, such as near-infrared (NIR) imaging, single-molecule imaging, super-resolution imaging, and multi-colour imaging, have been developed to meet the growing demands of scientific research and biomedical applications in diverse fields such as biology, physiology, medicine, pharmacology, and environmental sciences.11,41,44–47 The quality and efficiency of fluorescence imaging are mainly dominated by the cooperation between an optimal fluorescence probe and a perfectly matched sensing mechanism. In this section, we will focus on the optimization of fluorescent probes and develop sensing mechanisms for high-quality imaging applications.2.1. Optimization of the fluorescent probes
Fluorescent probes are crucial components in fluorescence imaging, playing a vital role in enhancing the sensitivity and specificity of imaging.48,49 Based on various classic fluorophores, such as xanthene, oxazine, BODIPY, coumarin, cyanine and hemicyanine (Fig. 1A), scientists are able to label and observe targeted structures and functional processes within biomolecules, cells, and tissues, gaining deep insights into disease-related mechanisms, cellular functions, and biological processes.14,18 Traditional fluorophores emit light based on a well-defined process explained by the Jabłoński diagram (Fig. 1B).50 When a fluorophore absorbs a photon, it transitions from its ground state (S0) to an excited state (S1 or higher Sn states). Following Kasha's rule and Franck–Condon principle, it relaxes to the lowest excited singlet state (S1) through vibrational relaxation and internal conversion.32,51 From S1, the fluorophore can return to the ground state (S0) by emitting a photon (fluorescence) or through non-radiative processes. Additionally, some fluorophores may enter the triplet state (T1) via intersystem crossing (ISC), leading to phosphorescence.52,53 Energy dissipation occurs in three ways: fluorescence, non-radiative decay, and transition to the T1 state. The Stokes shift, the difference between absorption and emission wavelengths, results from energy loss during vibrational relaxation. Photobleaching, a common issue with most of fluorophores, is found to be involved in the T1 state, can be induced by the degradation of some reactive oxygen species (ROS) like singlet oxygen (1O2), causing inevitable fluorescence quenching. Different fluorophores own different intrinsic polarity, size, and lipophilicity, these properties may limit their cell permeability for live cell imaging. Therefore, it can be observed from above fundamentals that traditional fluorophores suffer from shortcomings such as short absorption and emission wavelengths, low brightness, poor photostability, small Stokes shifts, or unsuitable permeability.14 To enhance the performance of traditional fluorophores for developing high-quality biological imaging technique, several key and specific improvements are necessary to be addressed. (1) Adjusting the imaging range to long-wavelength region. This can be achieved by red-shifting the absorption and emission spectra of fluorophores to the deep-red or near-infrared region. Recent advancements mainly focused on expanding the conjugated π-structure by either elongating the conjugated chain or adding more conjugated π-segments, or introducing heteroatoms (carbon, silicon, germanium, phosphorus, sulfur, selenium, or tellurium) in place of bridging oxygen to stabilize the LUMO level with the formed σ*–π* conjugation.30,32,52–55 For example, para-substituted styryl was incorporated into the 3′,6′-position of the xanthene core to expand the π-conjugation for enhanced NIR-II emission of a series of newly developed xanthene probes (VIXs). One of the xanthene probes named VIX-4 showed a red-shifted emission at 1210 nm and high brightness, and was successfully applied to measure blood flow volumes in femoral vessels with high spatiotemporal resolution and precision.56 (2) Improving the brightness. This can be resolved by increasing the water solubility through hydrophilic modifications with ionic or non-ionic multiple polar substituents to inhibit the formation of unwanted twisted intramolecular charge transfer (TICT) process.57–59 Developing fluorophores with aggregation-induced emission (AIE) properties has become mainstream to improving fluorescence output in high-concentration environments.60–62 For example, Sun et al. recently reported the very first mechanochemical uncaging strategy to activate the highly tunable AIE emission of engineered norborn-2-en-7-one (NEO) mechanophores with multicolor and white fluorescence.63 (3) Promoting the photostability. This can be realized by alleviating photobleaching via introducing electron-withdrawing groups, shielding susceptible ROS-binding sites, shortening the triplet-state lifetime by small-molecule quenchers (TSQs), etc.64–66 For example, Liu et al. developed a photostable probe (COT-Cy3.5) by conjugating the cyclooctatetraene (COT) with benzo-fused cyanine dyes, both of which guaranteed high photostability for live-cell stimulated emission depletion (STED) microscopy of mitochondria.67 (4) Enlarging the Stokes shift. This can be achieved by introducing asymmetric electronic structures into the HOMO and LUMO to facilitate or improve the process of internal conversion (IC), or amino groups to the middle position and rotors to suitable positions via single bonds.68,69 Recently, Jiang et al. designed a new probe named YL578 by equipping rhodamine with a quinoxaline motif with fine-tuned electron density, showing an enlarged Stokes shift (56 nm) with enhanced brightness and photostability for high-resolution STED imaging.70 (5) Avoiding aggregation-caused quenching (ACQ) effect. Developing fluorophores with aggregation-induced emission (AIE) properties, which enhance fluorescence upon aggregation, can improve the fluorescence output in high-concentration environments. (6) Promoting cell permeability. This can be resolved by reasonable designing of smaller and neutral fluorophores, modification with lipophilic or biologically labile protecting groups, connecting with cell-penetrating biomolecules as carriers, etc.71–73 For instance, Jiang et al. realized the efficient regulation of the cell uptake ability toward rhodamine probe through amino acetylation and esterification, which laid a crucial foundation for the following high-contrast imaging of biomarkers in vivo.74 By addressing these problems using those newly proposed strategies, the design and development of new fluorophores can be significantly optimized for biological imaging applications, leading to more accurate and reliable results in research and clinical diagnostics. | ||
| Fig. 1 (A) Structures of xanthene, cyanine, oxazine, boron-dipyrromethene (BODIPY), coumarin, and hemicyanine. (B) Jabłoński diagram.14 Copyright 2023, John Wiley and Sons. | ||
2.2. Fluorescence sensing mechanism for imaging
Fluorescence mechanisms endow fluorescent probes with a broader range of applications and significantly improve the quality and efficiency of fluorescence imaging. To date, various fluorescence sensing mechanisms have been explored including Förster resonance energy transfer (FRET), intramolecular charge transfer (ICT), photoinduced electron transfer (PeT), excited state intramolecular proton transfer (ESIPT), aggregation induced emission (AIE), or involve dual/triple sensing mechanisms (DSM/TSM).9,10 Understanding these mechanisms is vital for developing high-performance fluorophores for biomedical applications. This section delves into the fundamental principles behind the most prominent fluorescence mechanisms used in designing advanced fluorescent probes.(1) The mechanism for energy transfer can be through space (FRET) or through bonds (through-bond energy transfer, TBET). As shown in Fig. 2A, the FRET is a mechanism where energy transfer occurs between two light-sensitive molecules (a donor and an acceptor).75 When the donor molecule is excited by a photon, it can transfer energy to the acceptor molecule if it is within a close distance (<10 nm).76 TBET, on the other hand, transfers energy via the covalent bonds connecting the donor and acceptor. In TBET, energy is transmitted through the π-electron systems of the bonded components, independent of spectral overlap.77 (2) As shown in Fig. 2B, the ICT mechanism involves the electron transfer process from a donor to an acceptor via π bonds to form an extended and conjugated “D–π–A” structure.10 ICT-based fluorescent probes can interact with desired analyte to alter the strength of intramolecular electron push–pull effects, changing the energy gap between the HOMO and LUMO. This alteration causes the absorption and emission spectra to shift towards red or blue, thereby constructing efficient ratiometric fluorescent probes.78,79 (3) The PeT mechanism (Fig. 2C) occurs in a structure where fluorophore is conjugated with a recognition receptor via a short spacer. It can be divided into two processes, the a-PeT and d-PeT, depending on whether the excited state of fluorophore accepts or donates electrons to induce fluorescence quenching.80 The presence of a specific analyte can inhibit or enhance this electron transfer, leading to a change in fluorescence.81 (4) The ESIPT mechanism involves the transfer of a proton in the excited state to adjacent heteroatoms via intramolecular hydrogen bonds upon light excitation.82 This transfer results in a fast phototautomerization from the normal (enol) form to the proton-transferred (keto) forms. ESIPT-based probes, with large Stokes shifts and minimal overlap between excitation and emission spectra, are highly sensitive to environmental changes such as polarity and hydrogen bonding.12,83 (5) The AIE mechanism is a phenomenon where certain molecules are non-emissive in their monomeric state but become highly emissive upon aggregation. This behaviour is in contrast to the typical ACQ-fluorophores observed in many classic fluorescent dyes.62 AIE-active molecules are useful in biological imaging for detecting processes that involve molecular aggregation, such as protein folding, amyloid fibril formation, and cellular organelle dynamics.60,61 (6) The DSM/TSM mechanisms usually stand for the combination of two or more sensing mechanisms within a single fluorescent probe.12 This approach enhances the versatility and reliability of the probes, allowing for the simultaneous detection of multiple analytes or environmental changes, providing comprehensive insights into complex biological systems with high accuracy.
 | ||
| Fig. 2 Sensing mechanism of (A) Förster resonance energy transfer (FRET).75 Copyright 2020, Royal Society of Chemistry. Sensing mechanisms of (B) intramolecular charge transfer (ICT) and (C) photoinduced electron transfer (PeT).4 Copyright 2023, American Chemical Society. | ||
Different mechanisms offer distinct advantages for visualizing and detecting disease markers. By leveraging the unique properties of each mechanism, researchers can develop highly sensitive, specific, and versatile probes that address the limitations of traditional fluorophores. It is believed that the integration of optimized fluorescent probes with sensing mechanisms would significantly promote the development of advanced fluorescence imaging techniques in biomedical research and diagnostics.
3. Application of fluorescence imaging for different disease-related biomarkers
Benefiting from the optimized design/synthesis and efficient sensing mechanism, the performance of fluorescent probes can be significantly enhanced for high-quality bioimaging of biomarkers for early diagnosis and treatment monitoring of diseases like cancer, cardiovascular diseases, and neurodegenerative disorders.3,5,6,84–88 Modified fluorescent probe can specifically target biomarker to realize in vivo visualization of various pathological processes, which plays a crucial role in distinguishing healthy and diseased tissues to improve the precision and safety of surgeries. This section will discuss recent fluorescence imaging applications for different kinds of disease-related biomarkers, including those representative metal ions, reactive species, and enzymes used in modern fluorescence imaging.3.1. Imaging of ion-based biomarkers
Specific design of Na+/K+-responsive fluorescence probes offer new strategies for treating Na+/K+ related disorders such as cardiovascular diseases, neurodegenerative diseases, and muscle dysfunctions.92 For instance, Zou et al.93 developed a pH-insensitive fluorescent probe named RatiNa for accurately imaging the absolute levels of Na+ within acidic organelles with single-organelle resolution. RatiNa is a 45 base-pair double-stranded DNA (dsDNA) which consists of three single-stranded DNA (ssDNA) molecules designed for specialized functions. A novel Na+-sensitive fluorophore named Chicago Green (Fig. 3A) was featured with a 25-mer chain to act as the sensing component (D1) of RatiNa, which can bind with Na+ and sense concentration changes of Na+via the PET mechanism. Then, a 20-mer strand as the targeting module guides RatiNa to specific cellular compartments via a receptor-mediated endocytosis pathway. The fluorescence images confirm that RatiNa's response to Na+ is independent of pH variations and is highly specific with minimal interference from other physiological ions like K+, Li+, and Ca2+, highlighting its potential for monitoring Na+ within acidic cellular organelles in complex biological systems.
 | ||
| Fig. 3 (A) The sensing mechanism and imaging result of RatiNa for Na+ detection.93 Copyright 2021, American Chemical Society. (B) The sensing mechanism and imaging result of CCNa1 for Na+ detection.94 Copyright 2021, Royal Society of Chemistry. (C) The K+ sensing mechanism of RPS-1.96 Copyright 2021, John Wiley and Sons. | ||
Besides, a small-molecule fluorescent probe CCNa1 was designed based on cyclocyanine (CC) by Juvekar's team.94 As a two-photon (TP) probe, CCNa1 exhibited low solvent shift and strong TP fluorescence enhancement at 575 nm upon binding with Na+via the PET mechanism. CCNa1 featured a dissociation constant (Kd) of 22.2 mM and was insensitive to other metal ions and pH changes, making it particularly suitable for intracellular Na+ imaging. Results showed that the biocompatible CCNa1 probes can easily penetrate cells to provide bright two-photon microscopy (TPM) images up to 140 μm deep in mouse hippocampal tissue with high spatial resolution (Fig. 3B). This capability of CCNa1 makes it a valuable tool for detailed neuroscience research where understanding ionic changes within neurons is crucial.
K+ ions are among the most crucial substances in the body, involved in numerous physiological functions such as muscle contraction, nerve transmission, and kidney function.90,95 Wang et al.96 developed a first-generation ratiometric fluorescent probe named RPS-1 for K+ imaging via the PeT mechanism. As shown in Fig. 3C, the RPS-1 was composed of a dual-fluorophore system, including the K+-responsive PS525 and K+-insensitive Coumarin 343. Through esterase-directed cleavage, these two components were split apart into two independent fragments to enable ratiometric imaging of intracellular K+ levels within a range of 150 mM with high accuracy. Liu et al.95 present the development of a highly sensitive and specific nanosensor for K+ sensing by embedding an optical K+-responsive indicator called Asante Potassium Green-2 tetramethylammonium salt (APG) within mesoporous silica nanoparticles (MSN-APG), which were then shielded by a K+-permeable ultra-thin film. This design uniquely prevents the diffusion of other cations, thereby ensuring the specificity for potassium. The shielding of the nanosensor enables it to map the spatial distribution of K+ released in the hippocampal region of freely moving mice during events like epileptic seizures.
Changes in the levels of Na+ and K+ ions can indicate or influence various conditions such as heart diseases, kidney disorders, neurological conditions, and metabolic imbalances.91 Currently, simultaneous imaging and detection of ions are gaining increasing attention. Deng et al.92 presented a sophisticated Y-shaped DNA probe designed for the simultaneous detection and analysis of Na+ and K+ ions within cell membrane environments. The Y-shaped DNA probe was composed of three distinct DNA sequences (M1, M2, and M3). FAM-M1 as the Na+-specific enzymatic strand was first hybridized with a substrate chain (M2-BHQ) and then was cleaved in the presence of Na+, triggering a fluorescence signal via the FRET mechanism. Cyanine-M3 as the complementary strand to M2 is actually a G-quadruplex forming strand, thus the binding of K+ to G-quadruplex would cause conformational changes that facilitate the FRET process between Cy3 and Cy5 dyes. In these two separated but connected sensing pathways, the concentrations of Na+ and K+ were simultaneously detected with good specificity and stability within cell membranes.
Recognizing the crucial role of Zn2+ in synaptic signal transduction and their link to various neurological disorders, the team aimed to create a more effective tool for imaging Zn2+ in live tissue contexts.98 Wu et al.100 developed an innovative synaptic zinc imaging indicator called FRISZ by using an appropriate far-red fluorescent protein called mMaroon1 to replace the GFP in original fluorescent protein (FP) structure. As a qualified Zn-responsive indicator, FRISZ was then encoded onto the mammalian cell membrane using a pDISPLAY plasmid, proving its capability for imaging extracellular membrane Zn2+ change in the mouse brain slice experiments induced by electrical stimulation though the FRET mechanism. Along with further tests in awake mice, the ability of FRISZ was further demonstrated by monitoring synaptic Zn2+ changes in response to auditory stimuli. The satisfactory results confirmed the great potential of FRISZ as a significant probe for in vivo Zn2+ imaging in neurological research.
DNAzyme-based fluorescent probes have become important tools for metal-ion sensing due to their high selectivity and versatility. However, achieving precise temporal and spatial control in the activation of DNAzymes remains a challenge. Wang et al.101 demonstrated a sophisticated FRET method by using a high-intensity focused ultrasound (HIFU)-activated Zn2+-specific probe (8–17DNAzyme-HIFU) that can be by for specifically detecting and imaging zinc ions in HeLa cells and mice. This method, as shown in Fig. 4A, realized both spatial and temporal control over the precise activation of 8–17DNAzymevia the HIFU treatment. The results confirmed that the 8–17DNAzyme-HIFU probes exhibited minimal activity before HIFU activation, and significantly higher activity after exposure to HIFU ultrasound. This demonstration of non-invasive, high spatial and temporal control using the HIFU-activated probes expands their application in metal ion detection, making them a potent tool for in vivo imaging of metal ions.
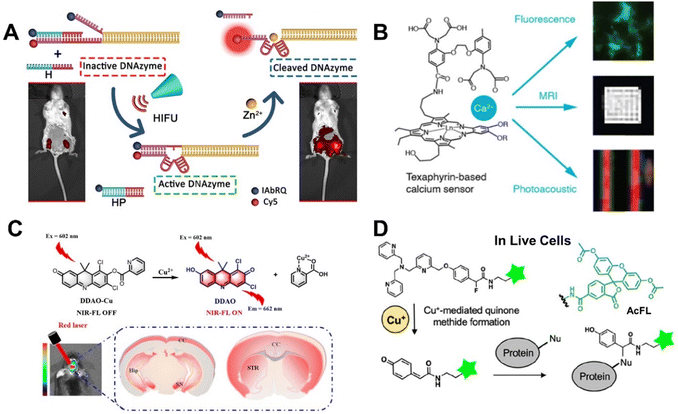 | ||
| Fig. 4 (A) The sensing mechanism of 8–17DNAzyme-HIFU for Zn2+ detection.101 Copyright 2022, American Chemical Society. (B) The multimodal probe of CaST-AM for Ca2+ detection.107 Copyright 2023, American Chemical Society. (C) The Cu2+ sensing mechanism of DDAO-Cu.112 Copyright 2020, Royal Society of Chemistry. (D) The sensing process of AcFL-P2 for Cu+ imaging in vivo.114 Copyright 2024, American Chemical Society. | ||
Changes in the levels of nuclear Zn2+ are also associated with neuronal dysfunction, oxidative stress, and inflammation. Liu et al.103 introduced an advanced CRISPR/Cas9 and 470 nm light-activated DNAzyme probe (TSDP) designed specifically for detecting nuclear Zn2+ in cell nuclei. The system uses CRISPR/Cas9 to create targeted DNA double-strand breaks (DSBs) in the nucleus, which forms a gated logic control system for the precise localization and controlled detection of nuclear Zn2+ along with 470 nm light-induced activation. The TSDP probe consisted of three long-length strands and remained inactive. When Cas9 was combined with sgRNA, the formed complex could cleavage TSDP to restore the DNAzyme activity that can then bind to nuclear Zn2+ for imaging. This method also incorporates light activation and Boolean logic gates to reduce non-specific activation before the probe enters the nucleus, enhancing dynamic monitoring and spatiotemporal imaging nuclear of Zn2+ control in both HeLa cells and mice.
Although some Ca2+-specific probes including BG3-Indo-1, BOCA-1-BG, RhoCa-Halo, and the near-infrared JF646-BAPTA, has been proposed, they suffer from limited cell permeability and solubility, and require washing steps to remove unreacted probes, which significantly limits their applicability. To deal with this, a series of rhodamine-based Ca2+ probes were developed by Mertes et al., characterized by high cellular permeability, localization, and varying calcium affinities.106 These probes offer the convenience of not requiring additional washing steps, making them highly practical for biological applications. The high-affinity probe MaPCa-656high and the low-affinity probe MaPCa-656low are used to detect Ca2+ changes triggered by single action potentials and to localize Ca2+ flow in the endoplasmic reticulum, respectively, providing important insights into the interactions of calcium across different organelles.
The ability to monitor intracellular Ca2+ concentrations using fluorescent probes has significantly advanced our understanding of biological signalling processes at the cellular level. However, a major challenge remains in linking these measurements with broader signal modalities that optical techniques alone cannot capture. To address this need, Thiabaud et al.107 utilized the stable, optically and magnetically active texaphyrin-based multimodal Ca2+ probe named CaST-AM. Unlike previous single- or dual-modality Ca2+ probes, CaST-AM produces MRI, PAT, and fluorescence signal changes upon Ca2+ stimulation (Fig. 4B). Future efforts pointed out that the sensitivity of CaST-related Ca2+ probes could be enhanced by altering the degree of coupling between the MGd and BAPTA components. Although functional imaging in vivo has not yet been performed, this work shows multimodal imaging contrast of CaST-AM probe in complex tissues, paving the way for studies bridging the gap between cellular physiology and biology at organ- or organism-scale in living subjects.
Parkinson's disease is the second most common neurodegenerative disorder.85 Cu2+ can exacerbate the aggregation of alpha-synuclein in the substantia nigra, leading to mitochondrial dysfunction and promoting the degeneration of dopaminergic neurons, thereby potentially accelerating the progression of Parkinson's disease.111 As displayed in Fig. 4C, Chen et al.112 designed a novel near-infrared fluorescent probe (DDAO-Cu) that can cross the blood–brain barrier (BBB) to specifically detect Cu2+ in the brains of mouse models of Parkinson's disease via the PeT mechanism. This study provides a crucial tool for systematic imaging of Cu2+ in Parkinson's models, contributing significantly to the understanding of potential pathological mechanisms related to copper in Parkinson's disease. Xiao et al. developed a rhodamine-based bifunctional probe MitoRhB that can target both mitochondrial membrane and Cu2+ to accurately detect changes in mitochondrial membrane potential (MMP) induced by Cu2+. The authors cleverly used the concentration of copper ions as a bridge to establish a quantitative relationship between the proportion of cells with low MMP and the fluorescence lifetime of MitoRhB. Additionally, using the previously reported AggTag probe Halo-P1, the study directly observed protein aggregation induced by Cu2+ in living cells using fluorescence lifetime imaging microscopy (FLIM), confirming that Cu2+-induced MMP depolarization exacerbates the degree of protein aggregation within cells.
Cai et al.113 designed a coumarin-based D–π–A typed AIE probe (Cm-p-TPA) by linking the lactone structure of coumarin to a propeller-like and electron-donating TPA group. Due to the good biocompatibility and specific recognition of Cu2+, the red-emitting Cm-p-TPA was successfully applied to monitor Cu2+ concentrations during the mitochondrial autophagy process in HeLa cells using the FLIM technique. This study demonstrates the feasibility of utilizing positional isomer strategies to regulate the performance of coumarin-based BioAIE probes from biomaterial derivatives, which is viable and effective for developing high quality imaging methods.
Fluorescent probes capable of detecting and tracking activated intracellular Cu+ provide valuable research tools for elucidating the physiological and pathological functions of Cu+. Recently, Cheng et al.114 designed a Cu+ responsive probe, where the fluorophore and copper chelator are connected via a methylene quinone (QM) precursor featuring a benzyl ether bond structure (Fig. 4D). The C–O bond of the benzyl ether is oxidatively cleaved through Cu+ coordination, subsequently releasing a QM active intermediate that can label nearby protein nucleophilic amino acid residues (Cys, Lys, and His) for imaging. It was found that the tris(2-pyridylmethyl) amine (TPA)-based FL-P2 probe can be more easily activated by Cu+ compared with other metal ions and ROSs. To enhance the cell membrane permeability of the probe, the luciferin structure in FL-P2 was replaced with diacetylluciferin (AcFL) to obtain the modified probe AcFL-P2. As expected, AcFL-P2 can be rapidly absorbed by living cells within five minutes. Once those acetyl groups were removed by endogenous esterases, AcFL-P2 emitted fluorescence and distributed uniformly throughout the cell. This modification allowed for unbiased spatial protein labeling and Cu+ detection in living cells, making it a highly suitable probe for real-time, in-cell studies of copper dynamics.
3.2. Imaging of reactive species-based biomarkers
Oxidative stress is intricately linked to the pathophysiology of various diseases like cancers, neurodegenerative disorders, epilepsy, depression, and diabetes.115–117 Reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS) playing crucial roles as both participants in and biomarkers of oxidative processes.118,119 However, due to the short lifespan and high reactivity of those reactive species in organisms, it is challenging to study their connections with diseases in detail.4 Fluorescence-based imaging technologies, combined with confocal imaging, two-photon imaging, and in vivo imaging, provide non-invasive, real-time, high-resolution imaging of cells and animals to explore the roles of ROS, RNS, and RSS in various pathological conditions. Recent advancements on fluorescence probes used to monitor the levels of ROS, RNS, and RSS will be shared in this section.Although some H2O2-responsive fluorescent probes based on classic fluorophores such as naphthalimide, rhodamine, BODIPY, coumarin, etc. have been reported, their applications in vivo are indeed limited due to short emission wavelengths (<650 nm).34 This shorter wavelength range can result in higher background fluorescence from biological tissues, leading to less effective tissue penetration and reduced imaging contrast in deeper tissues. Longer wavelengths, particularly in the near-infrared (NIR) range (650–900 nm), are more effective for in vivo applications because they allow for better tissue penetration and lower background interference. Therefore, Zhang et al.120 developed a NIR-I fluorescent probe (QX-B) for detecting H2O2 by linking the fluorescent quinolinium-xanthene to a borate ester as the response group. Through the ICT mechanism, the designed QX-B probe in Fig. 5A exhibited a significant NIR fluorescence at 772 nm and showed sensitive and specific response to H2O2 in diabetic mice over other interfering species.
 | ||
| Fig. 5 (A) The sensing mechanism of QX-B for H2O2 detection.120 Copyright 2021, American Chemical Society. (B) The sensing mechanism of MB-HClO for HClO detection in mice.124 Copyright 2023, Elsevier. (C) The sensing mechanism of PN910 for H2O2 and ONOO− sensing.132 Copyright 2021, Wiley and Sons. | ||
Similarly, Song et al.121 developed a specifically tailored NIR-I fluorescent probe (Mito-Bor) for monitoring mitochondrial H2O2 in the context of pulmonary fibrosis. The Mito-Bor probe was designed based on a near-infrared azo-BODIPY, featuring a 4-bromomethylphenyl boronic pinacol ester as the H2O2-responsive module. The addition of a lipophilic triphenylphosphine cation enhanced the Mito-Bor's cell membrane permeability and mitochondrial targeting, ensuring specificity and efficacy in real-time, high-resolution monitoring of mitochondrial H2O2. By employing the bleomycin-induced mouse model of pulmonary fibrosis, the critical role of NADPH oxidase 4 (NOX4) in regulating H2O2 levels was revealed. It was found that the direct inhibition of NOX4 markedly reduced H2O2 concentrations, highlighting the potential of Mito-Bor probe as a significant diagnostic and therapeutic tool for pulmonary fibrosis.
Compared with NIR, the NIR-II window (900–1800 nm) experiences less scattering in biological tissues, thereby offering deeper tissue penetration and higher imaging resolution. Tian et al.34 developed a novel NIR-II fluorescent probe (IR-990) with an acceptor–π–acceptor (A–π–A) structure, specifically designed for detecting H2O2in vivo to investigate biomarkers and mechanisms associated with drug-induced liver injury (DILI). Upon activation by H2O2, IR-990 transitioned to a donor–π–acceptor (D–π–A) configuration via the enhanced ICT mechanism, emitting intense NIR-II fluorescence at 990 nm with a 200 nm Stokes shift, which enabled high-contrast imaging. The IR-990 probe provided critical insights into the mechanisms of liver injury by monitoring H2O2 with a detection limit of 0.59 μM in HepG2 cell models of DILI. Furthermore, IR-990 probe was successfully applied in the DILI mouse models, facilitating real-time visualization of endogenous H2O2 dynamics during DILI and offering a powerful tool for studying oxidative stress-related disease mechanisms and therapeutic strategies.
Hypochlorous acid (HClO) is commonly grouped with ROS because it is a strong oxidizing agent produced by the body's immune cells, particularly during the respiratory burst in neutrophils.122,123 HClO is formed by the action of the enzyme myeloperoxidase, which uses H2O2 and chloride ions (Cl−) to produce HClO, which has been regarded as a potential biomarker for inflammation and infections.124 Jia et al.122 developed a specifically designed NIR fluorescent probe (NHF) for the selective detection of HClO in liver injury. The NHF probe was incorporated with a N-acetylgalactosamine (GalNAc) as a targeting ligand to facilitate active delivery to liver tissues in zebrafish and mice, showcasing deep tissue penetration with low autofluorescence interference. As the first hepatocyte-specific fluorescent probe, NHF showed high selectivity when monitoring endogenous HClO levels in HepG2 cells under oxygen–glucose deprivation/reperfusion conditions and in liver tissues affected by acetaminophen-induced damage. Similarly, Li et al.123 also developed a NIR hepatocyte-specific fluorescent probe (MBH-MT) for detecting HClO in hepatocellular carcinoma (HCC). Given the poor prognosis associated with HCC, early detection and precise surgical intervention are crucial. The MBH-MT probe exhibited excellent optical properties, strong NIR emission and low biotoxicity for deep tissue imaging of HClO in biological samples and has been effectively applied in distinguishing between normal and cancerous liver cells (such as HepG2) in vitro and targeted imaging in mouse models of liver cancer. The probe's application extends to guiding liver cancer surgery and could significantly enhance diagnostic and therapeutic outcomes for liver cancer patients, promising substantial impacts on clinical approaches and patient survival in liver disease.
Lysosomes play a vital role in cellular digestion and immune defence. Imaging of HClO within lysosomes is crucial for understanding intracellular signalling and host defence mechanisms. Li et al.124 focuses on developing a NIR probe (MB-HClO) for the rapid detection of HClO in lysosomes. Given the high morbidity and mortality associated with heatstroke, monitoring lysosomal HClO levels is crucial for preventing and treating this condition. In Fig. 5B, the MB-HClO probe showed good selectivity and sensitivity when monitoring both exogenous and endogenous levels of HClO in living cells, making it a promising tool for investigating the complex biological mechanisms of heatstroke.
ONOO− is a potent oxidant that can rapidly reacts with proteins, lipids, and DNA, playing a key role in the pathogenesis of numerous conditions, including cardiovascular diseases, neurodegenerative disorders, inflammation, and cancer. Detection of ONOO− is crucial for understanding and treating many pathological processes.125–127 Recently, Sun et al.128 developed a novel NIR probe (NIR-PN1), specifically designed for the rapid and sensitive detection of ONOO−, a key player in the pathogenesis of parkinson's disease (PD). The NIR-PN1 probe employed NIR fluorescence imaging technology to detect ONOO− within seconds with ultrafast response and high selectivity via the PeT mechanism, which has also been successfully applied to image ONOO− flux in various PD models, including PC12 cells, fruit flies, Caenorhabditis elegans, and mouse brains, providing new tools and insights for the study and potential treatment of PD.
Luo et al.129 developed a new NIR two-photon (TP) fluorescent probe (DCM-ONOO) to track and image ONOO−via the ICT mechanism. The DCM-ONOO probe was designed using a NIR dicyanomethylene-benzopyran (DCM) as the TP fluorophore, which emitted strong fluorescence (∼680 nm) after specifically reacting with ONOO−. Based on the excellent temporal and spatial resolution, DCM-ONOO probe enabled sensitive response to the changes in endogenous ONOO− concentration in living cells and in the brains of rats induced with epilepsy via kainic acid (KA). Another NIR imaging probe (ONP) was developed by Hu et al. for ONOO− imaging within the brain in vivo. The ONP probe was crafted to monitor dynamic changes in endogenous ONOO− levels in brains of epileptic models induced by KA, featuring high sensitivity and selectivity. Miao et al.130 developed a new fluorescent probe A2 for detecting ONOO−. The A2 probe was designed based on the N-oxidation and N-nitrosation reactions of aromatic tertiary amines, which exhibited an extremely rapid fluorescence turn-off response (within seconds), ultra-high sensitivity (detection limit below 2 nanomolar), and excellent selectivity against a range of biologically relevant reactive oxygen species and metal cations. Meanwhile, two derivatives of the A2 probe named Mito-A2 and Lyso-A2 were also fabricated to separately target mitochondria and lysosomes for specific subcellular imaging. Another ONOO−-responsive probe SiRTA was developed by incorporating a Si-rhodamine fluorophore with an aromatic tertiary amine functional group by Miao et al.131 The SiRTA probe exhibits rapid, sensitive, and specific fluorescence “off–on” response to endogenous ONOO− in living cells. Notably, the therapeutic effects of phenolic antioxidants in ischemia-reperfusion injury in endothelial cells, diabetes in pancreatic β-cells, and diabetic nephropathy in rats were successfully assessed by using the SiRTA probe.
Monitoring the dynamics of ROS and RNS in real-time during therapeutic interventions, especially antioxidant and anti-inflammatory treatments, helps in assessing treatment efficacy and adjusting therapeutic strategies.132 Wu et al.133 developed a bifunctional fluorescence probe (LW-OTf) for detecting and imaging ROS/RNS-related biomarkers involved in drug-induced liver injury (DILI). The LW-OTf probe employed two fluorescence mechanisms: near-infrared fluorescence (NIRF) and two-photon excited fluorescence (TPEF). The LW-OTf probe was first activated by O2˙− radicals to generate NIR fluorescence output. Then, peroxynitrite (ONOO−) promoted the cleavage activity of LW-OTf, releasing LW-XTD detected by TPEF. This probe can simultaneously monitor the activity of both biomarkers and is used to study the mechanisms of drug-induced liver damage, especially APAP-induced liver injury. By combining NIRF and TPEF technologies, the LW-OTf probe provides an effective tool for DILI research, capable of monitoring and differentiating the dynamics of ROS and RNS in both cellular and mouse models.
To achieve deeper tissue penetration and higher imaging resolution, Zhang et al.132 developed a dual-activatable NIR-II probe (PN910) for in vivo monitoring of both ROS and RNS species in alkaline conditions. As shown in Fig. 5C, PN910 exhibited high selectivity for H2O2 and ONOO− in a real-time and non-invasive manner, particularly suited for inflammation-prone areas, such as real-time monitoring of cystitis and colitis in animal models. The successful application of PN910 not only enhanced understanding of the dynamic changes in ROS/RNS biomarkers related to inflammation but also provided a new tool for clinical-related research. Besides, Murfin et al.134 introduced a novel two-photon fluorescent probe (Probe 1) composed of azulene coupled with a suitably substituted fluorene boronate ester receptor motif for the detection of ONOO− and H2O2 in mice tissues. Probe 1 showed notable features include excellent cell permeability, non-cytotoxicity, and superior photostability, making it suitable for cellular imaging. Utilizing the unique fluorescence properties of azulene and enhanced two-photon absorption, Probe 1 offers new possibilities for biological imaging, particularly in deep tissue imaging and real-time monitoring of dynamic biological processes.
In clinical applications, multi-component imaging can aid in the precise diagnosis of diseases by highlighting different pathological biomarkers or tissue states. Instead of two-component ROS/RNS imaging, Liu et al.135 developed a novel pH-sensitive fluorescent probe (Hcy-OH) for multi-component detection and imaging of different reactive species in varying pH environments. The Hcy-OH probe exhibits specific responses to different types of reactive species depending on the pH conditions—reacting to ONOO− under acidic conditions, under neutral conditions, and 1O2 under alkaline conditions. Featuring responsive fluorescence and a ratiometric curve, the Hcy-OH probe was utilized for visualizing both exogenous and endogenous ROS/RNS species at the cellular level. The sensing capabilities of Hcy-OH have been validated for imaging inflammatory tissues, particularly demonstrating accuracy under monitoring conditions like arthritis. The Hcy-OH probe serves as a powerful tool for elucidating the physiological and pathological aspects of inflammation by using multi-component fluorescence imaging strategy.
H2S, as a gasotransmitter, is recognized as a significant biomarker in numerous physiological and pathological processes occurring in cardiovascular disorders, neurodegenerative diseases, and cancers.138 Fluorescence imaging provides a non-invasive, real-time tool to observe H2S dynamics, offering insights into its regulatory effects on blood pressure, neural protection, cellular metabolism, and apoptosis. Chen et al.139 focuses on developing a novel probe H-Luc for monitoring endogenous H2S in a non-alcoholic fatty liver disease (NAFLD) mouse model. The probe was engineered by attaching an H2S-recognition component, 2,4-dinitrophenol, to the luciferase substrate D-luciferin, allowing for the release of cage-free D-luciferin in the presence of H2S through a nucleophilic aromatic substitution reaction. Combined with cellular delivery of firefly luciferase mRNA via lipid nanoparticles (LNP), this luciferase–luciferin system enables sensitive and selective detection of intracellular H2S, applicable both in vitro and in vivo. Wang et al.35 developed a dual-modal NIR-II nanoprobe (DCNP-Cu2O@PDA) to monitor high expression of H2S in colorectal cancer. DCNP-Cu2O@PDA was constructed via uniformly coating polydopamine (PDA) on the surface of Cu2O conjugated down-converting nanoparticles (DCNP). The high expression of endogenous H2S in colorectal tumors can convert Cu2O to copper sulfide (Cu2−xS), leading to a competitive absorption at ∼980 nm that quenched fluorescence signals. The dual-modal functionality (photoacoustic and fluorescence imaging) of DCNP-Cu2O@PDA offered a reliable method for accurate monitoring of H2S in colorectal cancer.
Compared to H2S, polysulfides (H2Sn) are more reactive than in the pathogenesis of diseases, such as neurodegenerative disorders by influencing neuroprotective and damaging pathways.136 Zheng et al.140 developed a novel dual-colour fluorescent probe specifically for detecting H2Sn as opposed to H2S. Through molecular engineering of a boron-dipyrromethene (BODIPY) luminophore with mPEG-DSPE-2000, a highly selective, water-soluble, and biocompatible nanoprobe BOD-CN-NP was designed. The H2Sn-acviated BOD-CN-NP nanoprobe exhibited a rare aggregation-induced dual-colour fluorescence (AADF) response, emitting bright lights at 588 and 750 nm, achieving high-integrity imaging of intracellular H2Sn. This approach of an H2Sn-activated dual-colour fluorescent probe provides new tools and insights for studying biological events mediated by H2Sn by multi-colour fluorescent imaging in the future.
Monitoring mitochondrial thiols provides insights into the oxidative stress status of cells, which is pivotal for understanding and diagnosing various pathological conditions. Yang et al.141 developed a novel fluorescent probe (NIR-HMPC) for specifically detecting and imaging thiols in mitochondria. Based on the chromene (benzopyran) molecule, the probe operates through a thiol-chromene “click” nucleophilic pyran ring-opening reaction, achieving rapid, sensitive, and specific imaging of thiols dynamics during oxidative stress and apoptosis.
Cysteine is a crucial amino acid involved in numerous metabolic and regulatory processes within the brain. Monitoring cysteine levels can provide insights into the redox state of the brain, essential for maintaining neuronal health and function. Zhang et al.142 developed a novel TP fluorescent probe TCS specifically for monitoring cysteine (Cys) in the brain. The probe is based on the specific recognition site of thio-benzyl ester, enabling it through a selective nucleophilic addition reaction with the Cys carbon-disulfide bonds to form a stable five-membered ring, triggering significant fluorescence enhancement. Uniquely, this reaction does not occur with other biological thiols, providing unprecedented selectivity for Cys. This probe is particularly crucial in studying the pathogenesis of depression, as it was successfully used to directly observe a marked reduction of Cys in the brains of mice with a depression phenotype. This not only advances our understanding of the pathogenic mechanisms of depression but also paves the way for precise imaging of Cys. Glutathione (GSH) is a vital antioxidant in cells, playing a crucial role in protecting against oxidative stress, which can damage cells and lead to diseases like cancer, and Alzheimer's, and heart diseases. Zhang et al.143 developed two novel “turn-off” NIR fluorescent probes, BCy-SeSe and BCy-SS, specifically for detecting changes in mitochondrial GSH during cerebral ischemia/reperfusion (I/R) processes. These probes, designed based on a new fluorophore BCy-Keto, exhibit significant fluorescence enhancement upon reacting with GSH. BCy-SeSe, selected for biological applications due to its faster response, successfully imaged GSH in real-time in live cells and a mouse model of focal cerebral ischemia.
Combined fluorescence imaging of RSS and ROS is essential for the comprehensive monitoring of oxidative and sulfidative stress in biological systems. Yang et al.144 developed a novel fluorescent probe named TCAB, designed for discriminative and dynamic detection of the signalling molecules H2O2 and H2S involved in oxidative stress. As shown in Fig. 6A, the TCAB probe exhibits high selectivity and sensitivity, producing distinct fluorescence signals for H2O2 and H2S. When both H2O2 and H2S are present, the probe can display colour changes based on the sequence of their reactions with the TCAB probe, enabling dynamic monitoring of their interactions. The development of this probe not only enhances our understanding of the redox processes involving H2O2 and H2S in living cells and organisms but also advances fluorescence imaging technology, providing a powerful tool for investigating the complex mechanisms of oxidative stress.
 | ||
| Fig. 6 (A) The sensing mechanism of TCAB for H2O2 and H2S detection.144 Copyright 2020, American Chemical Society. (B) The sensing mechanism of NIRII-HD5 platform for ONOO−/GSH/ALP detection.33 Copyright 2023, John Wiley and Sons. | ||
Gao et al.145 developed a fluorescent probe named HCy-ONO designed for simultaneous detection of superoxide anion (O2˙−) and polysulfides (H2Sn) in cells, key players in maintaining cellular redox balance. The HCy-ONO probe operates on a tandem reaction mechanism, enabling separate detection of O2•− and H2Sn in different fluorescence collection windows without spectral overlap, offering high sensitivity (detection limits of 90 and 100 nanomolar, respectively) and selectivity. This technology not only aids in studying cellular responses under continuous or intermittent hypoxia but also distinguishes inflamed from normal tissues in an acute peritonitis mouse model, in addition to dynamically imaging O2˙− and H2Sn in a tumor-bearing mouse model. Furthermore, this probe allows for an in-depth understanding of the molecular mechanisms involved in ischemia-reperfusion injury and other related pathological processes, holding significant implications for studying diseases related to oxidative stress.
Qin et al.33 developed a novel dye structure (NIRII-HD5), specifically optimized for developing a NIR-II fluorescent platform based on hemicyanine dyes (HD). Notably, the NIRII-HD5 probe exhibited excellent optical properties with an appropriate pKa value (∼6.5), excellent stability, and high NIR-II brightness for high-contrast in vivo imaging. Based on the NIRII-HD5 probe, three target-activatable NIR-II probes were designed for ONOO−, GSH, and alkaline phosphatase (ALP) in Fig. 6B. This advancement in NIR-II imaging technology demonstrates broad application prospects in disease diagnostics and biomedical research, particularly in deep tissue imaging and biosensing.
3.3. Imaging of enzymes-based biomarkers
Enzymes, such as γ-glutamyltransferase (GGT), β-galactosidase (β-Gal), nitroreductase (NTRP), aminopeptidase N, and alkaline phosphatase (ALP), play a central role in living systems by catalyzing biochemical reactions necessary for processes such as metabolism, DNA replication, and cellular signalling.8,146 Abnormal expression of enzymes has become a focal point for researchers due to their specificity and reliability in cellular and in vivo imaging.5,146 This method allows for real-time, non-invasive monitoring of these activities within cells and tissues, facilitating early diagnosis, monitoring of disease progression, and assessment of treatment efficacy. In this section, we will discuss recent advancements on novel fluorescent probes for bioimaging application of enzyme-related biomarkers. | ||
| Fig. 7 (A) The sensing mechanism of OTBP-G for GGT imaging.148 Copyright 2024, American Chemical Society. (B) The sensing mechanism of KSA01 and KSA02 platform for ratiometric fluorescence imaging.153 Copyright 2021, John Wiley and Sons. | ||
Hepatocellular carcinoma (HCC) is a major health threat to human beings while GGT is closely associated with its progression. Wang et al.149 developed structurally optimized probe (ETYZE-GGT) for bimodal imaging of GGT in HCC-related processes through the ICT mechanism, encompassing both far-red fluorescence (FL) and photoacoustic (PA) modes. ETYZE-GGT performed steady and practical monitoring capabilities across multiple HCC-related models including fibrosis, developed HCC processes, and premonitory induction stages like autoimmune hepatitis, drug-induced liver injury, and non-alcoholic fatty liver disease. The two imaging modes provided consistent and complementary results for GGT detection with high spatial resolution, precise apparatus, and excellent biocompatibility. Compared with existing techniques such as testing serum indexes and pathological staining, ETYZE-GGT facilitated a universal application for accurate pre-clinical diagnosis of as many HCC stages as possible.
Wang et al.150 developed double-locked TP fluorescent probe (C-HBrO-GGT) for the non-invasive and reliable detection of early-stage atherosclerosis, where GGT and hypobromous acid (HBrO) were two potential biomarkers. The double-locked C-HBrO-GGT probe was ingeniously designed with two sequential triggers pulled by GGT and HBrO in an orderly manner. In this way, it can be ensured that the C-HBrO-GGT probe can be activated by HBrO only after the hydrolysis by GGT. Using this strategy, the CLC-1-HBrO-catalase (CAT)-GGT signaling pathway at the cellular level was confirmed, and the formation of forthcoming atherosclerotic plaques was successfully predicted. The study presents a powerful tool for accurately indicating the location of mature plaques and providing early warnings for atherosclerotic plaques. Miao et al.151 developed an activatable dual-modality fluorescent–photoacoustic imaging probe (IP) for the specific visualization of liver fibrosis by using GGT as the responsive substrate. The IP probe was constructed based on a NIR thio-cyanine-semicyanine dye combined with GGT and integrin-targeting peptide (cRGD) modules. This unique molecular design allows the IP probe to specifically accumulate in areas of liver fibrosis through cRGD recognition of integrins, and its fluorescent-photoacoustic signal was activated upon interaction with overexpressed GGT, thus enabling precise monitoring of liver fibrosis. The study presents a potential strategy for designing dual-targeted fluorescent-photoacoustic imaging probes, providing a new approach for non-invasively detecting early-stage liver fibrosis.
Traditional probes for senescence-associated β-Gal (SA-β-gal) were used to primarily monitoring the enzyme accumulation in lysosomes, which cannot distinguish aging from other physiological processes involving β-gal. Gao et al.153 developed novel two-dimensional probes KSA01 and KSA02 for the precise tracking of cellular aging (Fig. 7B). The activity of β-Gal was detected by KSA01 and KSA02 probes through two dimensions: one was the selective cleavage of β-glycosidic C–O bond by probes to releasing the fluorophores KSAP1 and KSAP2, and another one was ratiometric fluorescence imaging by those KSAP1 and KSAP2 towards the pH variation in lysosomal aging cells. This novel design not only improved the distinction between SA-β-gal and cancer-associated β-gal but also enabled more accurate tracking of cellular and tissue aging. Interaction of the probe with an Escherichia coli β-gal mutant (E537Q) reveals the structural basis of its recognition, further confirming its specificity and efficacy.
Xu et al.154 developed a novel NIR AIE-based fluorescent probe (QM-TPA-Gal) for detecting β-Gal in ovarian cancer cells. The QM-TPA-Gal probe was operated by hydrolyzing a hydrophilic non-fluorescent substance through β-Gal to produce the hydrophobic QM-TPA-OH which aggregated in the nanoparticles within cells with NIR fluorescence. The QM-TPA-Gal probe performed high sensitivity and selectivity towards β-Gal with a detection limit of 0.21 U mL−1in vitro, which was further applied for β-Gal imaging in SKOV3 cells and live mouse models. All the successful results indicated the potential of QM-TPA-Gal as a crucial tool for the early diagnosis of ovarian cancer and other β-Gal-related diseases.
To enhance cellular permeability and solubility for precise imaging of intracellular β-Gal activity, a photochromic β-Gal-responsive probe (NpG) was hybridized with human serum albumin (HSA) to form a probe/protein hybrid (NpG@HSA).155 Upon binding with HSA, the fluorescent naphthalimide unit in NpG produced enhanced green fluorescence emission at 525 nm, while β-Gal-mediated cleavage of the galactoside unit triggered increased red fluorescence emission at 620 nm. By using STORM (stochastic optical reconstruction microscopy), this imaging system mapped the subcellular distribution of β-Gal within cells with nanoscale precision, which provided a universal platform for designing photochromic fluorescent probes to illuminate specific biomarkers in various cellular processes of diseases.
Zheng et al.159 developed a novel nano-enhancer, termed the cycling hypoxia-amplified nano-amplifier (CGH NAs), for precise theranostics within the tumor microenvironment (TME) with the assistance of NTR. The CGH NAs were formed by the encapsulation of CQ4T and GOx within an NTR-responsive hyaluronic acid-nitroimidazole (HA-NI) probe using a self-assembly method. This design leveraged a dual-triggered reaction process, including internal regulation and external stimulation, for precise activation and targeting of hypoxic zones. Upon accumulation and activation by NTR in the tumor region, CGH NAs cut off the tumor's energy supply and induce cancer starvation therapy by generating gluconic acid and H2O2. Concurrently, the release of CQ4T facilitated the rapid recovery of NIR-II FL signals for imaging-guided photodynamic therapy (PDT).
Xiang et al.160 proposed a nanoprobe (CuSe@BSA@NTRP) for in situ imaging and synergistic antibacterial treatment of bacterial keratitis by anchoring an NTR-responsive probe (NTRP) onto the CuSe@BSA nanoparticles. CuSe@BSA@NTRP generated specific fluorescence signals within 30 minutes due to the interaction between the released NTRP and the bacterial endogenous NTR. When combined with low-temperature photothermal therapy (PTT), the nanoparticles effectively eliminated bacteria with an antibacterial efficacy exceeding 95% and promote epithelial cell regeneration at the corneal wound site. Overall, CuSe@BSA@NTRP nanoparticles demonstrate potential for rapid, non-invasive diagnosis, treatment, and visual assessment of therapeutic outcomes in bacterial keratitis.
4. Artificial intelligence-enhanced optimization for fluorescent probes and imaging
Artificial intelligence (AI), have found success in diverse applications such as image recognition, speech processing, recommendation systems, and autonomous vehicles.28,40 To date, ML and DL algorithms have been applied for analyzing large datasets, predicting optimal molecular structures, proposing new chemical modifications that improve the performance of fluorescent probes and significantly enhanced image analysis.165,1664.1. AI-assisted optimization for fluorescent probes
To date, AI algorithms have been used to optimize the design of novel fluorescent probes with improved photostability, quantum yield, sensitivity, and specificity for particular biological targets.2,167 ML algorithms including ridge regression (RR), support vector machine (SVM), kernel ridge regression (KRR), feed-forward networks (FFN) and message-passing neural networks (MPNN) from different ML generations have been used to predict molecular properties. Bhat et al.167 developed a curated dataset containing information on 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 molecules and used density functional theory (DFT) and time-dependent DFT (TDDFT) related-AI algorithms to predict the electronic, redox, and optical properties of organic π-conjugated molecules in few seconds (Fig. 8A). De novo molecule generator (DNMG) is a computational algorithm designed to develop novel molecular structures without using those existing databases of molecules. DNMG usually integrates with AI to enhance its predictive capability. For instance, Sumita et al.168 combined DNMG with quantum chemical computations (QCs) to developed a massively parallelized ChemTS system (Fig. 8B) which generated large amounts of fluorescent molecules. Another significant advancement in the design of fluorescent probes by integrating AI into the process was reported for the designation of organelle-targeted fluorescent probes. In this work, Dong et al. constructed a multilevel prediction framework using AI algorithms to generate specific fluorescent probes for mitochondria bioimaging with high accuracy.
000 molecules and used density functional theory (DFT) and time-dependent DFT (TDDFT) related-AI algorithms to predict the electronic, redox, and optical properties of organic π-conjugated molecules in few seconds (Fig. 8A). De novo molecule generator (DNMG) is a computational algorithm designed to develop novel molecular structures without using those existing databases of molecules. DNMG usually integrates with AI to enhance its predictive capability. For instance, Sumita et al.168 combined DNMG with quantum chemical computations (QCs) to developed a massively parallelized ChemTS system (Fig. 8B) which generated large amounts of fluorescent molecules. Another significant advancement in the design of fluorescent probes by integrating AI into the process was reported for the designation of organelle-targeted fluorescent probes. In this work, Dong et al. constructed a multilevel prediction framework using AI algorithms to generate specific fluorescent probes for mitochondria bioimaging with high accuracy.
 | ||
| Fig. 8 (A) The input representation for an ML model and the model architecture for predicting π-conjugated molecules indicated in different color-coded arrows.167 Copyright 2023, Royal Society of Chemistry. (B) Workflow of fluorescent molecule generation by ChemTS based on the electronic structure theory by iterating this process.168 Copyright 2022, The American Association for the Advancement of Science. | ||
Since those existing ESIPT probes are not sufficiently tailored to meet specific requirements across different scenarios, and the traditional trial-and-error approach to developing these molecules is both time-consuming and costly. To deal with this, Huang et al.28 constructed the first high-quality ESIPT dataset and developed a multi-level prediction system which can accurately identifies ESIPT molecules from a large pool of compounds using a SHapley Additive exPlanations (SHAP) method. The study not only achieved the efficient identification of promising ESIPT molecules but also validated their safety and effectiveness, representing a substantial advancement in this area of research.
The pKcycl value represents the dissociation constant of a dye or fluorescent molecule, indicating how its properties such as brightness and stability might change under different pH conditions. There is an urgent need to develop effective AI-based methodologies that leverage existing dye data to guide the rational design of new molecules. Xiang et al.169 proposed a machine learning (ML)-assisted strategy for quantitatively predicting the pKcycl values of xanthene dyes. With an ML-assisted model, two suitable xanthene-based fluorophores Si-rhodamine-pH and Si-rhodamine-iapH with desired pKcycl values were obtained from 113 xanthene dyes. Machine learning models enable researchers to rapidly predict the behaviour of new dye molecules under various pH conditions, facilitating the development of new probes. This is particularly valuable in applications requiring precise control over fluorescence properties for specific bioimaging tasks.
In addition to optimizing molecular fluorophores, AI has also been applied to optimize the design and preparation of fluorescent nanoprobes. For instance, Hong et al.170 established a ML-assisted Extreme Gradient Boosting (XGBoost) model to guide the specific synthesis of customized fluorescent carbon dots (CDs) with predictable optical properties at room temperature. The XGBoost model can effectively predict the maximum fluorescence intensity and emission centers of the CDs, allowing for the production of CDs with high fluorescence intensity and tunable emission properties.
4.2. AI-assisted fluorescence microscopy techniques
Fluorescence microscopy, characterized by its high spatial resolution, high specificity, non-contact nature, and non-radiative properties, is crucial for imaging fine biological structures. However, image degradation caused by biological tissues and optical systems necessitates the improvement of spatial resolution in fluorescence microscopy.2,42 Chen et al.171 developed a high-throughput in vivo microscope based on wide-field NIR-II (900–1880 nm) fluorescence microscopy, using two-photon fluorescence microscopy techniques combined with a scale-recurrent network (SRN) to enhance optical performance. With significantly improved spatial resolution, high-speed and high-resolution dynamic imaging abilities, this microscopy system successfully reconstructed the details of opaque blood vessels in the brains of rodents in 3D and observed minute changes in brain vasculature in early acute respiratory failure mice at a speed of 30 frames per second. This technology is significant for a deeper understanding of dynamic processes in complex biological systems, particularly in neuroscience and pathological research.To further leverage deep learning to enhance the quality of fluorescence imaging, Baulin et al.165 successfully employed one of the best neural networks named IterNet to predict vessels structures in vivo by NIR-IIb (1500–1700 nm) microscopy. With the same purpose, Ma et al.172 trained a generative adversarial network (GAN)-based model named CycleGAN. Compared with IterNet, CycleGAN can transform fluorescence images from the shorter-wavelength NIR-I/IIa (700–1300 nm) window into images resembling those in the wanted NIR-IIb window. Compared with traditional microscopy, this technique exhibited significantly enhanced the signal-to-noise ratio and tumor-to-normal tissue ratio, and optimized tumor margin localization. Besides, CycleGAN also substantially improved the resolution and signal/background ratio of in vivo non-invasive NIR-II light-sheet microscopy (LSM). This advancement highlights the great potential of DL-enhanced fluorescence imaging for advanced clinical diagnostics and image-guided surgery applications. Another successful attempt is the development of a DL enhanced NIR-II microscopy technique based on a VNTCNN model by Song's team.29 The developed VNTCNN model can transform low-resolution visible images into high-resolution NIR-II images by using optimized lanthanide-doped nanocrystals with multimodal emissive properties, lighting up a new avenue for high-resolution bioimaging in vivo.
Structured illumination microscopy (SIM) is a type of super-resolution fluorescence imaging technique that has good spatial-resolution by illuminating the sample with specific optical interference patterns.173 However, spatial light gratings used in traditional SIM limits its imaging speed and multi-colour imaging. To date, AI has also been applied in SIM to enhance its imaging speed and optimizing multi-colour imaging capabilities of SIM. As shown in Fig. 9, Ward et al.174 proposed a machine learning (ML)-assisted SIM microscopy technique MAI-SIM, which achieved multi-colour super-resolution imaging with high-speed. The core component of MAI-SIM is a Michelson interferometer for projecting wavelength-dependent wedge-shaped fringes onto the sample. By using small steps of the galvanometer to translate the mirror beam, a fixed lateral shift is produced in the projected image plane, achieving pattern phase shifting. The detection path of this system is equipped with a commercial three-way image splitter, including dichroic mirrors and emission filters, allowing the simultaneous imaging of three different fluorophores on a single high-speed camera. MAI-SIM data are acquired with no temporal offsets between the channels, enabling the observation of organelle–organelle interactions in real time image reconstruction during the immediate visualization of a variety of biomarkers-related process in vivo.
 | ||
| Fig. 9 (A) Working principle of MAI-SIM model and its (B) schematic of detection optics. (C) The multi-colour imaging capability of the developed MAI-SIM-based microscopy.174 Copyright 2022, Springer Nature. | ||
4.3. AI-driven fluorescence imaging for biomarkers
AI is revolutionizing the imaging of disease biomarkers, leading to significant advancements in precision medicine. The developments of various advanced AI models can automatically detect and quantify the biomarkers concentration in imaging data, allowing for the early and accurate detection of various diseases Moreover, AI can integrate imaging data with other types of data, such as genomic and proteomic information, to provide a more comprehensive understanding of disease.1 This holistic approach enables the identification of complex biomarker patterns that can be used to develop personalized treatment plans, improving the effectiveness of therapies tailored to individual patients. In this section, several recent works on the topic of AI-enhanced bioimaging and sensing for biomarkers were addressed.Feng et al.175 proposed a deep-learning (DL) enhanced biosensing method based on a persistent luminescence (PersL) NIR-nanoprobe (ZGSO) for high-fidelity imaging of HCOl in Fig. 10A. This method employed a 3D deep convolutional neural network (3D-CNN) to accurately extract lifetime features from intensity-based decay profiles, which effectively overcame the signal attenuation caused by tissue absorption and scattering. The luminescence lifetime of ZGSO nanoparticles was precisely tuned for high-contrast lifetime-based HCOl imaging. The proposed DL-enhanced biosensing platform showed high sensitivity and precise imaging for both endogenous and exogenous hypochlorous acid, offering new possibilities for the development of high-fidelity biosensing in complex matrix systems. Hu et al.27 developed a deep learning-assisted artificial vision platform based on europium(III)-functionalized nanoprobe (Eu@TCBP-HOF) to sensitively detect two biomarkers, spermine (Spm) and N-acetylneuraminic acid (NANA) for breast and ovarian cancers. As shown in Fig. 10B, the ratio fluorescence sensor operates in a “Turn-on” mode selectively for Spm detection and transitions to a “Turn-off” mode in the presence of NANA. Utilizing the DenseNet algorithm, the system simulated the human visual system to differentiate the concentrations of Spm and NANA rapidly and accurately with an accuracy exceeding 99%. The system also exhibits high sensitivity and exceptional colour response in real saliva and serum samples.
 | ||
| Fig. 10 (A) The working principle of the persistent luminescence imaging network (PLI-Net) for high-fidelity imaging of HCOl.175 Copyright 2024, American Chemical Society. (B) The artificial visual platform DenseNet for detecting Spm and NANA using the Eu@TCBP-HOF as nanoprobe.27 Copyright 2023, American Chemical Society. | ||
Xiang et al.169 proposed a machine learning (ML)-assisted sensing strategy for detection pH and cathepsin in vivo based on a novel fluorescent probe named Si-Rhodamine-Cathepsin-pH (SiR-CTS-pH). By modifying with a peptide-linker (Val-Cit), the SiR-CTS-pH probe performed a cathepsin/pH sequentially activated capabilities to the changes in pH and cathepsin levels in tumor cells and tissues. Compared to single-activated pH or cathepsin probes, SiR-CTS-pH demonstrated a higher signal-to-noise ratio and could accurately differentiate between complex hepatocellular carcinoma tissues and normal tissues, showcasing significant clinical application potential.
5. Conclusions and perspectives
This review has primarily illuminated the significant advancements made recently in fluorescence bioimaging, spotlighting the development and utilization of innovative imaging and sensing strategies. These strategies employ highly efficient fluorescence probes for in situ visualization and detection of essential disease biomarkers at both cellular and in vivo levels. Remarkable efforts have been invested in developing novel fluorescent probes, especially in the NIR and NIR-II spectral regions, which optimize optical properties by addressing the limitations of traditional fluorophores through newly proposed chemical strategies. Compared to visible light, the NIR, especially the NIR-II region-based fluorescent probes, have become the mainstream of imaging emitters. They offer superior optical tissue penetration abilities, lower interference from biological autofluorescence, and higher in vivo imaging contrast, enabling more sensitive and accurate live detection. The development of novel fluorescent nanoparticles (NPs), such as carbon dots, quantum dots, polymer dots, AIE dots and up-conversion NPs, are also expanding the potential of NIR-II imaging. These nanoprobes are particularly promising due to their unique optical properties, including tunable emission wavelength, good stability and higher quantum yield, enhancing the capabilities of microscopy techniques for in situ fluorescence imaging.Integrating efficient sensing mechanisms such as FRET, ICT, PeT, ESIPT, and AIE with highly efficient fluorescence probes significantly improves the quality and functionality of imaging. FRET excels in studying biomolecular interactions within cells, making it ideal for detecting cellular changes linked to diseases. ICT's sensitivity to environmental changes allows for monitoring cellular conditions indicative of disease, although it may face interference in complex biological matrices. PeT is beneficial for targeted biomarker detection due to its specific analyte responsiveness, while ESIPT provides stable, high-contrast imaging useful in live-cell diagnostics, etc. The integration of these fluorescence mechanisms can enhance disease diagnostics by improving the visualization of biomarkers, offering a pathway to more sensitive, specific, and comprehensive disease detection and monitoring in vivo.
The clinical translational potential of these technologies has seen a significant increase, particularly after their groundbreaking application in liver cancer resection guided by surgical navigation in 2019. This marks a trend towards application-driven development for designing future NIR-II probes in super-resolution imaging, deep tissue imaging, or biomarker detection. However, the clinical translation of those newly reported fluorescence probes for imaging technologies is impeded by several challenges, including toxicity, stability, and biocompatibility. To mitigate toxicity, it is essential to develop probes using biologically inert compounds that are rapidly excreted and have enhanced probe brightness and targeting efficiency to reduce the required dosage. Stability issues, such as photobleaching and chemical degradation, can be addressed by chemically modifying the fluorophore structure for increased resistance to oxidative damage or by encapsulating fluorophores in protective delivery systems like micelles or liposomes. Improving biocompatibility involves modifying the probe surface with coatings such as polyethylene glycol (PEG) to reduce immune recognition and targeting specific cellular receptors to minimize non-target interactions. It is believed that by addressing these challenges through strategic research and development will facilitate the successful clinical adoption of fluorescence imaging probes in specific clinical scenarios.
An appealing prospect is the AI-driven optimization for fluorescent probes and microscopy techniques for automated, high-throughput, precise image analysis of disease biomarkers. Through the integration of machine learning and deep learning, AI offers significant advantages over traditional fluorescence probe design and imaging techniques. AI facilitates the design and optimization of fluorescence probes that are more efficient and precise in targeting specific biomarkers. These AI-driven technologies enhance imaging quality by refining image processing techniques, which improve clarity, reduce noise, and automate data analysis. This capability is crucial for accurately identifying subtle changes in biomarker expression that are indicative of disease states. As AI continues to evolve, it is set to become a central technology in biomedical research, providing powerful tools for real-time and personalized medical diagnostics through advanced fluorescence imaging.12,31
Looking to the future, the trend in fluorescence imaging and probe design for biomarker analysis is moving towards integrated AI systems that offer seamless automation from probe design to data interpretation. Despite the remarkable progress driven by AI in advancing the design of fluorescent molecules and the technology of fluorescence imaging, several issues must be addressed for future development. These include the reliance on high-quality, annotated datasets for training AI models, the need for interpretability and transparency of AI algorithms for trust and acceptance in clinical settings, and ethical considerations related to data privacy and potential biases in AI models.
In summary, the development of fluorescence bioimaging is anticipated to be significantly enhanced by the synergistic integration of advanced molecular designs, sophisticated fluorescence sensing mechanisms, and AI-driven innovative microscopy technologies. The ongoing advancements in this field are expected to inject new vitality into fluorescence imaging, further expanding its capabilities to drive more valuable innovation in the diagnosis, monitoring, and treatment of major diseases.
Data availability
Data sharing is not applicable here as no new data were created or analysed in this review article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22027807 and 22034004), the New Cornerstone Investigator Program, China Postdoctoral Science Foundation (2022TQ0171) and the Beijing Life Science Academy Initiative Scientific Research Program (No. 2023000CA0050).References
- F. Wang, Y. Zhong, O. Bruns, Y. Liang and H. Dai, In vivo NIR-II fluorescence imaging for biology and medicine, Nat. Photonics, 2024, 18, 535–547 Search PubMed.
- Y. Chen, S. Wang and F. Zhang, Near-infrared luminescence high-contrast in vivo biomedical imaging, Nat. Rev. Bioeng., 2023, 1, 60–78 Search PubMed.
- X. Wang, Q. Ding, R. R. Groleau, L. Wu, Y. Mao, F. Che, O. Kotova, E. M. Scanlan, S. E. Lewis, P. Li, B. Tang, T. D. James and T. Gunnlaugsson, Fluorescent probes for disease diagnosis, Chem. Rev., 2024, 124, 7106–7164 Search PubMed.
- H. Fang, Y. Chen, Z. Jiang, W. He and Z. Guo, Fluorescent probes for biological species and microenvironments: From rational design to bioimaging applications, Acc. Chem. Res., 2023, 56, 258–269 Search PubMed.
- W. T. Dou, H. H. Han, A. C. Sedgwick, G. B. Zhu, Y. Zang, X. R. Yang, J. Yoon, T. D. James, J. Li and X. P. He, Fluorescent probes for the detection of disease-associated biomarkers, Sci. Bull., 2022, 67, 853–878 Search PubMed.
- J. Chen, L. Chen, Y. Wu, Y. Fang, F. Zeng, S. Wu and Y. Zhao, A H2O2-activatable nanoprobe for diagnosing interstitial cystitis and liver ischemia-reperfusion injury via multispectral optoacoustic tomography and NIR-II fluorescent imaging, Nat. Commun., 2021, 12, 6870 Search PubMed.
- K. G. Fosnacht and M. D. Pluth, Activity-based fluorescent probes for hydrogen sulfide and related reactive sulfur species, Chem. Rev., 2024, 124, 4124–4257 Search PubMed.
- Q. Fu, X. Yang, M. Wang, K. Zhu, Y. Wang and J. Song, Activatable probes for ratiometric imaging of endogenous biomarkers in vivo, ACS Nano, 2024, 18, 3916–3968 Search PubMed.
- Y. Liu, D. Zhang, Y. Qu, F. Tang, H. Wang, A. Ding and L. Li, Advances in small-molecule fluorescent ph probes for monitoring mitophagy, Chem. Biomed. Imaging, 2023, 2, 81–97 Search PubMed.
- X. Tian, L. C. Murfin, L. Wu, S. E. Lewis and T. D. James, Fluorescent small organic probes for biosensing, Chem. Sci., 2021, 12, 3406–3426 Search PubMed.
- H. Xie, X. Han, G. Xiao, H. Xu, Y. Zhang, G. Zhang, Q. Li, J. He, D. Zhu, X. Yu and Q. Dai, Multifocal fluorescence video-rate imaging of centimetre-wide arbitrarily shaped brain surfaces at micrometric resolution, Nat. Biomed. Eng., 2024, 8, 740–753 Search PubMed.
- X. Tian, L. C. Murfin, L. Wu, S. E. Lewis and T. D. James, Fluorescent small organic probes for biosensing, Chem. Sci., 2021, 12, 3406–3426 Search PubMed.
- N. Gong, Y. Zhang, X. Teng, Y. Wang, S. Huo, G. Qing, Q. Ni, X. Li, J. Wang, X. Ye, T. Zhang, S. Chen, Y. Wang, J. Yu, P. C. Wang, Y. Gan, J. Zhang, M. J. Mitchell, J. Li and X. J. Liang, Proton-driven transformable nanovaccine for cancer immunotherapy, Nat. Nanotechnol., 2020, 15, 1053–1064 Search PubMed.
- G. Jiang, H. Liu, H. Liu, G. Ke, T. B. Ren, B. Xiong, X. B. Zhang and L. Yuan, Chemical approaches to optimize the properties of organic fluorophores for imaging and sensing, Angew. Chem., Int. Ed., 2024, 63, e202315217 Search PubMed.
- X. Ren, R. Deng, K. Zhang, Y. Sun, Y. Li and J. Li, Single-cell imaging of m6a modified RNA using m6a-specific in situ hybridization mediated proximity ligation assay (m6AISH-PLA), Angew. Chem., Int. Ed., 2021, 60, 22646–22651 Search PubMed.
- D. Cao, Z. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Lin, Coumarin-based small-molecule fluorescent chemosensors, Chem. Rev., 2019, 119, 10403–10519 Search PubMed.
- H. J. Chen, L. Wang, H. Zhu, Z. G. Wang and S. L. Liu, NIR-II fluorescence imaging for in vivo quantitative analysis, ACS Appl. Mater. Interfaces, 2024, 16, 28011–28028 Search PubMed.
- S. Samanta, K. Lai, F. Wu, Y. Liu, S. Cai, X. Yang, J. Qu and Z. Yang, Xanthene, cyanine, oxazine and bodipy: The four pillars of the fluorophore empire for super-resolution bioimaging, Chem. Soc. Rev., 2023, 52, 7197–7261 Search PubMed.
- C. Yang, Y. Yang, Y. Li, Q. Ni and J. Li, Radiotherapy-triggered proteolysis targeting chimera prodrug activation in tumors, J. Am. Chem. Soc., 2023, 145, 385–391 Search PubMed.
- G. Valenti, S. Scarabino, B. Goudeau, A. Lesch, M. Jović, E. Villani, M. Sentic, S. Rapino, S. Arbault, F. Paolucci and N. Sojic, Single cell electrochemiluminescence imaging: From the proof-of-concept to disposable device-based analysis, J. Am. Chem. Soc., 2017, 139, 16830–16837 Search PubMed.
- X. Dong, L. Sun, Z. Zhang, T. Zhu, J. Sun, J. Gao, C. Dong, R. Wang, X. Gu and C. Zhao, A dual-modality hydrogen sulfide-specific probe integrating chemiluminescence with nir fluorescence for targeted cancer imaging, Sci. China: Chem., 2023, 66, 1869–1876 Search PubMed.
- Y. Han, X. Ren, T. Wu, Y. L. Li, H. Ma, Z. Ru, Y. Jia, Z. F. Gao, Y. Du, D. Wu and Q. Wei, Effective enrichment of free radicals through nanoconfinement boosts electrochemiluminescence of carbon dots derived from luminol, Angew. Chem., Int. Ed., 2024, e202414073 Search PubMed.
- Y. Feng, N. Wang and H. Ju, Electrochemiluminescence biosensing and bioimaging with nanomaterials as emitters, Sci. China: Chem., 2022, 65, 2417–2436 Search PubMed.
- Y. Jia, X. Zhang, J. Fang, D. Jia, T. Tian, Y. Du, Q. Wei and F. Li, Analysis of okadaic acid using electrochemiluminescence imaging on microfluidic biosensing chip, Biosens. Bioelectron., 2024, 264, 116690 Search PubMed.
- Y. Zhou, Z. Mao and J.-J. Xu, Recent advances in near-infrared (NIR) electrochemiluminescence luminophores, Chin. Chem. Lett., 2024, 35, 109622 Search PubMed.
- F. Li, S. Liu, W. Liu, Z. Hou, J. Jiang, Z. Fu, S. Wang, Y. Si, S. Lu, H. Zhou, D. Liu, X. Tian, H. Qiu, Y. Yang, Z. Li, X. Li, L. Lin, H. Sun, H. Zhang and J. Li, 3D printing of inorganic nanomaterials by photochemically bonding colloidal nanocrystals, Science, 2023, 381, 1468–1474 Search PubMed.
- Z. Hu and B. Yan, Deep learning-assisted intelligent artificial vision platform based on dual-luminescence Eu(III)-functionalized hof for the diagnosis of breast and ovarian cancer, Anal. Chem., 2023, 95, 18889–18897 Search PubMed.
- W. Huang, S. Huang, Y. Fang, T. Zhu, F. Chu, Q. Liu, K. Yu, F. Chen, J. Dong and W. Zeng, AI-powered mining of highly customized and superior esipt-based fluorescent probes, Adv. Sci., 2024, 11, 2405596 Search PubMed.
- Y. Song, M. Lu, Y. Xie, G. Sun, J. Chen, H. Zhang, X. Liu, F. Zhang and L. Sun, Deep learning fluorescence imaging of visible to NIR-II based on modulated multimode emissions lanthanide nanocrystals, Adv. Funct. Mater., 2022, 32, 2206802 Search PubMed.
- Y.-F. Ou, T.-B. Ren, L. Yuan and X.-B. Zhang, Molecular design of NIR-II polymethine fluorophores for bioimaging and biosensing, Chem. Biomed. Imaging, 2023, 1, 220–233 Search PubMed.
- Y. Song, M. Lu, Y. Xie, G. Sun, J. Chen, H. Zhang, X. Liu, F. Zhang and L. Sun, Deep learning fluorescence imaging of visible to NIR-II based on modulated multimode emissions lanthanide nanocrystals, Adv. Funct. Mater., 2022, 32, 2206802 Search PubMed.
- Z. Lei and F. Zhang, Molecular engineering of NIR-II fluorophores for improved biomedical detection, Angew. Chem., Int. Ed., 2021, 60, 16294–16308 Search PubMed.
- Z. Qin, T. B. Ren, H. Zhou, X. Zhang, L. He, Z. Li, X. B. Zhang and L. Yuan, NIRII-HDs: A versatile platform for developing activatable NIR-II fluorogenic probes for reliable in vivo analyte sensing, Angew. Chem., Int. Ed., 2022, 61, e202201541 Search PubMed.
- Y. Tian, S. Liu, W. Cao, P. Wu, Z. Chen and H. Xiong, H2O2-activated NIR-II fluorescent probe with a large Stokes shift for high-contrast imaging in drug-induced liver injury mice, Anal. Chem., 2022, 94, 11321–11328 Search PubMed.
- S. Wang, X. Zhang, Y. Zhang, K. Zhu, X. Liu, J. Zhang, G. Wang, D. Liu, K. Zhao, X. Wang, J. Chen and J. Song, Activatable nanoprobes for dual-modal NIR-II photoacoustic and fluorescence imaging of hydrogen sulfide in colon cancer, Adv. Opt. Mater., 2023, 12(13), 2302796 Search PubMed.
- R. Riedl, Is trust in artificial intelligence systems related to user personality? Review of empirical evidence and future research directions, Electron. Markets, 2022, 32, 2021–2051 Search PubMed.
- Y. Li, Y. Su, M. Guo, X. Han, J. Liu, H. D. Vishwasrao, X. Li, R. Christensen, T. Sengupta, M. W. Moyle, I. Rey-Suarez, J. Chen, A. Upadhyaya, T. B. Usdin, D. A. Colon-Ramos, H. Liu, Y. Wu and H. Shroff, Incorporating the image formation process into deep learning improves network performance, Nat. Methods, 2022, 19, 1427–1437 Search PubMed.
- J. R. Lin, Y. A. Chen, D. Campton, J. Cooper, S. Coy, C. Yapp, J. B. Tefft, E. McCarty, K. L. Ligon, S. J. Rodig, S. Reese, T. George, S. Santagata and P. K. Sorger, High-plex immunofluorescence imaging and traditional histology of the same tissue section for discovering image-based biomarkers, Nat. Cancer, 2023, 4, 1036–1052 Search PubMed.
- J. Yang, M. Fan, Y. Sun, M. Zhang, Y. Xue, D. Zhang, T. Wang and X. Cui, A near-infrared fluorescent probe based on phosphorus-substituted rhodamine for deep imaging of endogenous hypochlorous acid in vivo, Sens. Actuators, B, 2020, 307, 127652 Search PubMed.
- J. Park, B. Bai, D. Ryu, T. Liu, C. Lee, Y. Luo, M. J. Lee, L. Huang, J. Shin, Y. Zhang, D. Ryu, Y. Li, G. Kim, H. S. Min, A. Ozcan and Y. Park, Artificial intelligence-enabled quantitative phase imaging methods for life sciences, Nat. Methods, 2023, 20, 1645–1660 Search PubMed.
- J. Kim, S. Rustam, J. M. Mosquera, S. H. Randell, R. Shaykhiev, A. F. Rendeiro and O. Elemento, Unsupervised discovery of tissue architecture in multiplexed imaging, Nat. Methods, 2022, 19, 1653–1661 Search PubMed.
- X. Li, Y. Li, Y. Zhou, J. Wu, Z. Zhao, J. Fan, F. Deng, Z. Wu, G. Xiao and J. He, Real-time denoising enables high-sensitivity fluorescence time-lapse imaging beyond the shot-noise limit, Nat. Biotechnol., 2023, 41, 282–292 Search PubMed.
- E. M. Christiansen, S. J. Yang, D. M. Ando, A. Javaherian, G. Skibinski, S. Lipnick, E. Mount, A. O’Neil, K. Shah and A. K. Lee, In silico labeling: Predicting fluorescent labels in unlabeled images, Cell, 2018, 173, 792–803 Search PubMed.
- S. Ye and Y. Bao, Recent advances in fluorescent polymers with color-tunable aggregate emission, Chem. Mater., 2024, 36, 5878–5896 Search PubMed.
- J. Yu, J. Xu, S. Ma, C. Wang, Q. Miao, L. Wang and G. Chen, Visible tracking of small molecules of gases with fluorescent donors, Chem. Biomed. Imaging, 2024, 2, 401–412 Search PubMed.
- Y. Zhou, J. Zhang, S. Sun, W. Chen, Y. Wang, H. Shi, R. Yang and Z. Qing, Amplified biosensors powered by endogenous molecules for intracellular fluorescence imaging, Anal. Chem., 2024, 96, 8078–8090 Search PubMed.
- Y. Dai, X. Teng and J. Li, Single-cell visualization of monogenic rna G-quadruplex and occupied G-quadruplex ratio through a module-assembled multifunctional probes assay (MAMPA), Angew. Chem., Int. Ed., 2022, 61, e202111132 Search PubMed.
- J. Ma, R. Sun, K. Xia, Q. Xia, Y. Liu and X. Zhang, Design and application of fluorescent probes to detect cellular physical microenvironments, Chem. Rev., 2024, 124, 1738–1861 Search PubMed.
- A. Martin and P. Rivera-Fuentes, A general strategy to develop fluorogenic polymethine dyes for bioimaging, Nat. Chem., 2024, 16, 28–35 Search PubMed.
- G. Feng, G. Q. Zhang and D. Ding, Design of superior phototheranostic agents guided by jablonski diagrams, Chem. Soc. Rev., 2020, 49, 8179–8234 Search PubMed.
- M. Kasha, Characterization of electronic transitions in complex molecules, Discuss. Faraday Soc., 1950, 9, 14–19 Search PubMed.
- Z. Wu, J. Nitsch, J. Schuster, A. Friedrich, K. Edkins, M. Loebnitz, F. Dinkelbach, V. Stepanenko, F. Wurthner, C. M. Marian, L. Ji and T. B. Marder, Persistent room temperature phosphorescence from triarylboranes: A combined experimental and theoretical study, Angew. Chem., Int. Ed., 2020, 59, 17137–17144 Search PubMed.
- S. Li, L. Fu, X. Xiao, H. Geng, Q. Liao, Y. Liao and H. Fu, Regulation of thermally activated delayed fluorescence to room-temperature phosphorescent emission channels by controlling the excited-states dynamics via j- and h-aggregation, Angew. Chem., Int. Ed., 2021, 60, 18059–18064 Search PubMed.
- Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, Evolution of group 14 rhodamines as platforms for near-infrared fluorescence probes utilizing photoinduced electron transfer, ACS Chem. Biol., 2011, 6, 600–608 Search PubMed.
- J. B. Grimm, B. P. English, J. Chen, J. P. Slaughter, Z. Zhang, A. Revyakin, R. Patel, J. J. Macklin, D. Normanno, R. H. Singer, T. Lionnet and L. D. Lavis, A general method to improve fluorophores for live-cell and single-molecule microscopy, Nat. Methods, 2015, 12, 244–250 Search PubMed.
- D. Liu, Z. He, Y. Zhao, Y. Yang, W. Shi, X. Li and H. Ma, Xanthene-based nir-ii dyes for in vivo dynamic imaging of blood circulation, J. Am. Chem. Soc., 2021, 143, 17136–17143 Search PubMed.
- M. S. Michie, R. Gotz, C. Franke, M. Bowler, N. Kumari, V. Magidson, M. Levitus, J. Loncarek, M. Sauer and M. J. Schnermann, Cyanine conformational restraint in the far-red range, J. Am. Chem. Soc., 2017, 139, 12406–12409 Search PubMed.
- D. H. Li, C. L. Schreiber and B. D. Smith, Sterically shielded heptamethine cyanine dyes for bioconjugation and high performance near-infrared fluorescence imaging, Angew. Chem., Int. Ed., 2020, 59, 12154–12161 Search PubMed.
- M. Sun, K. Mullen and M. Yin, Water-soluble perylenediimides: Design concepts and biological applications, Chem. Soc. Rev., 2016, 45, 1513–1528 Search PubMed.
- Q. Zheng and L. D. Lavis, Development of photostable fluorophores for molecular imaging, Curr. Opin. Chem. Biol., 2017, 39, 32–38 Search PubMed.
- R. R. Nani, J. A. Kelley, J. Ivanic and M. J. Schnermann, Reactive species involved in the regioselective photooxidation of heptamethine cyanines, Chem. Sci., 2015, 6, 6556–6563 Search PubMed.
- A. N. Butkevich, M. L. Bossi, G. Lukinavicius and S. W. Hell, Triarylmethane fluorophores resistant to oxidative photobluing, J. Am. Chem. Soc., 2019, 141, 981–989 Search PubMed.
- Y. Sun, K. Wang, X. Huang, S. Wei, E. Contreras, P. K. Jain, L. M. Campos, H. J. Kulik and J. S. Moore, Caged aiegens: Multicolor and white emission triggered by mechanical activation, J. Am. Chem. Soc., 2024, 146, 27117–27126 Search PubMed.
- C. F. Gomez-Duran, I. Garcia-Moreno, A. Costela, V. Martin, R. Sastre, J. Banuelos, F. Lopez Arbeloa, I. Lopez Arbeloa and E. Pena-Cabrera, 8-propargylaminobodipy: Unprecedented blue-emitting pyrromethene dye. Synthesis, photophysics and laser properties, Chem. Commun., 2010, 46, 5103–5105 Search PubMed.
- W. Chen, S. Xu, J. J. Day, D. Wang and M. Xian, A general strategy for development of near-infrared fluorescent probes for bioimaging, Angew. Chem., Int. Ed., 2017, 56, 16611–16615 Search PubMed.
- S. Sharma and K. S. Ghosh, Overview on recently reported fluorometric sensors for the detection of copper ion based on internal charge transfer (ICT), paramagnetic effect and aggregation induced emission (AIE) mechanisms, J. Mol. Struct., 2021, 1237, 130324 Search PubMed.
- T. Liu, T. Stephanc, P. Chene, J. K. Findeisenc, J. Chen, D. Riedel, Z. Yang, S. Jakobs and Z. Chen, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2215799119 Search PubMed.
- J. Wang, Q. Meng, Y. Yang, S. Zhong, R. Zhang, Y. Fang, Y. Gao and X. Cui, Schiff base aggregation-induced emission luminogens for sensing applications: A review, ACS Sens., 2022, 7, 2521–2536 Search PubMed.
- T. Wu, J. Huang and Y. Yan, Self-assembly of aggregation-induced-emission molecules, Chem. – Asian J., 2019, 14, 730–750 Search PubMed.
- G. Jiang, T.-B. Ren, E. D’Este, M. Xiong, B. Xiong, K. Johnsson, X.-B. Zhang, L. Wang and L. Yuan, A synergistic strategy to develop photostable and bright dyes with long Stokes shift for nanoscopy, Nat. Commun., 2022, 13, 2264 Search PubMed.
- J. L. Turnbull, B. R. Benlian, R. P. Golden and E. W. Miller, Phosphonofluoresceins: Synthesis, spectroscopy, and applications, J. Am. Chem. Soc., 2021, 143, 6194–6201 Search PubMed.
- L. Zhang, X. Wang, J. Zhao, B. Sun and W. Wang, Construction of targeting-peptide-based imaging reagents and their application in bioimaging, Chem. Biomed. Imaging, 2023, 2, 233–249 Search PubMed.
- C. Yang, Y. Li, Y. Yang, Q. Ni, Z. Zhang, Y. Chai and J. Li, Synthetic high-density lipoprotein-based nanomedicine to silence socs1 in tumor microenvironment and trigger antitumor immunity against glioma, Angew. Chem., Int. Ed., 2023, 62, e202312603 Search PubMed.
- G. Jiang, X. F. Lou, S. Zuo, X. Liu, T. B. Ren, L. Wang, X. B. Zhang and L. Yuan, Tuning the cellular uptake and retention of rhodamine dyes by molecular engineering for high-contrast imaging of cancer cells, Angew. Chem., Int. Ed., 2023, 62, e202218613 Search PubMed.
- L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X. P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents, Chem. Soc. Rev., 2020, 49, 5110–5139 Search PubMed.
- J. Miao, Y. Huo, X. Lv, Z. Li, H. Cao, H. Shi, Y. Shi and W. Guo, Fast-response and highly selective fluorescent probes for biological signaling molecule no based on n-nitrosation of electron-rich aromatic secondary amines, Biomaterials, 2016, 78, 11–19 Search PubMed.
- Y. Huo, J. Miao, J. Fang, H. Shi, J. Wang and W. Guo, Aromatic secondary amine-functionalized fluorescent no probes: Improved detection sensitivity for no and potential applications in cancer immunotherapy studies, Chem. Sci., 2019, 10, 145–152 Search PubMed.
- Z. R. Dai, G. B. Ge, L. Feng, J. Ning, L. H. Hu, Q. Jin, D. D. Wang, X. Lv, T. Y. Dou, J. N. Cui and L. Yang, A highly selective ratiometric two-photon fluorescent probe for human cytochrome P450 1A, J. Am. Chem. Soc., 2015, 137, 14488–14495 Search PubMed.
- J. Ning, W. Wang, G. Ge, P. Chu, F. Long, Y. Yang, Y. Peng, L. Feng, X. Ma and T. D. James, Target enzyme-activated two-photon fluorescent probes: A case study of CYP3A4 using a two-dimensional design strategy, Angew. Chem., Int. Ed., 2019, 58, 9959–9963 Search PubMed.
- D. Escudero, Revising intramolecular photoinduced electron transfer (PeT) from first-principles, Acc. Chem. Res., 2016, 49, 1816–1824 Search PubMed.
- P. Zhang, C. Fu, Q. Zhang, S. Li and C. Ding, Ratiometric fluorescent strategy for localizing alkaline phosphatase activity in mitochondria based on the esipt process, Anal. Chem., 2019, 91, 12377–12383 Search PubMed.
- W. Feng, S. Feng and G. Feng, A fluorescent esipt probe for imaging co-releasing molecule-3 in living systems, Anal. Chem., 2019, 91, 8602–8606 Search PubMed.
- J. E. Kwon and S. Y. Park, Advanced organic optoelectronic materials: Harnessing excited-state intramolecular proton transfer (ESIPT) process, Adv. Mater., 2011, 23, 3615–3642 Search PubMed.
- T. N. Francisco, D. Malafaia, L. Melo, A. M. S. Silva and H. M. T. Albuquerque, Recent advances in fluorescent theranostics for Alzheimer's disease: A comprehensive survey on design, synthesis, and properties, ACS Omega, 2024, 9, 13556–13591 Search PubMed.
- L. Gao, W. Wang, X. Wang, F. Yang, L. Xie, J. Shen, M. A. Brimble, Q. Xiao and S. Q. Yao, Fluorescent probes for bioimaging of potential biomarkers in Parkinson's disease, Chem. Soc. Rev., 2021, 50, 1219–1250 Search PubMed.
- A. K. Singh, A. V. Nair and N. D. P. Singh, Small two-photon organic fluorogenic probes: Sensing and bioimaging of cancer relevant biomarkers, Anal. Chem., 2022, 94, 177–192 Search PubMed.
- M. Sitara, W. Zhang, H. Gao, J. Li and J. Tian, Recent progress in molecular probes for imaging of acute kidney injury, Chem. Biomed. Imaging, 2024, 2, 526–541 Search PubMed.
- D. Su, W. Diao, J. Li, L. Pan, X. Zhang, X. Wu and W. Mao, Strategic design of amyloid-beta species fluorescent probes for Alzheimer's disease, ACS Chem. Neurosci., 2022, 13, 540–551 Search PubMed.
- C. Shi, Y. He, K. Hendriks, B. L. de Groot, X. Cai, C. Tian, A. Lange and H. Sun, A single nak channel conformation is not enough for non-selective ion conduction, Nat. Commun., 2018, 9, 717 Search PubMed.
- N. Zhou, F. Huo, Y. Yue and C. Yin, Specific fluorescent probe based on “protect-deprotect” to visualize the norepinephrine signaling pathway and drug intervention tracers, J. Am. Chem. Soc., 2020, 142, 17751–17755 Search PubMed.
- L. Li, P. Li, J. Fang, Q. Li, H. Xiao, H. Zhou and B. Tang, Simultaneous quantitation of Na+ and K+ in single normal and cancer cells using a new near-infrared fluorescent probe, Anal. Chem., 2015, 87, 6057–6063 Search PubMed.
- Z. Deng, P. Gao, H. Liu, Y. He, S. Zhong and Y. Yang, Cell-surface-anchored DNA sensors for simultaneously monitoring extracellular sodium and potassium levels, Anal. Chem., 2021, 93, 16432–16438 Search PubMed.
- J. Zou, K. Mitra, P. Anees, D. Oettinger, J. R. Ramirez, A. T. Veetil, P. D. Gupta, R. Rao, J. J. Smith, P. Kratsios and Y. Krishnan, A DNA nanodevice for mapping sodium at single-organelle resolution, Nat. Biotechnol., 2024, 42, 1075–1083 Search PubMed.
- V. Juvekar, M. K. Cho, H. W. Lee, D. J. Lee, H. Kang, J. M. Song, J. T. Je and H. M. Kim, A red-emissive two-photon fluorescent probe for mitochondrial sodium ions in live tissue, Chem. Commun., 2021, 57, 8929–8932 Search PubMed.
- J. Liu, F. Li, Y. Wang, L. Pan, P. Lin, B. Zhang, Y. Zheng, Y. Xu, H. Liao, G. Ko, F. Fei, C. Xu, Y. Du, K. Shin, D. Kim, S. S. Jang, H. J. Chung, H. Tian, Q. Wang, W. Guo, J. M. Nam, Z. Chen, T. Hyeon and D. Ling, A sensitive and specific nanosensor for monitoring extracellular potassium levels in the brain, Nat. Nanotechnol., 2020, 15, 321–330 Search PubMed.
- Z. Wang, T. C. Detomasi and C. J. Chang, A dual-fluorophore sensor approach for ratiometric fluorescence imaging of potassium in living cells, Chem. Sci., 2020, 12, 1720–1729 Search PubMed.
- H. Xu, C. Zhu, Y. Chen, Y. Bai, Z. Han, S. Yao, Y. Jiao, H. Yuan, W. He and Z. Guo, A fret-based fluorescent Zn2+ sensor: 3D ratiometric imaging, flow cytometric tracking and cisplatin-induced Zn2+ fluctuation monitoring, Chem. Sci., 2020, 11, 11037–11041 Search PubMed.
- T. Kambe, T. Tsuji, A. Hashimoto and N. Itsumura, The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism, Physiol. Rev., 2015, 95, 749–784 Search PubMed.
- J. Zhang, X. Peng, Y. Wu, H. Ren, J. Sun, S. Tong, T. Liu, Y. Zhao, S. Wang, C. Tang, L. Chen and Z. Chen, Red- and far-red-emitting zinc probes with minimal phototoxicity for multiplexed recording of orchestrated insulin secretion, Angew. Chem., Int. Ed., 2021, 60, 25846–25855 Search PubMed.
- T. Wu, M. Kumar, J. Zhang, S. Zhao, M. Drobizhev, M. McCollum, C. T. Anderson, Y. Wang, A. Pokorny, X. Tian and Y. Zhang, A genetically encoded far-red fluorescent indicator for imaging synaptically released Zn2+, Sci. Adv., 2023, 9, eadd2058 Search PubMed.
- X. Wang, G. Kim, J. L. Chu, T. Song, Z. Yang, W. Guo, X. Shao, M. L. Oelze, K. C. Li and Y. Lu, Noninvasive and spatiotemporal control of dnazyme-based imaging of metal ions in vivo using high-intensity focused ultrasound, J. Am. Chem. Soc., 2022, 144, 5812–5819 Search PubMed.
- R. M. Paredes, J. C. Etzler, L. T. Watts, W. Zheng and J. D. Lechleiter, Chemical calcium indicators, Methods, 2008, 46, 143–151 Search PubMed.
- R. Liu, D. Jiang, Y. Yun, Z. Feng, F. Zheng, Y. Xiang, H. Fan and J. Zhang, Photoactivatable engineering of CRISPR/Cas9-inducible dnazyme probe for in situ imaging of nuclear zinc ions, Angew. Chem., Int. Ed., 2024, 63, e202315536 Search PubMed.
- R. Roopa, N. Kumar, M. Kumar and V. Bhalla, Design and applications of small molecular probes for calcium detection, Chem. – Asian J., 2019, 14, 4493–4505 Search PubMed.
- J. Li, Z. Shang, J. H. Chen, W. Gu, L. Yao, X. Yang, X. Sun, L. Wang, T. Wang, S. Liu, J. Li, T. Hou, D. Xing, D. L. Gill, J. Li, S. Q. Wang, L. Hou, Y. Zhou, A. H. Tang, X. Zhang and Y. Wang, Engineering of nemo as calcium indicators with large dynamics and high sensitivity, Nat. Methods, 2023, 20, 918–924 Search PubMed.
- N. Mertes, M. Busch, M. C. Huppertz, C. N. Hacker, J. Wilhelm, C. M. Gurth, S. Kuhn, J. Hiblot, B. Koch and K. Johnsson, Fluorescent and bioluminescent calcium indicators with tuneable colors and affinities, J. Am. Chem. Soc., 2022, 144, 6928–6935 Search PubMed.
- G. D. Thiabaud, M. Schwalm, S. Sen, A. Barandov, J. Simon, P. Harvey, V. Spanoudaki, P. Muller, J. L. Sessler and A. Jasanoff, Texaphyrin-based calcium sensor for multimodal imaging, ACS Sens., 2023, 8, 3855–3861 Search PubMed.
- T. Tsang, C. I. Davis and D. C. Brady, Copper biology, Curr. Biol., 2021, 31, R421–R427 Search PubMed.
- P. A. Cobine and D. C. Brady, Cuproptosis: Cellular and molecular mechanisms underlying copper-induced cell death, Mol. Cell, 2022, 82, 1786–1787 Search PubMed.
- P. Tsvetkov, S. Coy, B. Petrova, M. Dreishpoon, A. Verma, M. Abdusamad, J. Rossen, L. J-Cohen, R. Humeidi, R. D. Spangler, J. K. Eaton, E. Frenkel, M. Kocak, S. M. Corsello, S. Lutsenko, N. Kanarek, S. Santagata and T. R. Golub, Copper induces cell death by targeting lipoylated TCA cycle proteins, Science, 2022, 375, 1254–1261 Search PubMed.
- J. Jiang, X. Wang, Y. Bao, F. Shen, G. Wang, K. Li and Y. Lin, Harnessing graphdiyne for selective Cu2+ detection: A promising tool for Parkinson's disease diagnostics and pathogenesis, ACS Sens., 2024, 9, 2317–2324 Search PubMed.
- J. Chen, R. Luo, S. Li, J. Shao, T. Wang, S. Xie, L. Xu, Q. You, S. Feng and G. Feng, A novel nir fluorescent probe for Cu2+ imaging in Parkinson's disease mouse brain, Chem. Sci., 2024, 15, 13082–13089 Search PubMed.
- X. M. Cai, S. Li, W. J. Wang, Y. Lin, W. Zhong, Y. Yang, F. E. Kuhn, Y. Li, Z. Zhao and B. Z. Tang, Natural acceptor of coumarin-isomerized red-emissive bioaiegen for monitoring Cu2+ concentration in live cells via flim, Adv. Sci., 2024, 11, e2307078 Search PubMed.
- R. Cheng, Y. Nishikawa, T. Wagatsuma, T. Kambe, Y. K. Tanaka, Y. Ogra, T. Tamura and I. Hamachi, Protein-labeling reagents selectively activated by Cu+, ACS Chem. Biol., 2024, 19, 1222–1228 Search PubMed.
- Y. Geng, Z. Wang, J. Zhou, M. Zhu, J. Liu and T. D. James, Recent progress in the development of fluorescent probes for imaging pathological oxidative stress, Chem. Soc. Rev., 2023, 52, 3873–3926 Search PubMed.
- K. Grover, A. Koblova, A. T. Pezacki, C. J. Chang and E. J. New, Small-molecule fluorescent probes for binding- and activity-based sensing of redox-active biological metals, Chem. Rev., 2024, 124, 5846–5929 Search PubMed.
- N. Gong, X. Ma, X. Ye, Q. Zhou, X. Chen, X. Tan, S. Yao, S. Huo, T. Zhang, S. Chen, X. Teng, X. Hu, J. Yu, Y. Gan, H. Jiang, J. Li and X. J. Liang, Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment, Nat. Nanotechnol., 2019, 14, 379–387 Search PubMed.
- L. Wu, A. C. Sedgwick, X. Sun, S. D. Bull, X. P. He and T. D. James, Reaction-based fluorescent probes for the detection and imaging of reactive oxygen, nitrogen, and sulfur species, Acc. Chem. Res., 2019, 52, 2582–2597 Search PubMed.
- X. Jiao, Y. Li, J. Niu, X. Xie, X. Wang and B. Tang, Small-molecule fluorescent probes for imaging and detection of reactive oxygen, nitrogen, and sulfur species in biological systems, Anal. Chem., 2018, 90, 533–555 Search PubMed.
- W. X. Wang, W. L. Jiang, G. J. Mao, M. Tan, J. Fei, Y. Li and C. Y. Li, Monitoring the fluctuation of hydrogen peroxide in diabetes and its complications with a novel near-infrared fluorescent probe, Anal. Chem., 2021, 93, 3301–3307 Search PubMed.
- X. Song, S. Bai, N. He, R. Wang, Y. Xing, C. Lv and F. Yu, Real-time evaluation of hydrogen peroxide injuries in pulmonary fibrosis mice models with a mitochondria-targeted near-infrared fluorescent probe, ACS Sens., 2021, 6, 1228–1239 Search PubMed.
- X. Jia, C. Wei, Z. Li, L. Liu, M. Wang, P. Zhang and X. Li, Selective imaging of HClO in the liver tissue in vivo using a near-infrared hepatocyte-specific fluorescent probe, Chem. – Asian J., 2021, 16, 1967–1972 Search PubMed.
- S. Li, P. Wang, K. Yang, Y. Liu, D. Cheng and L. He, Construction of HClO activated near-infrared fluorescent probe for imaging hepatocellular carcinoma, Anal. Chim. Acta, 2023, 1252, 341009 Search PubMed.
- S. Li, K. Yang, P. Wang, Y. Liu, D. Cheng and L. He, Monitoring heat stroke with a HClO-activatable near-infrared fluorescent probe, Sens. Actuators, B, 2023, 385, 133696 Search PubMed.
- X. Liu, J. Zhu, Q. Zhang, H. Hu, W. Zhang, H. Xu, Y. Huang, J. Xie, H. Liu, Y. Feng, J. Li and C. Jia, Multifunctional fluorescent probe for simultaneous revealing Cys and ONOO− dynamic correlation in the ferroptosis, Spectrochim. Acta, Part A, 2024, 315, 124248 Search PubMed.
- J. S. Hu, C. Shao, X. Wang, X. Di, X. Xue, Z. Su, J. Zhao, H. L. Zhu, H. K. Liu and Y. Qian, Imaging dynamic peroxynitrite fluxes in epileptic brains with a near-infrared fluorescent probe, Adv. Sci., 2019, 6, 1900341 Search PubMed.
- X. Li, R. R. Tao, L. J. Hong, J. Cheng, Q. Jiang, Y. M. Lu, M. H. Liao, W. F. Ye, N. N. Lu, F. Han, Y. Z. Hu and Y. H. Hu, Visualizing peroxynitrite fluxes in endothelial cells reveals the dynamic progression of brain vascular injury, J. Am. Chem. Soc., 2015, 137, 12296–12303 Search PubMed.
- Q. Sun, J. Xu, C. Ji, M. S. S. Shaibani, Z. Li, K. Lim, C. Zhang, L. Li and Z. Liu, Ultrafast detection of peroxynitrite in Parkinson's disease models using a near-infrared fluorescent probe, Anal. Chem., 2020, 92, 4038–4045 Search PubMed.
- X. Luo, Z. Cheng, R. Wang and F. Yu, Indication of dynamic peroxynitrite fluctuations in the rat epilepsy model with a near-infrared two-photon fluorescent probe, Anal. Chem., 2021, 93, 2490–2499 Search PubMed.
- J. Miao, Y. Huo, Q. Liu, Z. Li, H. Shi, Y. Shi and W. Guo, A new class of fast-response and highly selective fluorescent probes for visualizing peroxynitrite in live cells, subcellular organelles, and kidney tissue of diabetic rats, Biomaterials, 2016, 107, 33–43 Search PubMed.
- J. Miao, Y. Huo, H. Shi, J. Fang, J. Wang and W. Guo, A Si-rhodamine-based near-infrared fluorescent probe for visualizing endogenous peroxynitrite in living cells, tissues, and animals, J. Mater. Chem. B, 2018, 6, 4466–4473 Search PubMed.
- X. Zhang, Y. Chen, H. He, S. Wang, Z. Lei and F. Zhang, Ros/rns and base dual activatable merocyanine-based NIR-II fluorescent molecular probe for in vivo biosensing, Angew. Chem., Int. Ed., 2021, 60, 26337–26341 Search PubMed.
- L. Wu, J. Liu, X. Tian, R. R. Groleau, S. D. Bull, P. Li, B. Tang and T. D. James, Fluorescent probe for the imaging of superoxide and peroxynitrite during drug-induced liver injury, Chem. Sci., 2021, 12, 3921–3928 Search PubMed.
- L. C. Murfin, M. Weber, S. J. Park, W. T. Kim, C. M. Lopez-Alled, C. L. McMullin, F. Pradaux-Caggiano, C. L. Lyall, G. Kociok-Kohn, J. Wenk, S. D. Bull, J. Yoon, H. M. Kim, T. D. James and S. E. Lewis, Azulene-derived fluorescent probe for bioimaging: Detection of reactive oxygen and nitrogen species by two-photon microscopy, J. Am. Chem. Soc., 2019, 141, 19389–19396 Search PubMed.
- H. Liu, W. Chen, W. Yuan, J. Gao, Q. Zhang, P. Zhang and C. Ding, A NIR pH sensitive fluorescent strategy for ratiometric detection of reactive oxygen species and its application in the imaging of arthritis, Sens. Actuators, B, 2023, 379, 133262 Search PubMed.
- X. Ni, S. S. Kelly, S. Xu and M. Xian, The path to controlled delivery of reactive sulfur species, Acc. Chem. Res., 2021, 54, 3968–3978 Search PubMed.
- A. Doman, E. Doka, D. Garai, V. Bogdandi, G. Balla, J. Balla and P. Nagy, Interactions of reactive sulfur species with metalloproteins, Redox Biol., 2023, 60, 102617 Search PubMed.
- Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria, Angew. Chem., Int. Ed., 2013, 52, 1688–1691 Search PubMed.
- Z. Chen, Y. Yang, Y. Tian, J. Yang and H. Xiong, Diagnosis of nonalcoholic fatty liver disease via a H2S-responsive bioluminescent probe combined with firefly luciferase mRNA delivery, Anal. Chem., 2024, 96, 9236–9243 Search PubMed.
- Z. Zheng, J. Gao, R. Wang, C. Dong, X. Dong, J. Sun, L. Sun, X. Gu and C. Zhao, Molecular engineering of luminogens for high-integrity imaging of hydrogen polysulfides via activatable aggregation-induced dual-color fluorescence, ACS Nano, 2023, 17, 22060–22070 Search PubMed.
- Y. Yang, T. Zhou, M. Jin, K. Zhou, D. Liu, X. Li, F. Huo, W. Li and C. Yin, Thiol-chromene “click” reaction triggered self-immolative for nir visualization of thiol flux in physiology and pathology of living cells and mice, J. Am. Chem. Soc., 2020, 142, 1614–1620 Search PubMed.
- Y. Zhang, X. Wang, X. Bai, P. Li, D. Su, W. Zhang, W. Zhang and B. Tang, Highly specific cys fluorescence probe for living mouse brain imaging via evading reaction with other biothiols, Anal. Chem., 2019, 91, 8591–8594 Search PubMed.
- X. Zhang, Y. Huang, X. Han, Y. Wang, L. Zhang and L. Chen, Evaluating the protective effects of mitochondrial glutathione on cerebral ischemia/reperfusion injury via near-infrared fluorescence imaging, Anal. Chem., 2019, 91, 14728–14736 Search PubMed.
- L. Yang, Y. Zhang, X. Ren, B. Wang, Z. Yang, X. Song and W. Wang, Fluorescent detection of dynamic H2O2/H2S redox event in living cells and organisms, Anal. Chem., 2020, 92, 4387–4394 Search PubMed.
- M. Gao, X. Zhang, Y. Wang, Q. Liu, F. Yu, Y. Huang, C. Ding and L. Chen, Sequential detection of superoxide anion and hydrogen polysulfides under hypoxic stress via a spectral-response-separated fluorescent probe functioned with a nitrobenzene derivative, Anal. Chem., 2019, 91, 7774–7781 Search PubMed.
- H. Li, D. Kim, Q. Yao, H. Ge, J. Chung, J. Fan, J. Wang, X. Peng and J. Yoon, Activity-based NIR enzyme fluorescent probes for the diagnosis of tumors and image-guided surgery, Angew. Chem., Int. Ed., 2021, 60, 17268–17289 Search PubMed.
- Q. Liu, J. Yuan, R. Jiang, L. He, X. Yang, L. Yuan and D. Cheng, γ-glutamyltransferase-activatable fluoro-photoacoustic reporter for highly sensitive diagnosis of acute liver injury and tumor, Anal. Chem., 2023, 95(3), 2062–2070 Search PubMed.
- H. Shen, L. Du, C. Xu, B. Wang, Q. Zhou, R. Ye, R. T. K. Kwok, J. W. Y. Lam, G. Xing, J. Sun, T. M. Liu and B. Z. Tang, A near-infrared-II excitable pyridinium probe with 1000-fold on/off ratio for γ-glutamyltranspeptidase and cancer detection, ACS Nano, 2024, 18(31), 20268–20282 Search PubMed.
- K. Wang, X. Y. Chen, R. W. Zhang, Y. Yue, X. L. Wen, Y. S. Yang, C. Y. Han, Y. Ma, H. J. Liu and H. L. Zhu, Multifunctional fluorescence/photoacoustic bimodal imaging of γ-glutamyltranspeptidase in liver disorders under different triggering conditions, Biomaterials, 2024, 310, 122635 Search PubMed.
- H. Wang, X. Zhang, P. Li, F. Huang, T. Xiu, H. Wang, W. Zhang, W. Zhang and B. Tang, Prediction of early atherosclerotic plaques using a sequence-activated fluorescence probe for the simultaneous detection of γ-glutamyl transpeptidase and hypobromous acid, Angew. Chem., Int. Ed., 2024, 63, e202315861 Search PubMed.
- M. Miao, J. Miao, Y. Zhang, J. Zhang, M. She, M. Zhao, Q. Miao, L. Yang, K. Zhou and Q. Li, An activatable near-infrared molecular reporter for fluoro-photoacoustic imaging of liver fibrosis, Biosens. Bioelectron., 2023, 235, 115399 Search PubMed.
- J. Zhang, P. Cheng and K. Pu, Recent advances of molecular optical probes in imaging of β-galactosidase, Bioconjugate Chem., 2019, 30, 2089–2101 Search PubMed.
- Y. Gao, Y. Hu, Q. Liu, X. Li, X. Li, C. Y. Kim, T. D. James, J. Li, X. Chen and Y. Guo, Two-dimensional design strategy to construct smart fluorescent probes for the precise tracking of senescence, Angew. Chem., Int. Ed., 2021, 60, 10756–10765 Search PubMed.
- L. Xu, H. Gao, Y. Deng, X. Liu, W. Zhan, X. Sun, J. J. Xu and G. Liang, β-galactosidase-activated near-infrared AIEgen for ovarian cancer imaging in vivo, Biosens. Bioelectron., 2024, 255, 116207 Search PubMed.
- X. Chai, H. H. Han, A. C. Sedgwick, N. Li, Y. Zang, T. D. James, J. Zhang, X. L. Hu, Y. Yu, Y. Li, Y. Wang, J. Li, X. P. He and H. Tian, Photochromic fluorescent probe strategy for the super-resolution imaging of biologically important biomarkers, J. Am. Chem. Soc., 2020, 142, 18005–18013 Search PubMed.
- P. Pimviriyakul, Y. Kapaothong and T. Tangsupatawat, Heterologous expression and characterization of a full-length protozoan nitroreductase from leishmania orientalis isolate pcm2, Mol. Biotechnol., 2023, 65, 556–569 Search PubMed.
- J. Sun, Z. Hu, R. Wang, S. Zhang and X. Zhang, A highly sensitive chemiluminescent probe for detecting nitroreductase and imaging in living animals, Anal. Chem., 2019, 91, 1384–1390 Search PubMed.
- S. Zhang, M. Ma, C. Zhao, J. Li, L. Xu, Z. Zhang, Q. Diao, P. Ma and D. Song, A novel low-background nitroreductase fluorescent probe for real-time fluorescence imaging and surgical guidance of thyroid cancer resection, Biosens. Bioelectron., 2024, 261, 116514 Search PubMed.
- Z. Zheng, X. Chen, R. Dai, S. Wu, W. Kang, Y. Qin, S. Ren, R. Zhang and Z. Cheng, Cyclic enhancement of hypoxic microenvironment via an intelligent nanoamplifier for activated NIR-II fluorescence/photoacoustic imaging-guided precise synergistic therapy, Mater. Today Bio, 2022, 17, 100478 Search PubMed.
- J. Xiang, R. Zou, P. Wang, X. Wang, X. He, F. Liu, C. Xu and A. Wu, Nitroreductase-responsive nanoparticles for in situ fluorescence imaging and synergistic antibacterial therapy of bacterial keratitis, Biomaterials, 2024, 308, 122565 Search PubMed.
- M. Li, B. Gurram, S. Lei, N. T. Blum, P. Huang and J. Lin, Recent advances in fluorescence imaging of alkaline phosphatase, Chin. Chem. Lett., 2021, 32, 1316–1330 Search PubMed.
- X. Gao, G. Ma, C. Jiang, L. Zeng, S. Jiang, P. Huang and J. Lin, In vivo near-infrared fluorescence and photoacoustic dual-modal imaging of endogenous alkaline phosphatase, Anal. Chem., 2019, 91, 7112–7117 Search PubMed.
- L. H. Xiong, L. Yang, J. Geng, B. Z. Tang and X. He, All-in-one alkaline phosphatase-response aggregation-induced emission probe for cancer discriminative imaging and combinational chemodynamic-photodynamic therapy, ACS Nano, 2024, 18, 17837–17851 Search PubMed.
- Y. Wang, Y. Zhang, M. Li, X. Gao and D. Su, An efficient strategy for constructing fluorescent nanoprobes for prolonged and accurate tumor imaging, Anal. Chem., 2024, 96, 2481–2490 Search PubMed.
- V. A. Baulin, Y. Usson and X. Le Guevel, Deep learning: Step forward to high-resolution in vivo shortwave infrared imaging, J. Biophotonics, 2021, 14, e202100102 Search PubMed.
- Y. Qian, O. T. Celiker, Z. Wang, B. Guner-Ataman and E. S. Boyden, Temporally multiplexed imaging of dynamic signaling networks in living cells, Cell, 2023, 186, 5656–5672 Search PubMed.
- V. Bhat, P. Sornberger, B. S. S. Pokuri, R. Duke, B. Ganapathysubramanian and C. Risko, Electronic, redox, and optical property prediction of organic π-conjugated molecules through a hierarchy of machine learning approaches, Chem. Sci., 2022, 14, 203–213 Search PubMed.
- M. Sumita, K. Terayama, N. Suzuki, S. Ishihara and K. Tsuda, De novo creation of a naked-eye-detectable fluorescent molecule based on quantum-chemical computation and machine learning, Sci. Adv., 2022, 8, eabj3906 Search PubMed.
- F. F. Xiang, H. Zhang, Y. L. Wu, Y. J. Chen, Y. Z. Liu, S. Y. Chen, Y. Z. Guo, X. Q. Yu and K. Li, Machine-learning-assisted rational design of Si horizontal line rhodamine as cathepsin-ph-activated probe for accurate fluorescence navigation, Adv. Mater., 2024, 36, e2404828 Search PubMed.
- Q. Hong, X. Y. Wang and Y. T. Gao, Customized carbon dots with predictable optical properties synthesized at room temperature guided by machine learning, Chem. Mater., 2022, 34 Search PubMed.
- R. Chen, S. Peng and L. Z. M. F. F. Z. Qian, Enhancing total optical throughput of microscopy with deep learning for intravital observation, Small Methods, 2023, 7, 2300172 Search PubMed.
- Z. Ma, F. Wang, W. Wang, Y. Zhong and H. Dai, Deep learning for in vivo near-infrared imaging, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2021446118 Search PubMed.
- D. Shterman, B. Gjonaj and G. Bartal, Experimental demonstration of multi moiré structured illumination microscopy, ACS Photonics, 2018, 5, 1898–1902 Search PubMed.
- E. N. Ward, L. Hecker, C. N. Christensen, J. R. Lamb, M. Lu, L. Mascheroni, C. W. Chung, A. Wang, C. J. Rowlands, G. S. K. Schierle and C. F. Kaminski, Machine learning assisted interferometric structured illumination microscopy for dynamic biological imaging, Nat. Commun., 2022, 13, 7836 Search PubMed.
- Y. Feng, X. Yang, Q. Rao, L. Zhang, Y. Su and Y. Lv, Persistent luminescence lifetime-based near-infrared nanoplatform via deep learning for high-fidelity biosensing of hypochlorite, Anal. Chem., 2024, 96, 7240–7247 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
