Yue Yuan, Jong-Wha Jung and Seung-Yong Seo
Org. Biomol. Chem., 2019,17, 44-48
DOI:
10.1039/C8OB02364F,
Communication
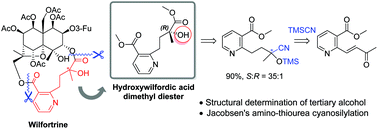
An enantioselective synthetic route to hydroxywilfordic acid, a key subunit of sesquiterpene pyridine alkaloids such as wilfortrine, was developed. Asymmetric cyanation using Jacobsen's (R,R)-amino-thiourea and hydrolysis were performed to afford chiral α-hydroxy-α-methyl acid as the (S)-isomer. Naturally derived hydroxywilfordate prepared by methanolysis of wilfortrine was found to be the (R)-isomer upon comparison with the synthetic compound.