A novel 3,6-diamino-1,8-naphthalimide derivative as a highly selective fluorescent “turn-on” probe for thiols†
Cheng Donga,
Chun-Qiong Zhou*a,
Jian-Wei Yanga,
Ting-Cong Liaoa,
Jin-Xiang Chena,
Cai-Xia Yinb and
Wen-Hua Chen*a
aGuangdong Provincial Key Laboratory of New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou 510515, P. R. China. E-mail: huagongzhoucq@163.com; whchen@smu.edu.cn; Fax: +86 20 61648533
bKey Laboratory of Chemical Biology and Molecular Engineering of Ministry of Education, Institute of Molecular Science, Shanxi University, Taiyuan 030006, P. R. China
First published on 1st April 2015
Abstract
A novel 1,8-naphthalimide derivative bearing 3-amino and 6-(2,4-dinitrobenzenesulfonamido) groups was synthesized and found to exhibit a fluorescent “turn-on” response to biothiols, including Cys, Hcy and GSH. This compound exhibited high selectivity and low detection limit, in particular toward Cys. The sensing mechanism involved the cleavage of the 2,4-dinitrobenzenesulfonamido group and the formation of an electron-donating amino group. In addition, its properties for cellular imaging were also briefly discussed.
Biologically important thiol-containing molecules, such as cysteine (Cys), homocysteine (Hcy) and glutathione (GSH), play an essential role in many biological processes. Abnormal levels of these species are closely related to certain diseases, such as liver damage, skin lesions, Alzheimer's disease, cardiovascular disease, heart disease and AIDS.1 This biological and practical significance has spurred great efforts to develop efficient approaches to detect and quantify biothiols in physiological media. Remarkable among them are synthetic molecular sensors that can change optical properties predominantly in response to the presence of the analytes of interest.
In this regard, 1,8-naphthalimide has served as an attractive fluorophore. Owing to its intense fluorescence, high sensitivity and photostability,2,3 1,8-naphthalimide has been widely used to synthesize various probes for the colorimetric and fluorescent detection of chemical and biological species from inorganic metal ions,4 anions,5 H2S,6,7 biothiols,8–13 reactive oxygen species,14 nucleobases to cancer cells.15–17 These fluorescent probes have been largely confined to 4-substituted 1,8-naphthalimide derivatives,2–7,13–17 for example 4-amino-1,8-naphthalimides.6,7,13,15,16,18–20 As a matter of facts, all the amino-substituted 1,8-naphthalimides, including 4-amino, 3-amino and 3,6-diamino-substituted derivatives, emit strong fluorescence through a typical intramolecular charge transfer (ICT) process and in principle can be used in the field of fluorescence sensing.21,22‡ However, few molecular sensors based on 3-mono or 3,6-bis-substituted 1,8-naphthalimides have been reported to date.21
In the work reported herein, we designed a new 1,8-naphthalimide derivative as a fluorescent “turn-on” probe for thiols, that is, compound 1 (Scheme 1). This compound bears 3-amino and reactive 6-(2,4-dinitrobenzenesulfonamido) (DNs) groups. Because of this structural feature, compound 1 may be readily converted into strongly fluorescent 3,6-diamino-1,8-naphthalimide derivative 2 (Scheme 1), leading to immediate fluorescence regeneration and enabling the efficiency of fluorescence activation to be optimized.23–25 Thus, rapid and sensitive detection of targeting biothiol molecules is feasible by use of compound 1 rather than its corresponding analog having two DNs groups. In addition, the left free amino group may adjust the cellular permeability of the molecule itself.25 Herein we report the synthesis of compound 1 and its sensing properties towards biothiols. In addition, its properties for cellular imaging are also briefly discussed.
 | ||
| Scheme 1 Synthesis of compound 1. Reagents and conditions: (a) H2/Pd/C, EtOH; (b) 2,4-dinitrobenzenesulfonyl chloride (5.0 equiv.), 2,6-dimethylpyridine (5.0 equiv.), anhydrous CH2Cl2, rt, 45 h. | ||
Compound 1 was prepared according to the synthetic route in Scheme 1. Thus, palladium/carbon-catalyzed reduction of N-butyl-3,6-dinitro-1,8-naphthalimide 3 gave N-butyl-3,6-diamino-1,8-naphthalimide 2 in an 85% yield. Mono-acylation of compound 2 with 2,4-dinitrobenzenesulfonyl chloride in the presence of 2,6-dimethylpyridine as a base afforded compound 1 in a 28% yield. Compound 3 was prepared according to the reported protocols.26 The structure of compound 1 was confirmed by NMR (1H and 13C) and ESI-MS (LR and HR) (ESI†).§
Fig. 1 shows the fluorescence changes that compound 1 undergoes upon the addition of various amino acids, including Cys, GSH, Hcy, Ala, Arg, Gln, Glu, Gly, His, Lys, Met, Phe, Ser, IIe, Val, Leu, Asp, Thr, Asn, Pro, Trp and Tyr, in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO-PBS buffer (0.01 M, pH 7.4).¶ It can be seen that compound 1 was essentially non-fluorescent, and addition of amino acids without thiol groups induced negligible changes in the fluorescence intensity. However, addition of thiol-containing Cys, Hcy and GSH caused up to 83-fold fluorescence enhancements with new strong emission at 560 nm. Of those three biothiols, Cys caused the highest increase in the fluorescence intensity, with a dramatic fluorescent color change from dark to orange (Fig. 1b). This result suggests that compound 1 exhibited fluorescent “turn-on” response toward Cys with much higher selectivity than toward the other amino acids (Scheme 2).
1 DMSO-PBS buffer (0.01 M, pH 7.4).¶ It can be seen that compound 1 was essentially non-fluorescent, and addition of amino acids without thiol groups induced negligible changes in the fluorescence intensity. However, addition of thiol-containing Cys, Hcy and GSH caused up to 83-fold fluorescence enhancements with new strong emission at 560 nm. Of those three biothiols, Cys caused the highest increase in the fluorescence intensity, with a dramatic fluorescent color change from dark to orange (Fig. 1b). This result suggests that compound 1 exhibited fluorescent “turn-on” response toward Cys with much higher selectivity than toward the other amino acids (Scheme 2).
In order to ensure the accurate detection of Cys, the sensing behavior of compound 1 should not be interfered by other chemical species. Given the complexity of intracellular environments, we studied the response of compound 1 toward a wide range of common species, including reactive sulfur species (HS−, S2− and HSO3−), reactive oxygen species (H2O2), nucleophiles (KI) and metal ions (K+, Ca2+, Na+, Mg2+, Al3+, Zn2+, Fe3+, Cu2+, Mn2+ and Cd2+). As shown in Fig. 2, compound 1 triggered no fluorescent feedback in the presence of NaHSO3, KI, H2O2 or metal ions. NaHS and Na2S led to a slight fluorescence increase. These results suggest that compound 1 exhibited fluorescent response to Cys with remarkably high selectivity.
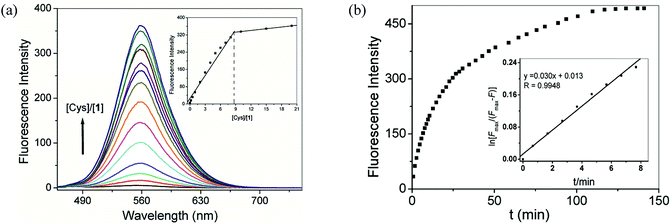
To ensure practical application, a sensor should have low detection limit and reasonably short response time. Therefore we firstly measured the detection limit of compound 1 toward Cys, GSH and Hcy by means of spectrofluorimetric titrations. As shown in Fig. 3a, the fluorescent intensity increased with the increase in the concentrations of Cys until saturation was observed. The stoichiometric ratio of Cys to compound 1 was found to be 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the detection limit was calculated to be 2.0 × 10−7 M based on S/N = 3 (Fig. S10†).27 Under the same conditions, compound 1 exhibited response to GSH and Hcy with the detection limits being 4.3 × 10−7 M and 1.2 × 10−6 M, respectively (Fig. S11 and S12†).
1, and the detection limit was calculated to be 2.0 × 10−7 M based on S/N = 3 (Fig. S10†).27 Under the same conditions, compound 1 exhibited response to GSH and Hcy with the detection limits being 4.3 × 10−7 M and 1.2 × 10−6 M, respectively (Fig. S11 and S12†).
Then we studied the time course of the response of compound 1 to Cys, GSH and Hcy under pseudo-first-order conditions and at pH 7.4 and 25 °C (Fig. 3b, S13 and S14†). Non-linear fitting of the initial concentration changes according to a pseudo-first-order kinetics equation afforded the rate constants of 3.0 × 10−2 min−1 for Cys, 9.5 × 10−3 min−1 for GSH and 5.9 × 10−3 min−1 for Hcy, respectively. Thus, the pseudo-first-order rate constant of Cys was 3- and 5-fold higher than those of GSH and Hcy, respectively.28 These data underlie the selectivity of compound 1 to small-molecular-weight biological thiols in the order of Cys > GSH > Hcy.
The above-observed new strong emission peak at 560 nm is thought to be due to the cleavage of the DNs group to produce an electron-donating amino group. In other words, the sensing mechanism involves the nucleophilic attack of thiols on the DNs group of compound 1 to give highly fluorescent N-butyl-3,6-diamino-1,8-naphthalimide 2, leading to immediate fluorescence regeneration.23,24 To verify this, the response of compound 1 to Cys was investigated by 1H NMR spectra. Upon the addition of Cys, the resonances at 7.82 ppm (Ha), 7.81 ppm (Hd), 7.66 ppm (Hc) and 7.19 ppm (Hb) of compound 1 disappeared. Meanwhile, two new resonances appeared at 7.61 ppm corresponding to H′a and H′d and 7.08 ppm corresponding to H′b and H′c of compound 2, respectively (Fig. 4b and c and S6†). This result is indicative of the cleavage of the DNs group and the production of compound 2. This was further supported by ESI-MS spectra, in which a peak at m/z 284.2 corresponding to [2 + H]+ (calcd = 284.2) was clearly observed (Fig. S7 and S8†).
 | ||
| Fig. 4 Partial 1H NMR spectra of compound 1 before the addition (a) and 72 h after the addition (b) of Cys, and of compound 2 (c) in d6-DMSO/D2O (5/2, v/v). | ||
To evaluate whether compound 1 has sufficient cellular permeability and can be activated by intracellular biothiols in living cells, its permeability and subsequent monitoring of thiols in living cells were investigated.29 Thus, human hepatocarcinoma HepG2 cells were incubated with compound 1 (5 μM) for 2 h. Strong red-fluorescence was exhibited inside the cells (Fig. 5d). In a control experiment, when cells were pretreated with N-ethylmaleimide (NEM, 20 μM, 0.5 h, a trapping reagent of thiol species), a remarkable decrease in fluorescence intensity was observed (Fig. 5g), indicating the specific detection of thiols by compound 1. These results suggest that compound 1 could enter cells and make fluorescence labeling.
In summary, we have successfully developed a novel 3,6-diamino-1,8-naphthalimide-based fluorescent “turn-on” probe for biothiols. It functions via the cleavage of the DNs group and the formation of an electron-donating amino group. This compound exhibits high selectivity toward biothiols over the other natural amino acids and a wide range of common species, with an obvious fluorescent color change from dark to orange. This compound may also be applied in the biological imaging of biothiols in living cells.
Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (no. 21402085, 21102086, 21472118), Guangdong Provincial Department of Science and Technology of China (2012B050100007), Shanxi Province Science Foundation for Youths (nos 2012021009-4 and 2013011011-1), Shanxi Province Foundation for Returnee (no. 2012-007) and Shanxi Province Outstanding Youth Fund (no. 2014021002).Notes and references
- C. X. Yin, F. J. Huo, J. J. Zhang, R. Martínez-Máñez, Y. T. Yang, H. G. Lv and S. D. Li, Chem. Soc. Rev., 2013, 42, 6032 RSC.
- W. Sun, W. H. Li, J. Li, J. Zhang, L. P. Du and M. Y. Li, Tetrahedron, 2012, 68, 5363 CrossRef CAS PubMed.
- Y. B. Liu, Y. W. Liu, W. Liu and S. C. Liang, Spectrochim. Acta, Part A, 2015, 137, 509 CrossRef CAS PubMed.
- J. Jiang, H. Jiang, W. Liu, X. L. Tang, X. Zhou, W. S. Liu and R. T. Liu, Org. Lett., 2011, 13, 4922 CrossRef CAS PubMed.
- X. J. Zheng, W. C. Zhu, D. Liu, H. Ai, Y. Huang and Z. Y. Lu, ACS Appl. Mater. Interfaces, 2014, 6, 7996 CAS.
- X. L. Liu, X. J. Du, C. G. Dai and Q. H. Song, J. Org. Chem., 2014, 79, 9481 CrossRef CAS PubMed.
- W. M. Xuan, R. Pan, Y. T. Cao, K. J. Liu and W. Wang, Chem. Commun., 2012, 48, 10669 RSC.
- Y. B. Zhang, F. J. Huo, C. X. Yin, Y. K. Yue, J. S. Hao, J. B. Chao and D. S. Liu, Sens. Actuators, B, 2015, 207, 59 CrossRef CAS PubMed.
- F. J. Huo, J. Kang, C. X. Yin, Y. B. Zhang and J. B. Chao, Sens. Actuators, B, 2015, 207, 139 CrossRef CAS PubMed.
- Y. T. Yang, F. J. Huo, C. X. Yin, J. B. Chao and Y. B. Zhang, Dyes Pigm., 2015, 114, 105 CrossRef CAS PubMed.
- F. J. Huo, Y. Q. Sun, J. Su, Y. T. Yang, C. X. Yin and J. B. Chao, Org. Lett., 2010, 12, 4756 CrossRef CAS PubMed.
- Y. T. Yang, F. J. Huo, C. X. Yin, A. M. Zheng, J. B. Chao, Y. Q. Li, Z. X. Nie, R. Martínez-Máñez and D. S. Liu, Biosens. Bioelectron., 2013, 47, 300 CrossRef CAS PubMed.
- B. C. Zhu, X. L. Zhang, Y. M. Li, P. F. Wang, H. Y. Zhang and X. Q. Zhuang, Chem. Commun., 2010, 46, 5710 RSC.
- Y. Wen, K. Y. Liu, H. R. Yang, Y. Li, H. C. Lan, Y. Liu, X. Y. Zhang and T. Yi, Anal. Chem., 2014, 86, 9970 CrossRef CAS PubMed.
- L. M. Zhang, L. E. Guo, X. M. Li, Y. G. Shi, G. F. Wu, X. G. Xie, Y. Zhou, Q. H. Zhao and J. F. Zhang, Tetrahedron Lett., 2014, 55, 6131 CrossRef CAS PubMed.
- W. C. Silvers, B. Prasai, D. H. Burk, M. L. Brown and R. L. McCarley, J. Am. Chem. Soc., 2013, 135, 309 CrossRef CAS PubMed.
- S. U. Hettiarachchi, B. Prasai and R. L. McCarley, J. Am. Chem. Soc., 2014, 136, 7575 CrossRef CAS PubMed.
- Q. L. Qiao, M. Zhao, H. J. Lang, D. Q. Mao, J. N. Cui and Z. C. Xu, RSC Adv., 2014, 4, 25790 RSC.
- Y. Y. Guo, T. Zeng, G. Q. Shi, Y. Q. Cai and R. L. Xie, RSC Adv., 2014, 4, 33626 RSC.
- M. H. Lee, J. H. Han, P. S. Kwon, S. Bhuniya, J. Y. Kim, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2012, 134, 1316 CrossRef CAS PubMed.
- Z. Ma, W. Sun, L. Z. Chen, J. Li, Z. Z. Liu, H. X. Bai, M. Y. Zhu, L. P. Du, X. D. Shi and M. Y. Li, Chem. Commun., 2013, 49, 6295 RSC.
- L. Zhang, S. Li, M. Hong, Y. Xu, S. Wang, Y. Liu, Y. Qian and J. Zhao, Org. Biomol. Chem., 2014, 12, 5115 CAS.
- A. Shibata, K. Furukawa, H. Abe, S. Tsuneda and Y. Ito, Bioorg. Med. Chem. Lett., 2008, 18, 2246 CrossRef CAS PubMed.
- S. M. Ji, J. Yang, Q. Yang, S. S. Liu, M. D. Chen and J. Z. Zhao, J. Org. Chem., 2009, 74, 4855 CrossRef CAS PubMed.
- M. Yoshida, M. Kamiya, T. Yamasoba and Y. Urano, Bioorg. Med. Chem. Lett., 2014, 24, 4363 CrossRef CAS PubMed.
- M. S. Alexiou and J. H. P. Tyman, J. Chem. Res., Synop., 2001, 2, 59 CrossRef.
- Y. Q. Sun, M. L. Chen, J. Liu, X. Lv, J. F. Li and W. Guo, Chem. Commun., 2011, 47, 11029 RSC.
- L. Yuan, W. Y. Lin and Y. T. Yang, Chem. Commun., 2011, 47, 6275 RSC.
- Y. H. Chen, J. Z. Zhao, H. M. Guo and L. J. Xie, J. Org. Chem., 2012, 77, 2192 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra03849a |
‡ 3-Amino-1,8-naphthalimides have lower fluorescence quantum yields, but larger stokes shift than the 4-amino analogue. For example, N-butyl-4-amino-1,8-naphthalimide exhibits maximum absorption at 430 nm (ε = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 M−1 cm−1) and emission at 541 nm (Φ = 0.13), whereas compound 2 exhibits maximum absorption at 418 nm (ε = 8300 M−1 cm−1) and emission at 560 nm (Φ = 0.0594). See ref. 22 and ESI† for more details. 600 M−1 cm−1) and emission at 541 nm (Φ = 0.13), whereas compound 2 exhibits maximum absorption at 418 nm (ε = 8300 M−1 cm−1) and emission at 560 nm (Φ = 0.0594). See ref. 22 and ESI† for more details. |
| § Structural data for compound 1: 1H NMR (400 MHz, d6-DMSO) δ 8.89 (d, J = 2.0 Hz, 1H), 8.58 (m, 1H), 8.30 (d, J = 8.4 Hz, 1H), 7.87 (d, J = 2.4 Hz, 1H), 7.86 (d, J = 2.4 Hz, 1H), 7.14 (d, J = 2.0 Hz, 1H), 3.97 (t, J = 7.2 Hz, 2H), 1.56 (m, 2H), 1.32 (m, 2H), 0.91 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, d6-DMSO) δ 163.7, 163.5, 150.6, 149.0, 148.3, 136.4, 135.4, 134.6, 132.1, 127.7, 123.6, 123.0, 121.6, 120.8, 120.6, 119.4, 118.5, 111.2, 30.0, 20.2, 14.1; ESI-MS m/z: 514.2 ([M + H]+) and HR-ESI-MS for C22H19N5O8S ([M + H]+) m/z: calcd 514.1033, found 514.1029. |
| ¶ We studied the effect of pH on the sensing properties of compound 1 toward Cys, and found that the emission intensity increased with the increase of pH from 3.0 to 7.5 and kept constant from pH 7.5 to 9.0 (Fig. S9†). Therefore we chose pH 7.4 as the operational pH under which the highest sensitivity can be achieved. |
| This journal is © The Royal Society of Chemistry 2015 |