A novel crosslinked polyelectrolyte synthesized via a one-step hydrothermal process as a humidity sensor
Kai Jiang,
Teng Fei* and
Tong Zhang*
State Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, PR China. E-mail: zhangtong@jlu.edu.cn; feiteng@jlu.edu.cn; Fax: +86 431 85168270; Tel: +86 431 85168385
First published on 5th September 2014
Abstract
A crosslinked polyelectrolyte was synthesized by reaction of lithium benzene-1,3,5-tris(olate) and terephthalic aldehyde using a one-step hydrothermal process. The structure and morphology of the resultant polymer poly(lithium benzene-1,3,5-tris(olate)) (PLBTO) were investigated by Fourier transform infrared spectrum (FTIR), thermal gravimetric analysis (TGA), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). A humidity sensor based on PLBTO was fabricated and humidity sensitive properties were explored. The impedance of PLBTO sensor changed four orders of magnitude with a good linearity over a relative humidity (RH) range from 33% to 95%. The conductive mechanism of the sensor was discussed based on complex impedance plots. The results indicate the quantity of adsorption water molecules would determine the category of conductive particles, and cause the impedance change under different RH. Moreover, the PLBTO sensor showed little hysteresis, rapid response/recovery and good long-term stability, which have benefited from the crosslinked structure of PLBTO.
1. Introduction
Polyelectrolytes, which can greatly enhance their electrical conductivity when they adsorb water molecules to ionize the ions contained in their molecular structure, have been widely used to fabricate resistance-type humidity sensors in recent years.1–7 Although humidity sensors based on polyelectrolytes have the advantages of low cost, easy preparation, high sensitivity and rapid response,8–10 long-term stability of polymeric humidity sensors at high humidity is not satisfactory owing to their intrinsic property of being soluble in water. Several methods have been proposed to make polyelectrolytes insoluble in water to manufacture dependable humidity sensors, including copolymerizing or grafting with hydrophobic monomers, forming crosslinked or interpenetrating network structures and constructing inorganic/organic hybrids.6,11–19 Forming a crosslinked structure in the sensitive films is a promising technique to improve the long-term stability of the humidity sensors under humid environments. Generally, the preparation of crosslinked polyelectrolytes needs two steps: polymerization and crosslinking. Crosslinking is usually through the reaction of polyelectrolytes and cross-linker in the solid state by heating.2,14 Hence the course of the crosslinking reaction is difficult to keep under control, which will result in indeterminate structural formulae of the crosslinked polyelectrolytes.In this report, Friedel–Crafts alkylation has been put forward to synthesize the crosslinked polyelectrolyte by a one-step hydrothermal process. Terephthalic aldehyde acts as an external cross-linker to polymerize with the monomer lithium benzene-1,3,5-tris(olate) to obtain the crosslinked polyelectrolyte with a clear structural formula. This method can effectively overcome the shortcoming of crosslinked polyelectrolytes mentioned above. The resultant polyelectrolyte was used as a humidity sensitive material to fabricate a humidity sensor. The obtained sensor showed good linearity, little hysteresis, long-term stability and rapid response/recovery. In order to research the sensing mechanism, the complex impedance plots were explored.
2. Experimental
2.1 Materials
1,3,5-trihydroxybenzene, 1,4-dioxane, ethanol and tetrahydrofuran were obtained from Tianjin Guangfu Technology Decelopment Co., Ltd., China. Terephthalic aldehyde and lithium hydroxide were purchased from Sinopharm Chemical Reagent Co., Ltd., China. All chemicals were used as received without further purification. The distilled water was purified through a Millipore system.2.2 Synthesis of lithium benzene-1,3,5-tris(olate) (LBTO)
An amount of 1.26 g (10 mmol) of 1,3,5-trihydroxybenzene, 0.96 g (40 mmol) of lithium hydroxide and 20 mL of distilled water were added into a round-bottom flask. The mixture was stirred at 50 °C for 2 h. After cooling to room temperature, 50 mL of ethanol was added into the flask and unreacted lithium hydroxide was precipitated to a white solid. The white solid was filtered out to collect filter liquor, and the filter liquor was concentrated to get a solid under vacuum. The product was dried in the vacuum oven at 50 °C for 24 h to obtain pure LBTO.2.3 Synthesis of polyelectrolyte poly(lithium benzene-1,3,5-tris(olate)) (PLBTO)
The crosslinked polyelectrolyte was synthesized according to the reported literature.20 In a round-bottom flask, 0.576 g (4 mmol) of LBTO and 0.402 g (3 mmol) of terephthalic aldehyde were added into 25 mL of dioxane. The mixture was stirred at 70 °C for 1 h. The obtained solution was then transferred to a Teflon lined autoclave which was purged by N2 for 10 minutes (a flow rate of 200 mL min−1), and the Teflon lined autoclave placed in an oven at 220 °C for 4 days. After cooling down to room temperature, white solid was collected by filtration and washed thrice with tetrahydrofuran. The solid was dried under vacuum at 50 °C for 24 h to get PLBTO.2.4 Characterizations
Fourier transform infrared spectrum (FTIR) was recorded with Perkin-Elmer spectrometer, using KBr pellets as reference. Thermal gravimetric analysis (TGA) was undertaken on a Perkin-Elmer thermal analysis system at a heating rate of 10 °C min−1 and a nitrogen flow rate of 200 mL min−1. The morphology of the film was performed on a JEOL JSM-6700F scanning electron microscopy (SEM). The microstructure of the film was checked using a Hitachi H-800 transmission electron microscopy (TEM), with an accelerating voltage of 200 kV.2.5 Fabrication and measurement of the humidity sensor
The humidity sensor based on PLBTO was prepared by mixing PLBTO with deionized water in a weight ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to form a paste. Then the paste was dip-coated onto a ceramic substrate (6 mm × 3 mm, 0.5 mm in thickness) with five pairs of Ag–Pd interdigitated electrodes (electrodes width and distance: 0.15 mm) to form a sensing film (0.05 mm in thickness). The film was dried in air at 20 °C for 5 h. Finally, the humidity sensor was obtained after aging at 95% relative humidity (RH) with a voltage of 1 V, 100 Hz for 24 h to improve stability and durability.
1 to form a paste. Then the paste was dip-coated onto a ceramic substrate (6 mm × 3 mm, 0.5 mm in thickness) with five pairs of Ag–Pd interdigitated electrodes (electrodes width and distance: 0.15 mm) to form a sensing film (0.05 mm in thickness). The film was dried in air at 20 °C for 5 h. Finally, the humidity sensor was obtained after aging at 95% relative humidity (RH) with a voltage of 1 V, 100 Hz for 24 h to improve stability and durability.
The characteristic curves of humidity sensitivity were measured on a ZL-5 model LCR analyzer (Shanghai, China). The data were collected by a computer automatically through an IEEE-488 GPIB Bus (82350A GPIB, Agilent, America). Special application software was developed using the LabVIEW program development environment (Version 7.1) to govern the work of the whole system. The voltage applied in our studies was AC 1 V, and the frequency varied from 20 Hz to 100 kHz. The measuring temperature was controlled at 20 °C by air-conditioner. The atmospheres of different RH were produced by different saturated salt solutions in several chambers. Their equilibrium states stood for 33% RH (MgCl2), 54% RH (Mg(NO3)2), 75% RH (NaCl), 85% RH (KCl) and 95% RH (KNO3) at 20 °C, respectively. The uncertainty of the RH values was about ± 1%.21 The measurement system of humidity sensor is shown in Fig. 1.
3. Results and discussion
In the reported literature, 1,3,5-trihydroxybenzene, which has reactive sites suitable for electrophilic aromatic substitution, is used to react with terephthalic aldehyde by elimination of water molecules.20 In our work, 1,3,5-trihydroxybenzene is converted into lithium benzene-1,3,5-tris(olate) (LBTO) firstly (Scheme 1a), which can be used for constructing polyelectrolyte and also enhance the reaction activity of electrophilic substitution. LBTO is used to react with terephthalic aldehyde in 1,4-dioxane by elimination of water molecules under high temperature and pressure without any catalyst to produce the crosslinked polyelectrolyte PLBTO as shown in Scheme 1b.Thermal stability of PLBTO is investigated by TGA as shown in Fig. 2. The TGA trace shows that PLBTO is stable by heating under N2 until 300 °C, and it retains more than 65% of its mass at 600 °C. Mass loss nearly 2% up to 150 °C owes to residual solvents (dioxane, THF, and H2O), which have not been removed during the drying procedure.
The FTIR spectra of 1,3,5-trihydroxybenzene, LBTO and PLBTO are shown in Fig. 3. The FTIR spectrum of 1,3,5-trihydroxybenzene (Fig. 3(a)) shows strong vibrational peaks at 3200, 1310 and 1162 cm−1, which illustrate the stretching of phenolic hydroxyl group. These peaks disappear in Fig. 3(b), and the new absorption peak at 864 cm−1 corresponds to the stretching of O–Li bond, which indicates hydroxyl groups were thoroughly converted.22 Seen from Fig. 3(c), the absorption peak at 2920 cm−1 is assigned to the stretching of alkyl, which indicates that LBTO is reacted with terephthalic aldehyde by elimination of water molecules. The stretching of aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C shows strong vibrational peaks at 1582 and 1422 cm−1. The absorption peak at 1014 cm−1 comes from the stretching of C–O bond. The absorption peak at 864 cm−1 demonstrates the stretching of O–Li bond. The absorption peaks at 822, 759 and 533 cm−1 correspond to the stretching of C–H bond in the benzene ring. Above results demonstrate the desired PLBTO is successfully prepared.
C shows strong vibrational peaks at 1582 and 1422 cm−1. The absorption peak at 1014 cm−1 comes from the stretching of C–O bond. The absorption peak at 864 cm−1 demonstrates the stretching of O–Li bond. The absorption peaks at 822, 759 and 533 cm−1 correspond to the stretching of C–H bond in the benzene ring. Above results demonstrate the desired PLBTO is successfully prepared.
The morphology of PLBTO film is examined with SEM as shown in Fig. 4(a). As can be seen, PLBTO film is stacked by irregular submicron particles, which is different from the reported polyelectrolytes.16 In the literature, the surface of crosslinked copolymer looks like a membranous porous structure in accordance with mostly polymers. Fig. 4(b) is the TEM image of PLBTO film. It can be seen clearly that abundant pores exist in PLBTO film probably resulting from accumulation of nanoparticles and crosslinked structure of the polyelectrolyte, which may allow free passages of water molecules.
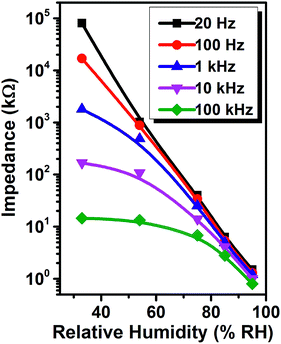
The humidity sensor based on PLBTO is fabricated and the impedance of PLBTO sensor is measured at AC 1 V from 33% to 95% RH. In order to research the influence of working frequency on humidity sensing property of PLBTO sensor, the impedance as a function of RH is measured at different frequencies as shown in Fig. 5. The impedance of PLBTO sensor decreases remarkably with raising frequency at low RH (33%) and the difference of impedance between adjacent two curves becomes gradually smaller as RH increased. The impedance versus RH curve shows a good linearity at 100 Hz, and the impedance changes four orders of magnitude from 33% to 95% RH.
In order to further research humidity sensing properties of PLBTO films, the sensitive mechanism of PLBTO sensor is researched by complex impedance plots (CIP). CIP are measured from 20 Hz to 100 kHz at different RH as shown in Fig. 6. ReZ and −ImZ, which stand for the real and imaginary parts of the CIP, are the horizontal axis and vertical axis, respectively. Table 1 shows equivalent circuits (EC) and main conductive particles (MCP) for various RH. At low RH (33%), the CIP is an arc with large curvature radius, which looks like a straight line, and the EC can be displayed by a constant phase element (CPE).23 At this point, a few water molecules are adsorbed on the surface of PLBTO films, and the MCP are protons (H+), which migrated by hopping from site to site across the surface of film leading to comparatively high impedance.24 When the RH increases to 54%, a semicircle is observed from CIP proving EC comprised of parallel resistor (Rf) and capacitor (Cf).25 More water molecules are adsorbed, synchronously, and H+ combines with H2O to form H3O+. According to the ion transfer mechanism of Grotthuss,26 H2O + H3O+ → H3O+ + H2O, the initial and final states are the same. The energy is also equivalent, so that the easy transfer of H3O+ leads to the quick decrease of impedance. As the RH further increased to 75%, CIP consists of the most part of semicircle at the high frequency range and a short line at the low frequency range. The short line represents Warburg impedance (Zw), which is caused by the diffusion process of ions or charge carriers at the interface between sensing film and electrode.27 In this process, several serial water layers form, which make a contribution to ionization of Li+. The dissociated Li+ can contribute to conduction along with H+ and H3O+. CIP at 85% RH is also comprised of the part of semicircle and a short line (not shown here), which is similar with 75% RH. With the RH increasing to 95%, the semicircle disappears and only a line remains in CIP. Due to a great quantity of ionization of Li+, the impedance of sensor was dominated by Zw, hence film resistance can ignore.23 Moreover, film conduction mainly comes from Li+ resulted in a sharp decrease of impedance of the sensor with four orders of magnitude compared to the initial impedance.
There are some other important humidity properties such as humidity hysteresis, response/recovery time and long-time stability for humidity sensor. Humidity hysteresis, defined as the maximum difference of humidity sensor between the adsorption and desorption process, is generally used to estimate the reliability of humidity sensors.28 The humidity hysteresis plots of PLBTO sensor are shown in Fig. 7. As can be seen, the maximum hysteresis is about 4% RH at the range of 33% to 95% RH, indicating a good reliability of the humidity sensor.
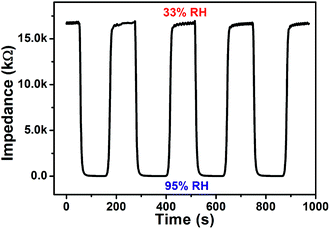
Fig. 8 displays response and recovery curves of PLBTO sensor. The measurements are repeated for four cycles, and the result demonstrates good repeatability of the sensor. The time taken by a sensor to achieve 90% of the total impedance change is defined as the response or recovery time.29 The response time is about 10 s when RH ranged from 33% to 95%, and the recovery time is about 19 s when RH decreased from 95% to 33% RH. The rapid response and recovery is related to the crosslinking structure of PLBTO. Water molecules are adsorbed on the surface of films by chemisorption and physisorption.30 At low RH, the chemisorption takes place firstly then physisorption follows. Lithium ions at the surface of film combine with OH− ionized by water molecules to form the chemisorbed water layer, and the chemisorbed water layer makes a contribution to forming physisorption water layer by hydrogen bonds as shown in Fig. 9. The physisorption water layers grow with the RH increasing. The adsorption/desorption rate at physisorption water layers directly influences the response/recovery time. The crosslinking structure of PLBTO is beneficial for the transportation of water molecules, hence PLBTO sensor has a fast response and recovery.
 | ||
| Fig. 9 The schematic diagram of the physisorption and chemisorption of water molecules on the film surface. | ||
In order to research temperature dependence of PLBTO sensor, the impedances of PLBTO sensor are measured at different temperature (from 10 to 50 °C). As shown in Fig. 10, the impedances of PLBTO sensor show little change at low humidities (33 and 54% RH) with the increase of temperature. At high humidities (75, 85 and 95% RH), the impedances of PLBTO sensor decrease a little from 10 to 50 °C due to enhanced ionization of water molecules. The results demonstrate the humidity sensing property is affected by temperature variation, but the influence is limited.
 | ||
| Fig. 10 The impedances of PLBTO sensor to the RH under different temperature (10, 20, 30, 40, 50 °C). | ||
In order to exam the long-time stability, PLBTO sensor was put into the glass container of 95% RH for repeated measurements every 5 days for 30 days. PLBTO sensor exhibits good high humidity stability and durability owing to slight variation in impedance with the passing of time as shown in Fig. 11. The results indicate that PLBTO sensor is promising for practical application.
4. Conclusions
In summary, a novel crosslinked polyelectrolyte PLBTO was synthesized by a one-pot hydrothermal process. The humidity sensor based on PLBTO was fabricated and the humidity sensitive properties of PLBTO sensor were discussed. As the introduction of lithium in the polymer networks, PLBTO sensor showed excellent humidity sensing properties, including good linearity, small hysteresis, rapid response and recovery. The crosslinking structure of PLBTO ensured good long-time stability of the sensor.Acknowledgements
This work was supported by the Natural Science Foundation Committee (NSFC, no. 51103053), Postdoctoral Science Foundation of China (PSFC, no. 2011M500608 and 2013T60324), Doctoral Fund of Ministry of Education of China (no. 20110061120053) and Program from Changjiang Scholars and Innovative Research Team in University (no. IRT13018).Notes and references
- M. K. Bernard, J. Am. Ceram. Soc., 1991, 74, 697 CrossRef PubMed.
- C. W. Lee, Y. Kim, S. W. Joo and M. S. Gong, Sens. Actuators, B, 2003, 88, 21 CrossRef CAS.
- Y. Li, M. J. Yang, N. Camaioni and G. Casalbore-Miceli, Sens. Actuators, B, 2001, 77, 625 CrossRef CAS.
- Y. Sakai and M. Matsuguchi, Electrochim. Acta, 2001, 46, 1509 CrossRef CAS.
- P. G. Su, I. C. Chen and R. J. Wu, Anal. Chim. Acta, 2001, 449, 103 CrossRef CAS.
- C. W. Lee, S. W. Joo and M. S. Gong, Sens. Actuators, B, 2005, 105, 150 CrossRef CAS PubMed.
- M. S. Gong, J. U. Kim and J. G. Kim, Sens. Actuators, B, 2010, 147, 539 CrossRef CAS PubMed.
- X. Lv, Y. Li, P. Li and M. J. Yang, Sens. Actuators, B, 2009, 135, 581 CrossRef CAS PubMed.
- M. S. Gong, S. W. Joo and B. K. Choi, Sens. Actuators, B, 2002, 86, 81 CrossRef CAS.
- B. Adhikari and S. Majumdar, Prog. Polym. Sci., 2004, 29, 699 CrossRef CAS PubMed.
- Y. Sakai, Y. Sadaoka, M. Matsuguchi, V. L. Rao and M. Kamigaki, Polymer, 1989, 30, 10681 CrossRef.
- S. Z. Wu, Y. L. Zhu, F. X. Li and J. R. Shen, J. Appl. Polym. Sci., 1999, 74, 1992 CrossRef CAS.
- Y. Sakai, M. Matsuguchi, Y. Sadaoka and K. Hirayama, J. Electrochem. Soc., 1993, 140, 432 CrossRef CAS PubMed.
- Y. Sakai, M. Matsuguchi and T. Hurukawa, Sens. Actuators, B, 2000, 66, 135 CrossRef CAS.
- C. W. Lee, H. S. Park, J. G. Kim, B. K. Choi, S. W. Joo and M. S. Gong, Sens. Actuators, B, 2005, 109, 315 CrossRef CAS PubMed.
- Y. Li, M. J. Yang and Y. She, Sens. Actuators, B, 2005, 107, 252 CrossRef CAS PubMed.
- Y. Li, L. J. Hong and M. J. Yang, Talanta, 2008, 75, 412 CrossRef CAS PubMed.
- M. S. Gong, S. W. Joo and B. K. Choi, J. Mater. Chem., 2002, 12, 902 RSC.
- Y. Sakai, Y. Sadaoka and M. Matsuguchi, J. Electrochem. Soc., 1989, 136, 171 CrossRef CAS PubMed.
- A. P. Katsoulidis and M. G. Kanatzidis, Chem. Mater., 2011, 23, 1818 CrossRef CAS.
- L. Greenspan, Journal of Research of NBS Section A Physics and Chemistry, 1977, 81, 89 Search PubMed.
- H. P. Ma, H. Ren, X. Q. Zou, F. X. Sun, Z. J. Yan, K. Cai, D. Y. Wang and G. S. Zhu, J. Mater. Chem. A, 2013, 1, 752 CAS.
- W. C. Geng, Q. Yuan, X. M. Jiang, J. C. Tu, L. B. Duan, J. W. Gu and Q. Y. Zhang, Sens. Actuators, B, 2012, 174, 513 CrossRef CAS PubMed.
- J. H. Anderson and G. A. Parks, J. Phys. Chem., 1968, 72, 3662 CrossRef CAS.
- X. F. Song, Q. Qi, T. Zhang and C. Wang, Sens. Actuators, B, 2009, 138, 368 CrossRef CAS PubMed.
- F. M. Ernsberger, J. Am. Ceram. Soc., 1983, 66, 747 CrossRef CAS PubMed.
- C. D. Feng, S. L. Sun, H. Wang, C. U. Segre and J. R. Stetter, Sens. Actuators, B, 1997, 40, 217 CrossRef CAS.
- R. Wang, X. W. Liu, Y. He, Q. Yuan, X. T. Li, G. Y. Lu and T. Zhang, Sens. Actuators, B, 2010, 145, 386 CrossRef CAS PubMed.
- S. Agarwal and G. L. Sharma, Sens. Actuators, B, 2002, 85, 205 CrossRef CAS.
- X. W. Liu, R. Wang, Y. Xia, Y. He and T. Zhang, Sens. Lett., 2011, 9, 1 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |