Effect of reducing catalyst coke by La loading in hydrocracking of Jatropha oil
Kai Fana,
Xiaoyi Yangb,
Jing Liuc and
Long Rong*a
aKey Laboratory for Biomechanics and Mechanobiology of Ministry of Education, School of Biological Science and Medical Engineering, Beihang University, Beijing 100191, P. R. China. E-mail: ronglong@buaa.edu.cn; Fax: +86 10 8233 9157; Tel: +86 10 8233 9157
bEnergy and Environment International Center, Beihang University, Beijing 100191, P. R. China
cBeijing Key Laboratory of Lignocellulosic Chemistry, Beijing Forestry University, Beijing 100083, P. R. China
First published on 1st April 2015
Abstract
In order to assess the La effect on reducing carbonaceous deposition (coke) on catalysts for the hydrocracking of Jatropha oil, NiW/nHA, NiW/Al2O3 and NiW/HY catalysts modified with loaded La were synthesized and studied. The catalysts were characterized by N2 adsorption–desorption, powder X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), temperature-programmed desorption of ammonia (NH3-TPD), temperature programmed desorption of hydrogen (TPD-Hads), and temperature-programmed desorption of carbon dioxide (CO2-TPD). The species and the amount of coke on the hydrocracking catalyst were measured by Fourier transform infrared (FT-IR) spectroscopy, solid state 13C nuclear magnetic resonance (NMR) and thermogravimetric analysis (TGA), demonstrating that the coke was constituted of polyaromatic hydrocarbons and that the La catalyst loading had a very positive effect on coke reduction. The amount of coke on NiW/nHA, NiW/Al2O3 and NiW/HY catalysts decreased respectively by 0.94%, 1.19%, 1.91% after 8 h and 1.66%, 1.89%, 3.78% after 180 h. The catalytic stability and the catalyst lifetime were also improved by La loading.
Introduction
In the past decades, the increasing world energy crisis and the growing concerns on environmental protection have made renewable biofuels an important alternative to fossil fuels.1,2 There are many benefits of biofuels, such as high quality, decreased greenhouse gas emissions, decreased dependence on fossil fuels and increased national energy security. Hydrocracking of vegetable oil, which transforms triglyceride into hydrocarbons, with hydrogen at elevated temperature and pressure in the presence of heterogeneous catalysts is one of the processes widely used and studied for the production of new biofuels.3,4The common industrial catalysts for hydrocracking production involve an alumina support and bifunctional catalyst (HY, HZSM, SAPO) loaded with a sulfurated transition metal (Ni, Mo and W).5–7 During the hydrocracking process, the produced carbonaceous deposition (coke) deposits on the catalyst. Although much research has focused on improving the process of hydrocracking, little work has been concerned with the formation of coke. As the amount of coke increases, it will cover the active center and block the catalyst pores, deactivating the catalyst over long term operation. For practical applications, it is necessary to study in detail the mechanism of reducing coke formation.
In our previous work, the rare earth element La was used for getting rid of sulfur.8 Finally, the effects of La loading not only lead to desulfuration, but also reduced the coke formation on catalyst. To further investigate this effect of reducing coke, traditional catalysts including Al2O3 and HY should be tested in La loading. Besides, we have also successfully synthesized the nano-hydroxyapatite (nHA) for hydrocracking,9 this support should be tested to see whether it has the same effect. Thus, in this work, La was loaded into NiW/Al2O3, NiW/nHA and NiW/HY catalysts for hydrocracking of Jatropha oil, the coke species will be investigated and the effect of reducing coke by La loading will also be discussed.
Experimental
Catalyst preparation and characterization
The Al2O3 and nHA support were prepared by a precipitation method.9,10 All the chemical reagents used in this work were analytic reagent and purchased from Sinopharm Chemical Reagent Co.,Ltd, China.The HY support was purchased from Catalyst Plant of Nankai University. The Ni, W, La (Ni 5.6%, W 6.4%, La 5%) component were loaded by impregnation of aqueous solutions of Ni(NO3)2·6H2O, (NH4)6W7O24·6H2O and La(NO3)3·6H2O. Impregnated samples were dried at 100 °C over night and calcined at 400 °C for 6 h.
To determine specific surface areas, the N2 adsorption–desorption were measured using a V-Sorb 2800 TP Surface Area and Pore Distribution Analyzer instrument (BeiJing Gold APP Instruments Co., Ltd). At first, the samples were degassed in a vacuum at 300 °C for 4 h, then the specific surface area was obtained by the Brunauer, Emmett and Teller (BET) procedure. The t-plot method and Barret–Joyner–Halenda (BJH) method were used to determine the area of the micropores and pore size distribution, respectively.
The X-ray diffraction (XRD) patterns used Cu-Kα radiation at 40 kV and 30 mA, recording on a D/max2500VB2+/PC XRD analyzer (Japan Electronics Science Co., Ltd.). The samples were measured at a scan speed of 2° min−1, in the 2θ range from 10° to 80°.
X-ray photoelectron spectroscopy (XPS) measurements were carried out at 280 eV pass energy, using a ESCALAB 250Xi instrument (Thermofisher). Binding energies were corrected for sample charging, using the C 1s peak at 284.6 eV for adventitious carbon as a reference. The peak areas of the samples were determined by measuring the Ni 2p and W 4f peak areas (after linear subtraction of the background).
The temperature-programmed desorption of ammonia (NH3-TPD) were using a TP-5080 Multi-functional automatic Adsorption Instrument (Tianjin Golden Eagle Technology Co., Ltd) to determine the acidities of the catalysts. Before started, all samples were pretreated in N2 (25 mL min−1) at 300 °C for 2 h. The desorption step was performed at a heating rate of 10 °C min−1 from 100 °C to 700 °C.
Temperature programmed desorption of hydrogen (TPD-Hads) experiments were performed using a TX 200 equipment from Tianjin Golden Eagle Technology Co., Ltd., a mixed stream of H2 (20 mL min−1) and N2 (38 mL min−1) was used for 100 mg of catalyst samples. Adsorption of hydrogen was carried out initially at constant temperature of 200 °C for 0.25 h, then gradually decreasing temperature from the initial value to 20 °C, finally at constant temperature of 20 °C for 1 h. After that, the examined sample was flushed with a N2 stream (38 mL min−1, 20 °C, 0.25 h) to remove adsorbed hydrogen by TPD method. The TPD-Hads examination was carried out in an N2 stream of 38 mL min−1 at linearly increasing temperature of 10 °C min−1 from 20 to 900 °C.
The CO2-TPD measurement was used for surface basicity testing of catalyst samples. All samples (100 mg) were first treated in He at 400 °C for 1 h, cooled to 50 °C. And then exposed to CO2 (10 mL min−1) for 0.5 h, purged in He for 2 h at 50 °C and heated linearly at 10 °C min−1 to 800 °C in 30 mL min−1 He. CO2 (m/z = 44) in effluent was recorded continuously as functions of temperature.
The thermogravimetric analysis (TGA) were performed on a NETZSCH STA449F3 analyzer to determine the amount of coke on the past-reacted catalysts. Samples were first heated from 30 °C to 550 °C with a heating rate of 30 °C min−1 in N2 flow of 100 mL min−1, at a constant temperature of 550 °C for 15 min, then heated linearly at 30 °C min−1 to 800 °C in 100 mL min−1 O2 flow. The weight loss of samples was calculated by microcomputer.
Chemical analysis of the fresh and used catalyst support were carried out by a Fourier transform infrared (FT-IR) spectrophotometer (GANGDONG FTIR-650) in the range from 4000 cm−1 to 400 cm−1 at 1.5 cm−1 resolution averaging 32 scans.
The solid-state 13C NMR experiments were performed on Bruker AVANCE III 600 spectrometer at a resonance frequency of 150.9 MHz to analysis the component of coke on the catalyst. The 13C NMR spectra were recorded using a 4 mm MAS probe and a spinning rate of 14 kHz. A contact time of 3 ms, a recycle delay of 5 s, and 4000 accumulations were used for the 13C measurement. The chemical shifts of 13C was externally referenced to TMS.
Catalytic activity measurements
Jatropha oil was purchased from Jiangsu Donghu Bioenergy Co., Ltd, the content includes: myristic acid 0.1%, arachic acid 0.5%, linolenic acid 0.9%, palmitoleic acid 1.2%, stearic acid 7.3%, palmitic acid 14.8%, linoleic acid 36.2%, oleic acid 38.3%.The experiments were performed in a fixed-bed reactor equipped with electrically heating system (JF-2, Tianjing Golden Eagle Technology Co., Ltd, China). The equipment for continuous hydrotreatment included feed system, heating section, tubular reactor, condensation section, storage section, instrumentation and control section. The reaction temperature was controlled by microcomputer while the system pressure was maintained by a back-pressure regulator. Jatropha oil was delivered from a feed pot into the reactor by a high-pressure pump. The hydrogen input rate was controlled by a separate mass-flow meter.
The catalyst samples (10 g) were loaded respectively into the tubular reactor and activated prior to the experiments with H2 flow at 400 °C and 3 MPa for 3 h. The reaction conditions for catalytic hydrocracking experiment were as follows: temperature 360 °C, pressure 3 MPa, LHSV 2 h−1, and H2 to feed ratio of 600 mL H2 gas/mL liquid feed.
After 8 h of stabilization of reaction conditions, the product oil was analyzed by a gas chromatograph equipped with a flame-ionization detector (FID). The capillary column (AT.SE-30, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences) dimensions were 0.32 mm i.d. × 30 m with a film thickness of 0.5 μm. The normal paraffin standards (purchased from Sigma-Aldrich, LLC) were used to estimate the relative percentages and distributions of the products with respect to their carbon numbers. The conversion of Jatropha oil was calculated as:
| C = 100% − C(JO) | (1) |
Results and discussion
Characterization of catalyst
The BET data of different catalysts were presented in Table 1. The nHA exhibited larger total pore volume (0.51 cm3 g−1) and average pore diameter (22.3 nm), the HY had larger specific surface areas (452 m2 g−1). The La loading amount (5%) was based on our previous work. After impregnation, all samples' specific surface areas and total pore volume decreased while the average pore diameter increased, suggesting some pores of catalysts were blocked by the metal La.| Catalyst | Specific surface areas, m2 g−1 | Total pore volume, cm3 g−1 | Average pore diameter, nm |
|---|---|---|---|
| nHA | 161 | 0.51 | 22.3 |
| NiWLa/nHA | 114 | 0.43 | 23.1 |
| Al2O3 | 378 | 0.54 | 5.6 |
| NiWLa/Al2O3 | 325 | 0.49 | 6.4 |
| HY | 452 | 0.42 | 0.8 |
| NiWLa/HY | 397 | 0.38 | 0.9 |
The XRD patterns of different catalysts were shown in Fig. 1. All samples exhibited their own standard XRD spectrum of hydroxyapatite (JCPDS no. 9-0432), γAl2O3 (JCPDS no. 10-0425) and zeolite Y (JCPDS no. 81-2467), respectively.11–13 After impregnation, the main crystal structure of catalysts remained, suggesting the frameworks of catalysts were undamaged. It was obvious that after impregnation of Ni and W, the diffraction peaks of Ni oxide phase (2θ = 37.3°, 43.6° and 63.4°, JCPDS no. 4-835) and WO3 (2θ = 55.9°, JCPDS no. 43-1035) appeared at the figure,14,15 demonstrating the metal was successfully loaded. However, after impregnation of La, the La2O3 phase was considered to disperse highly on the surface, which is not detected by XRD. Meanwhile the peaks of NiO and WO3 became weaker after La loading, indicating highly dispersion of Ni and W, this was in agreement with the reported literature by Bouarab et al.16 Moreover, as the literature reported, the coke was supposed to occur more easily on larger metal particles than on smaller ones.17 Therefore, the high dispersion of Ni, W could contribute to the resistance of coke.
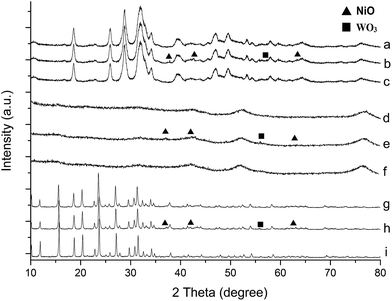 | ||
| Fig. 1 XRD patterns of (a) nHA (b) NiW/nHA (c) NiWLa/nHA (d) Al2O3 (e) NiW/Al2O3 (f) NiWLa/Al2O3 (g) HY (h) NiW/HY and (i) NiWLa/HY. | ||
To investigate the variation of Ni and W oxidation state caused by La, the NiW and NiWLa were examined using XPS. The Ni 2p core level signal of NiW was shown in Fig. 2(a), the binding energy values at 851.2 and 852.6 eV were associated with the Ni0 of NiW and NiWLa respectively,18 other peaks were associated with the Ni2+. The W 4f core level signal was shown in Fig. 2(b), the peaks of low binding energy value at 36.4 eV and 36.6 eV were attributed to Wx+ (Wx+ represents nonstoichiometric WOx/W) of NiW and NiWLa respectively, other peaks at high energy values were attributed to W6+.19
As calculated from Table 2, the content of the Ni0 significantly increased after La loading. The ratio of Ni0/Ni2+ was increased from 11.6% to 48.4%, while that of Wx+/W6+ was decreased from 55.5% to 43.9%. These demonstrated the La loading could promote the reduction of Ni2+ and the oxidation of Wx+, these also implied the W species donate partial electrons to Ni oxide species. Therefore, the reduction state of Ni increased, the hydrogenation capacity of catalysts were enhanced.20,21
| Catalyst | Ni (%) | W (%) | ||||
|---|---|---|---|---|---|---|
| Ni0 | Ni2+ | Ni0/Ni2+ | Wx+ | W6+ | W6+/Wx+ | |
| NiW | 10.4 | 89.6 | 11.6 | 69.5 | 30.5 | 43.9 |
| NiWLa | 32.6 | 67.4 | 48.4 | 64.3 | 35.7 | 55.5 |
The NH3-TPD profiles of different catalyst samples were shown in Fig. 3, the total acidities of them were recorded in Table 3. It can be concluded that before La loading, the acidities of catalysts followed the order: NiW/nHA < NiW/Al2O3 < NiW/HY. The nHA and Al2O3 exhibited mild acidity while HY had strong acidity. After La loading, all the acidities of catalysts decreased. It was probably due to some of the La cations replaced the framework cation (Al3+ and Ca2+) to balance the negative framework charge by ion exchange, leading the original acidity of catalyst partly disappeared, this results were in accord with the reported literature.22,23
 | ||
| Fig. 3 NH3-TPD profiles of (a) NiW/nHA and NiWLa/nHA (b) NiW/Al2O3 and NiWLa/Al2O3 (c) NiW/HY and NiWLa/HY. | ||
| Catalyst | Total acidity (mmol g−1) |
|---|---|
| NiW/nHA | 0.12 |
| NiWLa/nHA | 0.09 |
| NiW/Al2O3 | 0.28 |
| NiWLa/Al2O3 | 0.2 |
| NiW/HY | 0.97 |
| NiWLa/HY | 0.78 |
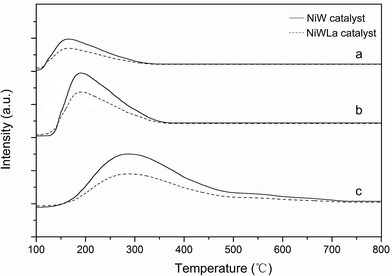
The TPD-Hads was used to determinate the metal function (hydrogenation capacity) of catalysts. The TPD-Hads spectra of catalysts were shown in Fig. 4, before La loading, it was obvious that there are two peaks, indicating two adsorbing sites on the surface of the catalysts. The low temperature desorption peaks are assigned to hydrogen on metallic Ni, while the high temperature desorption peaks are due to the H2 spillover species absorbed on the region between the active sites and support.24 After La loading, the area of the lower temperature peak gradually increased while the area of the high temperature peak increased but shifted to higher temperatures. The total amount of H2 desorption increased, revealing that the La loading increased the hydrogenation capacity of catalyst. These were consistent with the reported literature by Hou et al.25
 | ||
| Fig. 4 TPD-Hads spectra of (a) NiW/HY (b) NiWLa/HY (c) NiW/Al2O3 (d) NiWLa/Al2O3 (e) NiW/nHA and (f) NiWLa/nHA. | ||
The CO2-TPD profiles of different catalysts were shown in Fig. 5. Before La loading, little desorption peak of CO2 can be only observed for NiW/nHA and NiW/Al2O3, it was due to the alkalinity for both nHA and Al2O3 exhibiting. Yet the HY was typical acidy support, it had no absorption for CO2. After La loading, it can be seen that the total amount of desorbed CO2 obviously increased for all catalysts, indicating the corresponding basicity of catalysts was increasing due to La loading. Then the increased basicity of catalysts shall suppress the reaction, which need acid sites of catalyst to provide protons.16,26
Coke analysis
According to the reference of ISO 6964-1986,27 the amount of coke on the used catalysts were determined by TGA. As shown in Fig. 6(A), the amount of coke on NiW catalyst after use for 8 h was following the order: NiW/nHA < NiW/Al2O3 < NiW/HY, the same with the acidity order of catalyst. After La loading, the coke amount on NiWLa catalyst after use for 8 h was significantly lower than that on NiW catalyst, decreasing 1.91%, 0.94% and 1.19% than that on NiW/HY, NiW/nHA and NiWLa/Al2O3 respectively. As shown in Fig. 6(B), the data was similar with Fig. 6(A), the coke amount on all catalysts after use for 180 h was also decreased 3.78%, 1.66% and 1.89% on NiW/HY, NiW/nHA and NiWLa/Al2O3, respectively.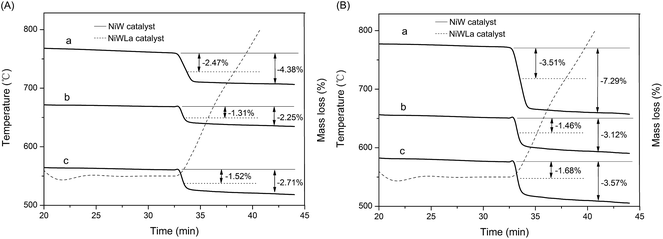 | ||
| Fig. 6 TGA profiles of (a) NiW/HY and NiWLa/HY (b) NiW/nHA and NiWLa/nHA (c) NiW/Al2O3 and NiWLa/Al2O3 after use for (A) 8 h and (B) 180 h at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1. | ||
The FT-IR spectrum of fresh and used catalysts were shown in Fig. 7. For the nHA, the characteristic peaks corresponding to OH− (3434.2 and 627.3 cm−1) and vibrations due to PO42− (1105.3, 1031.9, 954.8, 601.8, 561.3, and 464.9 cm−1) were observed.28 For the Al2O3, the band at 3745.2 cm−1 is assigned to the OH vibration. For the HY, the intensity at 1700–2000 cm−1 was assigned to overtones and combination modes of the zeolitic framework.29 It was obvious that the used catalysts all exhibited new bands at 1400–1600 cm−1 were characteristic of the vibration absorption of C in aromatic rings, demonstrating the coke of hydrocracking catalyst was the polyaromatic compound.30 Moreover, the signal of polyaromatic compound for used La loading catalyst was weaker, illustrating the coke amount was decreased, in accord with the TGA profiles.
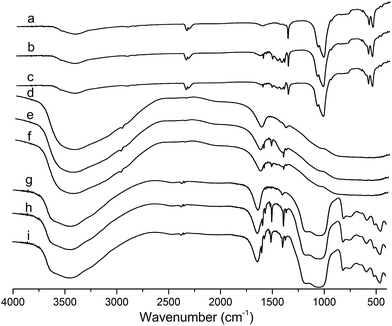 | ||
| Fig. 7 The FT-IR spectrum of (a) NiW/nHA (b) used NiW/nHA (c) used NiWLa/nHA (d) NiW/Al2O3 (e) used NiW/Al2O3 (f) used NiWLa/Al2O3 (g) NiW/HY (h) used NiW/HY and (i) used NiWLa/HY. | ||
The 13C NMR profiles of fresh and used catalysts were shown in Fig. 8. The used catalysts all exhibited new peaks around 125 ppm, corresponding to aromatic C–H bonds, while other peaks around 17 and 58 ppm were associated with the alkyl groups on the benzene ring.31,32 After La loading, the decreased signal of polyaromatic compound agreed with coke amount determinated by TGA. From these results, it can be proved again that the coke of hydrocracking catalyst is polyaromatic hydrocarbon and the La loading could reduce the coke amount.
 | ||
| Fig. 8 The solid-state 13C NMR profiles of (a) NiW/Al2O3 (b) used NiW/Al2O3 (c) used NiWLa/Al2O3 (d) NiW/nHA (e) used NiW/nHA (f) used NiWLa/nHA (g) NiW/HY (h) used NiW/HY and (i) used NiWLa/HY. | ||
Hydrocracking results
The GC charts of the product oil from hydrocracking of Jatropha oil over catalysts at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1 were shown in Fig. 9. Chemically, 3 mole fatty acid shall be produced from 1 mole of raw materials. As the literature had proved that after the oxygen removing reaction in hydrogen atmosphere, the fatty acid transformed to n-paraffins.33 There are two routes of the oxygen removing, one is hydrodeoxygenation (HDO) yielding paraffins with even carbon number (mainly C16 and C18) and H2O; the other is hydrodecarboxylation (HDC), including decarbonylation and decarboxylation, yielding paraffins with odd carbon number (mainly C15 and C17) and CO, CO2. | ||
| Fig. 9 GC charts of product oil over different catalysts at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1. | ||
After that, the isomerization of paraffins shall happen by the cooperation of metal and acid sites of catalyst.34 According to the classical carbenium ion principle, the full process was as follows: (I) through hydride elimination, the n-paraffin transformed to n-olefin. (II) Carbenium ion intermediate was formed by proton addition. (III) The formed normal carbenium ion intermediate isomerized to a branched carbenium ion intermediate. (IV) The iso-olefin was formed by elimination of proton. (V) Through hydrogenation, the iso-olefin transformed to iso-paraffin. However, at step III, the cracking reaction might happen, producing light paraffins. Therefore, as the GC charts illustrated, the main contents of product oil were n-paraffins and iso-paraffins ranging from C15 ∼ C18, besides, there are still some cracking light paraffins existing.
As the literature reported, the formation of carbenium ion intermediates need proton, the isomerization reaction relates to the acidity of catalyst,35,36 it can be seen from Table 4, the cracking paraffins content decreased as the acidity of catalysts decreased after La loading. However, the iso/n ratio increased even if the acidity of catalyst dropped off, this fascinating phenomenon was also reported by Li et al.,37 the mechanism was due to the enhanced hydrogenation capacity and decreased acidity of catalyst caused by La loading, increasing the metal/acid function ratio. Then it suppressed the probability of the intermediates staying on an acid site and then the further cracking of the carbenium ion intermediates. Therefore, we inferred that the La loading could balance the metal/acid function ratio to enhance the isomerization and weaken cracking reaction.
| Catalyst | NiW/nHA | NiWLa/nHA | NiW/Al2O3 | NiWLa/Al2O3 | NiW/HY | NiWLa/HY |
|---|---|---|---|---|---|---|
| Conversion (%) | 89.3 | 100 | 78.4 | 95.2 | 82.3 | 92.4 |
| Iso/n ratio | 0.21 | 0.25 | 0.35 | 0.4 | 0.57 | 0.82 |
| C15 ∼ C18 | 88.3 | 92.1 | 86.5 | 89.7 | 65.4 | 76.3 |
| <C15 | 11.7 | 7.9 | 13.5 | 10.3 | 34.6 | 23.7 |
It was proved by FT-IR and solid-state 13C NMR, the components of coke on the catalyst at high temperature were polyaromatic hydrocarbons. Based on the literature,31,32,38,39 the polyaromatic coke was formed from cracking paraffin, which was produced by cracking reaction of isomerization process. The cracking paraffin aromatization process can be divided in two parts. First, the cracking paraffin shall transform to olefin. Second, through oligomerization, cyclization and hydrogen transfer, the olefin shall transform to polyaromatic hydrocarbon, involving carbenium ions as transition states.
Since the acid/metal function was adjusted by La loading, the increased metal function could enhance the isomerization and weaken the cracking reaction, less cracking paraffin was obtained. On the other hand, as the NH3-TPD and CO2-TPD revealed, the acidity of catalyst was decreased while the alkalinity of catalyst was increased after La loading, it will slow down the formation of carbenium ion intermediate during aromatization process, suppressing the formation of polyaromatic hydrocarbon.
Reaction of time-on-stream
The conversion of Jatropha oil over different catalyst samples as a function of reaction time at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1 were shown in Fig. 10. It was obvious that after La loading, all catalysts' conversion increased and remained constant, suggesting the catalytic stability can be improved by the La loading, this is consistent with Gao et al.40 Combing with the TGA profiles, the coke of all catalyst samples decreased by La loading, avoiding the coke covering the active sites to deactivate the catalyst and guaranteeing the catalyst had a long lifetime for hydrocracking of Jatropha oil. Especially for the NiW/HY, its original strong acidity shall lead to more coke (over 5%), this is the reason of its conversion kept dropping off after use for 40 h. After La loading, its coke amount evidently decreased, then the conversion became stable. | ||
| Fig. 10 Conversion of Jatropha oil over different catalysts at 360 °C, 3 MPa, H2/oil (v/v) = 600, LHSV = 2 h−1 as a function of reaction time. | ||
Conclusion
The La was respectively loaded into NiW/nHA, NiW/Al2O3 and NiW/HY catalyst to value its effect of reducing coke (polyaromatic hydrocarbon) in hydrocracking of Jatropha oil. In all cases, the amount of coke was significantly decreased by La loading. The La loading could enhance the hydrogenation capacity to reduce the obtained cracking paraffin, decrease the acidity and increase the alkalinity of catalyst to suppress the aromatization process. For long term reaction, the La loading kept conversion of Jatropha oil stable and extend the lifetime of catalyst.References
- G. W. Huber, P. O'Connor and A. Corma, Appl. Catal., A, 2007, 329, 120–129 CrossRef CAS PubMed.
- D. Kubička and L. Kaluža, Appl. Catal., A, 2010, 372, 199–208 CrossRef PubMed.
- M. Krár, S. Kovács, D. Kalló and J. Hancsók, Bioresour. Technol., 2010, 101, 9287–9293 CrossRef PubMed.
- R. Tiwari, B. S. Rana, R. Kumar, D. Verma, R. Kumar, R. K. Joshi, M. O. Garg and A. K. Sinha, Catal. Commun., 2011, 12, 559–562 CrossRef CAS PubMed.
- R. Kumar, B. S. Rana, R. Tiwari, D. Verma, R. Kumar, R. K. Joshi, M. O. Garg and A. K. Sinha, Green Chem., 2010, 12, 2232–2239 RSC.
- M. Toba, Y. Abe, H. Kuramochi, M. Osako, T. Mochizuki and Y. Yoshimura, Catal. Today, 2011, 164, 533–537 CrossRef CAS PubMed.
- A. C. Barrón, J. Melo-Banda, J. E. Dominguez, E. M. Hernández, R. R. Silva, A. T. Reyes and M. M. Meraz, Catal. Today, 2011, 166, 102–110 CrossRef PubMed.
- J. Liu, C. Liu, G. Zhou, S. Shen and L. Rong, Green Chem., 2012, 14, 2499–2505 RSC.
- G. Zhou, Y. Hou, L. Liu, H. Liu, C. Liu, J. Liu, H. Qiao, W. Liu, Y. Fan and S. Shen, Nanoscale, 2012, 4, 7698–7703 RSC.
- P. Priecel, L. Čapek, D. Kubička, F. Homola, P. Ryšánek and M. Pouzar, Catal. Today, 2011, 176, 409–412 CrossRef CAS PubMed.
- J. Hu, J. Russell, B. Ben-Nissan and R. Vago, J. Mater. Sci. Lett., 2001, 20, 85–87 CrossRef CAS.
- D. Martin and D. Duprez, J. Phys. Chem., 1996, 100, 9429–9438 CrossRef CAS.
- D. Jin, B. Zhu, Z. Hou, J. Fei, H. Lou and X. Zheng, Fuel, 2007, 86, 2707–2713 CrossRef CAS PubMed.
- T. Hayakawa, S. Suzuki, J. Nakamura, T. Uchijima, S. Hamakawa, K. Suzuki, T. Shishido and K. Takehira, Appl. Catal., A, 1999, 183, 273–285 CrossRef CAS.
- S. Pokhrel, C. E. Simion, V. S. Teodorescu, N. Barsan and U. Weimar, Adv. Funct. Mater., 2009, 19, 1767–1774 CrossRef CAS.
- R. Bouarab, O. Cherifi and A. Auroux, Thermochim. Acta, 2005, 434, 69–73 CrossRef CAS PubMed.
- E. Ruckenstein and H. Wang, J. Catal., 2002, 205, 289–293 CrossRef CAS.
- T. Lehmann, T. Wolff, V. Zahn, P. Veit, C. Hamel and A. Seidel-Morgenstern, Catal. Commun., 2011, 12, 368–374 CrossRef CAS PubMed.
- A. Vimont, A. Travert, C. Binet, C. Pichon, P. Mialane, F. Sécheresse and J.-C. Lavalley, J. Catal., 2006, 241, 221–224 CrossRef CAS PubMed.
- M. J. Ledoux and B. Djellouli, Appl. Catal., 1990, 67, 81–91 CrossRef CAS.
- Y. Yoshimura, H. Shimada, T. Sato, M. Kubota and A. Nishijima, Appl. Catal., 1987, 29, 125–140 CrossRef CAS.
- A. Yasukawa, K. Gotoh, H. Tanaka and K. Kandori, Colloids Surf., A, 2012, 393, 53–59 CrossRef CAS PubMed.
- M. Jiang, S. J. Ye and S. C. Shan, Chin. J. Chem. Phys., 1997, 10, 377–382 CAS.
- J. B. Zheng, Z. Q. Xia, J. J. Li, W. K. Lai, X. D. Yi, B. H. Chen, W. P. Fang and H. L. Wan, Catal. Commun., 2012, 21, 18–21 CrossRef CAS PubMed.
- Y. Hou, Y. Wang, F. He, S. Han, Z. Mi, W. Wu and E. Min, Mater. Lett., 2004, 58, 1267–1271 CrossRef CAS PubMed.
- J. Gao, Z. Hou, J. Guo, Y. Zhu and X. Zheng, Catal. Today, 2008, 131, 278–284 CrossRef CAS PubMed.
- J. Zhu, X. Peng, L. Yao, J. Shen, D. Tong and C. Hu, Int. J. Hydrogen Energy, 2011, 36, 7094–7104 CrossRef CAS PubMed.
- R. Chakraborty and S. K. Das, Ind. Eng. Chem. Res., 2012, 51, 8404–8414 CrossRef CAS.
- B. Xu, F. Rotunno, S. Bordiga, R. Prins and J. A. van Bokhoven, J. Catal., 2006, 241, 66–73 CrossRef CAS PubMed.
- C. L. Li, O. Novaro, E. Munoz, J. L. Boldu, X. Bokimi, J. A. Wang, T. Lopez and R. Gomez, Appl. Catal., A, 2000, 199, 211–220 CrossRef CAS.
- M. A. Callejas, M. T. Martinez, T. Blasco and E. Sastre, Appl. Catal., A, 2001, 218, 181–188 CrossRef CAS.
- H. S. Cerqueira, G. Caeiro, L. Costa and F. R. Ribeiro, J. Mol. Catal. A: Chem., 2008, 292, 1–13 CrossRef CAS PubMed.
- Y. Liu, R. Sotelo-Boyás, K. Murata, T. Minowa and K. Sakanishi, Energy Fuels, 2011, 25, 4675–4685 CrossRef CAS.
- T. Degnan and C. Kennedy, AIChE J., 1993, 39, 607–614 CrossRef CAS.
- M. Roussel, S. Norsic, J.-L. Lemberton, M. Guisnet, T. Cseri and E. Benazzi, Appl. Catal., A, 2005, 279, 53–58 CrossRef CAS PubMed.
- M. Roussel, J.-L. Lemberton, M. Guisnet, T. Cseri and E. Benazzi, J. Catal., 2003, 218, 427–437 CrossRef CAS.
- D. Li, F. Li, J. Ren and Y. Sun, Appl. Catal., A, 2003, 241, 15–24 CrossRef CAS.
- M. Guisnet and P. Magnoux, Appl. Catal., A, 2001, 212, 83–96 CrossRef CAS.
- G. Caeiro, R. H. Carvalho, X. Wang, M. Lemos, F. Lemos, M. Guisnet and F. R. Ribeiro, J. Mol. Catal. A: Chem., 2006, 255, 131–158 CrossRef CAS PubMed.
- J. Gao, Z. Hou, J. Guo, Y. Zhu and X. Zheng, Catal. Today, 2010, 151, 412–412 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |


