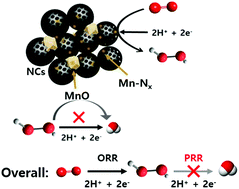
Electrochemical hydrogen peroxide (H2O2) production by the direct two-electron (2e−) oxygen reduction reaction (ORR) has received much attention as a promising alternative to the industrially developed anthraquinone fabrication process. Transition metal (M) and nitrogen doped carbon (M–N–C, M = Fe or Co) catalysts are known to be active for four electron ORR pathways via two + two electron transfer, where the former is for the ORR and the latter for the peroxide reduction reaction (PRR). Here, we report mesoporous N-doped carbon/manganese hybrid electrocatalysts composed of MnO and Mn–Nx coupled with N-doped carbons (Mn–O/N@NCs), which have led to the development of electrocatalysis towards the 2e− ORR route. Based on the structural and electrochemical characterization, the number of transferred electrons during the ORR on the Mn–O/N@NCs was found to be close to the theoretical value of the 2e− process, indicating their high activity toward H2O2. The favored ORR process arose due to the increased number of Mn–Nx sites within the mesoporous N-doped carbon materials. Furthermore, there was a strong indication that the PRR is significantly suppressed by adjacent MnO species, demonstrating its highly selective production of H2O2 (>80%) from the oxygen electrochemical process. The results of a real fuel cell device test demonstrated that an Mn–O/N@NC catalyst sustains a very stable current, and we attributed its outstanding activity to a combination of site-dependent facilitation of 2e− transfer and a favorable porosity for mass transport.