 Open Access Article
Open Access ArticleScalable depolymerizing transesterification and amidation of (poly)lactic acid (PLA) enabled by resonant acoustic mixing (RAM)†
Anton S.
Makarov
 and
Magnus
Rueping
and
Magnus
Rueping
 *
*
KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. E-mail: magnus.rueping@kaust.edu.sa
First published on 10th December 2024
Abstract
This study presents a scalable mechanochemical method for the upcycling of (poly)lactic acid (PLA) into industrially valuable alkyl lactate esters via organocatalytic depolymerizing transesterification enabled by resonant acoustic mixing (RAM). The process is characterized by its simplicity, requiring neither grinding media nor a co-solvent and utilizing nearly stoichiometric amounts of an alcohol reaction partner in the presence of an inexpensive, easily accessible catalyst. Additionally, mechanochemistry is successfully extended to the upcycling of post-consumer PLA for the synthesis of various substituted esters and lactamides.
Introduction
The market for (poly)lactic acid (PLA) is experiencing significant growth, driven largely by a fundamental shift in consumer preferences toward more sustainable alternatives to fossil fuel-derived polymeric materials.1 PLA, being a bio-based compostable polymer acknowledged for its far greater biocompatibility compared to conventional plastics,2 has been preferentially used for manufacturing packaging materials and disposable cutlery.3,4 Clearly, its integration into such a large sector of modern economy will inevitably lead to an increase in PLA waste formation in the near future.Although considered biodegradable, PLA requires controlled conditions at specialized facilities in order to decompose within reasonable time, which would otherwise take much longer when simply burred in soil.5 High production expenses, however, make it economically impractical to compost PLA, nor does the incineration of PLA waste seem pragmatic.6 Mechanical recycling, in its turn, leads to notable deterioration and thus can only be performed a few times for a selected amount of PLA-made products.7 Consequently, the most financially feasible way of PLA waste treatment could be seen in chemical upcycling to produce value-added chemicals.8
The presence of an ester group in a repeat unit determines the major pathways for chemical upcycling of PLA. So-called closed-loop upcycling methods aim at exploiting the chemical transformations for accessing initial monomers, namely lactic acid9 and lactide,10 or for direct repolymerization of PLA.11 Conversely, open-loop methods target other chemicals that usually cannot be directly utilized for polymerization yet find important applications in other segments of the chemical industry. Apart from various protocols for PLA conversion into pyruvic12 and acrylic acids,13 or into propylene glycol,14 depolymerizing transesterification of PLA to obtain valuable lactic acid esters receives marked attention.
Low molecular weight lactic acid esters are recognized for their biocompatibility and relatively safe levels of toxicity.15 Their low vapor pressure, excellent solubilizing properties, and water miscibility make certain lactate esters, particularly ethyl lactate, highly sought after in industries such as food and beverages, pharmaceuticals, agrochemicals, cosmetics, electronics, and paints and inks, not to mention their use as “green” solvents for chemical reactions.16 Moreover, alkyl lactates serve as precursors for lactide,17 thereby closing the loop for the circular economy of PLA.18
Traditional liquid-phase methods for the conversion of PLA into alkyl lactates, despite their high efficiency, often require a large excess of an alcohol19 or addition of a co-solvent,20 or performing reactions at elevated temperatures, or utilization of reactive catalysts,21 which might limit their further implementation within the framework of the basic principles of green chemistry (Fig. 1a).22 Very recently, Borchardt and Kim proposed a practical and sustainable approach for the synthesis of methyl lactate from PLA via ball milling demonstrating that grinding a sample containing 2.2 g of post-consumer PLA and 20-fold excess of MeOH in a planetary mill with stainless-steel balls afforded nearly quantitative amount of methyl lactate within 6 h.23 This result clearly shows that mechanochemistry does have the potential to become a tool for environmentally benign and cost-effective recycling of PLA (Fig. 1b).24,25
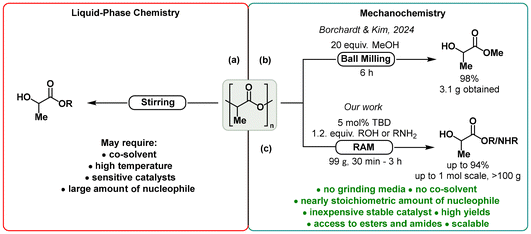 | ||
| Fig. 1 Established strategies for chemical upcycling of PLA into alkyl lactates: (a) liquid-phase methods, (b) mechanochemical method proposed by Borchardt and Kim, and (c) our method. | ||
To further expand the mechanochemical toolbox for sustainable production of alkyl lactates from PLA, we directed our attention to resonant acoustic mixing (RAM), a recently replenished technology that applies a low-frequency, high-intensity acoustic field to create micro-mixing zones, enabling efficient, media-free mixing and blending without the need for bulk solvents or grinding agents.26 Apart from its original purposes, RAM has been tested in organic mechanosynthesis, having proven to be scalable, economical, and easily manageable to perform chemical reactions.27 In addition, we recently demonstrated the use of RAM for mechanochemical High Throughput Experimentation (HTE).27e
We theorized that performing the depolymerizing transesterification of PLA under RAM conditions could significantly decrease the amount of alcohol required as a reaction partner, eliminate the need for a co-solvent, reduce the risks associated with the potential uncontrollable impact of grinding media on the reaction progress, and remove the need for post-use treatment of the media (Fig. 1c). Herein, we report our results aimed at developing an ecological, expeditious, and scalable method for upcycling PLA into alkyl lactate esters facilitated by RAM, which could further be extended to access alkyl amides of lactic acid.
Results and discussion
We commenced our research with the optimization of a model reaction of a commercially available sample of virgin PLA (Mw 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Đ 2.10) with EtOH on a 0.1 mmol scale under RAM conditions (Table 1). Triazabicyclodecene (TBD) was quickly identified as a suitable catalyst that afforded an acceptable degree of PLA (1) conversion into ethyl lactate (2a) at various alcohol loadings.28 TBD is recognized for its ability to promote transesterification reactions via a dual activation mode, acting as both a proton donor and proton acceptor due to the presence of sp2 and sp3 nitrogen atoms positioned at an optimal distance from each other to effectively interact with an ester group. This activation mechanism is thought to facilitate the nucleophilic addition and proton transfer steps in the depolymerizing transesterification of PLA as well.29 Tuning the process parameters led us to spot highly effective conditions for the formation of ethyl lactate at the selected reaction scale: mixing the suspension of PLA, 1.2 equiv. of EtOH and 13 mol% TBD at 99 g for 6 h provided ethyl lactate with a 92% analytical yield (entry 1). Close structural analogs of TBD, such as 7-methyl-triazabicyclodecene (MTBD) and 2-tert-butyl-tetramethylguanidine (BTMG), appeared to be slightly less effective, providing the target ester 2a with a yield of around 75% (entries 2 and 3). Switching to DBU, tetramethylguanidine (TMG), or guanidine decreased the yield of ethyl lactate dramatically (entries 4–6), whereas, in the absence of a base, the reaction did not proceed at all (entry 7). Neither altering TBD and EtOH loadings (entries 8–11) nor reducing the acceleration or shortening the reaction time (entries 12 and 13) improved the yield of ethyl lactate.
000, Đ 2.10) with EtOH on a 0.1 mmol scale under RAM conditions (Table 1). Triazabicyclodecene (TBD) was quickly identified as a suitable catalyst that afforded an acceptable degree of PLA (1) conversion into ethyl lactate (2a) at various alcohol loadings.28 TBD is recognized for its ability to promote transesterification reactions via a dual activation mode, acting as both a proton donor and proton acceptor due to the presence of sp2 and sp3 nitrogen atoms positioned at an optimal distance from each other to effectively interact with an ester group. This activation mechanism is thought to facilitate the nucleophilic addition and proton transfer steps in the depolymerizing transesterification of PLA as well.29 Tuning the process parameters led us to spot highly effective conditions for the formation of ethyl lactate at the selected reaction scale: mixing the suspension of PLA, 1.2 equiv. of EtOH and 13 mol% TBD at 99 g for 6 h provided ethyl lactate with a 92% analytical yield (entry 1). Close structural analogs of TBD, such as 7-methyl-triazabicyclodecene (MTBD) and 2-tert-butyl-tetramethylguanidine (BTMG), appeared to be slightly less effective, providing the target ester 2a with a yield of around 75% (entries 2 and 3). Switching to DBU, tetramethylguanidine (TMG), or guanidine decreased the yield of ethyl lactate dramatically (entries 4–6), whereas, in the absence of a base, the reaction did not proceed at all (entry 7). Neither altering TBD and EtOH loadings (entries 8–11) nor reducing the acceleration or shortening the reaction time (entries 12 and 13) improved the yield of ethyl lactate.
| Entry | Deviation | Yield of 2a,b % |
|---|---|---|
| a Reactions were performed in standard GC-vials, the temperature of the reaction content was 25–30 °C according to infrared thermometer readings taken after RAM stopped. b GC yields, internal standard – dodecane. | ||
| 1 | No deviation | 92 |
| 2 | Base = MTBD | 77 |
| 3 | Base = BTMG | 71 |
| 4 | Base = DBU | 34 |
| 5 | Base = TMG | 30 |
| 6 | Base = guanidine | 21 |
| 7 | No base | 0 |
| 8 | 12 mol% TBD | 85 |
| 9 | 14 mol% TBD | 92 |
| 10 | 1.1 equiv. of EtOH | 80 |
| 11 | 1.3 equiv. of EtOH | 92 |
| 12 | Reaction time = 5 h | 85 |
| 13 | Acceleration = 90 g | 74 |
As a further step in our research project, we moved on to scaling up the process. The possibility of reducing TBD and EtOH loadings was questioned upon tuning the reaction conditions on a 1 mmol scale (Table 2).
| Entry | TBD loading, mol% | EtOH loading, equiv. | Yield of 2a,b % |
|---|---|---|---|
| a Reactions were performed in standard GC-vials, the temperature of the reaction content was 25–30 °C according to infrared thermometer readings taken after RAM stopped. b NMR yields, internal standard – TBD. | |||
| 1 | 13 | 1.2 | 93 |
| 2 | 10 | 1.2 | 92 |
| 3 | 5 | 1.2 | 94 |
| 4 | 10 | 1.0 | 81 |
| 5 | 10 | 1.05 | 80 |
| 6 | 10 | 1.1 | 84 |
| 7 | 5 | 1.0 | 80 |
| 8 | 5 | 1.05 | 79 |
| 9 | 5 | 1.1 | 82 |
We found that the amount of TBD could be reduced up to 5 mol% while maintaining the chemoselectivity at the same level (entries 1–3). Apparently, the presence of a larger quantity of the substrate creates additional mechanical friction in the reaction mixture, peeling the PLA particles and facilitating the reaction. Reducing the amount of EtOH in the presence of 10 or 5 mol% TBD resulted in a non-significant decrease in ethyl lactate yield (entries 4–9). To our delight, even with just 1 equiv. of EtOH per repeat unit, the yield of ethyl lactate remained practical.
The reaction run on a 10 mmol scale took 3 hours and provided ethyl lactate with 94% analytical yield. However, we obtained different yields when we attempted to isolate the target ester. It seemed likely that TBD, known for initiating ring-opening polymerization of lactide,30 can catalyze oligomerization of ethyl lactate. This effect might become particularly significant when the reaction mixture is heated and concentrated during distillation. Indeed, when a sample of pure ethyl lactate was subjected to RAM for 3 h in the presence of 5 mol% TBD, the resulting mixture contained a detectable amount of dimer 3 alongside EtOH (Fig. 2). Ethyl lactate conversion was estimated to be ca. 5–10% based on NMR data. The obtained data may represent the state of the system under equilibrium. Factors driving the observed process, including the presence of PLA or short-chain oligomers, as well as reaction time, have not been thoroughly investigated thus far.
 | ||
| Fig. 2 1H NMR spectra of (a) ethyl lactate and (b) reaction mixture containing 1 equiv. of ethyl lactate and 5 mol% TBD after mixing at 99 g for 3 h under RAM conditions (400 MHz, CDCl3). | ||
In order to prevent ethyl lactate oligomerization, the reaction was quenched with acetic acid upon completion, which led to generating rather consistent reproducible quantitative results. Following the established protocol, we ran a set of preparative experiments utilizing both pure and post-consumer samples of PLA (Table 3). On a 10 mmol scale, a sample of virgin PLA was converted into ethyl lactate with 91% isolated yield (entry 1). The other samples obtained from PLA cups (Mw 179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Đ 2.01) and a 3D printing filament (Mw 205
000, Đ 2.01) and a 3D printing filament (Mw 205![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Đ 1.85) provided comparable results (entries 2 and 3). The process demonstrated excellent scalability: we were able to obtain the target product with about 90% isolated yield from both the cups and the filament on a 100 mmol scale (entries 4 and 5). Finally, when we performed the reaction on a 1 mol scale, we did not observe any decrease in the yield of ethyl lactate (entry 6). Notably, in this case, the reaction took only 30 minutes. We believe that factors such as increased particle friction in the reaction media and a moderate rise in temperature mutually influenced the reaction kinetics. We presume that it may be possible to further decrease the reaction time and TBD loading when scaling up the process to larger volumes. The base could be quenched with trifluoroacetic acid to provide a stable solid salt that could be easily separated after distillation of the main reaction content and used for regeneration (see the ESI† for details).
000, Đ 1.85) provided comparable results (entries 2 and 3). The process demonstrated excellent scalability: we were able to obtain the target product with about 90% isolated yield from both the cups and the filament on a 100 mmol scale (entries 4 and 5). Finally, when we performed the reaction on a 1 mol scale, we did not observe any decrease in the yield of ethyl lactate (entry 6). Notably, in this case, the reaction took only 30 minutes. We believe that factors such as increased particle friction in the reaction media and a moderate rise in temperature mutually influenced the reaction kinetics. We presume that it may be possible to further decrease the reaction time and TBD loading when scaling up the process to larger volumes. The base could be quenched with trifluoroacetic acid to provide a stable solid salt that could be easily separated after distillation of the main reaction content and used for regeneration (see the ESI† for details).
| Entry | Scale | Material | Yield of 2a,b % |
|---|---|---|---|
| a Reactions were performed in 5 mL PTFE jars (10 mmol), 100 mL PTFE jars (100 mL) and 250 ml glass jars (250 mL); the reaction mixture was treated with acetic acid (5 mol%) upon completion. b Isolated yield. c Temperature upon completion was 30–35 °C. d Temperature upon completion was 35–40 °C. e When the reaction was quenched with TFA instead of AcOH, TBD·TFA was isolated with 90% yield. f Temperature upon completion was 45 °C. g Reaction time – 30 min. h When the reaction was quenched with TFA instead of AcOH, TBD·TFA was isolated with 92% yield. | |||
| 1c | 10 mmol | Virgin PLA | 91 |
| 2c | 10 mmol | PLA cups | 90 |
| 3c | 10 mmol | PLA 3D printing filament | 90 |
| 4d | 100 mmol | PLA cups | 89e |
| 5d | 100 mmol | PLA 3D printing filament | 88 |
| 6f,g | 1 mol | PLA cups | 91h |
We studied the reaction progress under RAM conditions compared to conventional stirring on a 10 mmol scale. The shape of the reaction vessels, as well as their contents, was identical, with the only difference being the mixing mode. The reactions under conventional stirring were maintained at 35 °C throughout the entire experiment to replicate the temperature conditions observed during the RAM experiments. As can be seen from Fig. 3, the formation of ethyl lactate proceeds at a significantly slower rate throughout the duration of the experiment in the case of conventional stirring. Considering the particularly low solubility of PLA in EtOH or ethyl lactate,31 the overall process most likely proceeds through surface erosion of the PLA sample,32 which is heavily dependent on the extent of contact between PLA, EtOH, and TBD. Accounting for the proposed mechanism, the shape of the reaction vessel may have a substantial impact on the reaction rate upon conventional stirring. However, under RAM conditions, where the formation of micro-mixing zones across the entire vessel promotes the bulk movement of materials, the shape of a reaction vessel seems to be not relevant.
 | ||
| Fig. 3 Ethyl lactate formation over time under RAM conditions vs. conventional stirring, monitored by 1H NMR. | ||
To assess the potential sustainability and economic impact of the described method, we calculated the process mass intensity (PMI) for our protocol in comparison with one of the reported purely liquid-phase protocols for PLA upcycling into alkyl lactates.19b PMI is one of the environmental process metrics33 that is defined as the total mass (in kg) of raw materials used to produce 1 kg of the product. When comparing two processes, the one with a lower PMI reflects greater environmental benefits and efficiency, identifying it as the greener option. The PMI value for our process is 1.3, compared to 10.5 for the previously reported method, indicating the advantage of the RAM protocol from both environmental and economic perspectives (see the ESI† for details).
The established conditions were proven to be applicable for the synthesis of some other industrially important alkyl lactates. By engaging the respective alcohols, we synthesized methyl, iso-propyl, butyl, iso-butyl and pentenyl esters in high isolated yields without changing any reaction parameters or a reactants-to-catalyst ratio starting from the post-consumer PLA sample (Table 4, compounds 2b–f).
| a All reactions were performed on a 10 mmol scale; the yields of the product refer to isolated yields. |
|---|
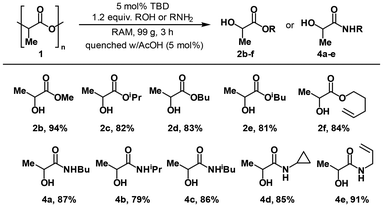
|
We also found that the method could be further extended toward substituted lactamides. Thus, mixing PLA with butyl-, iso-propyl-, iso-butyl-, cyclopropyl-, or allylamine in the presence of TBD led to the high-yielding formation of the corresponding products (Table 4, compounds 4a–e).
Conclusions
In summary, we demonstrated that resonant acoustic mixing can be efficiently adopted for the economical and scalable upcycling of (poly)lactic acid into industrially valuable alkyl lactate esters via organocatalytic depolymerizing transesterification. The process does not require any grinding media or co-solvent and utilizes nearly stoichiometric amounts of an alcohol reaction partner along with an inexpensive, one-step accessible catalyst. We also demonstrated that the method can be employed to access substituted lactamides. Our findings suggest that this method holds the potential for the development of sustainable industrial technologies aimed at producing low molecular weight esters and amides from post-consumer PLA, providing a versatile platform for green chemistry and circular economy initiatives.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the King Abdullah University of Science and Technology (KAUST) (grant number BAS/1/1385). We thank Dr Shrikant B. Nikam (KAUST) for performing the GPC studies and Alice Nanni (KAUST) for assisting with the RAM experiment setup.References
- (a) European Bioplastics, Bioplastics Market Development Update 2023, https://www.european-bioplastics.org/bioplastics-market-development-update-2023-2/ (accessed August 28, 2024); ; (b) E. Balla, V. Daniilidis, G. Karlioti, T. Kalamas, M. Stefanidou, N. D. Bikiaris, A. Vlachopoulos, I. Koumentakou and D. N. Bikiaris, Polymers, 2021, 13, 1822 CrossRef CAS.
- S. Lambert and M. Wagner, Chem. Soc. Rev., 2017, 46, 6855–6871 RSC.
- (a) E. Castro-Aguirre, F. Iñiguez-Franco, H. Samsudin, X. Fang and R. Auras, Adv. Drug Delivery Rev., 2016, 107, 333 CrossRef CAS PubMed; (b) S. Farah, D. G. Anderson and R. Langer, Adv. Drug Delivery Rev., 2016, 107, 367 CrossRef CAS; (c) M. Rabnawaz, I. Wyman, R. Auras and S. Cheng, Green Chem., 2017, 19, 4737 RSC.
- For biomedical applications of PLA, see: M. S. Singhvi, S. S. Zinjarde and D. V. Gokhale, J. Appl. Microbiol., 2019, 127, 1612 CrossRef CAS.
- (a) T. Ohkita and S. H. Lee, J. Appl. Polym. Sci., 2006, 100, 3009–3017 CrossRef CAS; (b) W. Sikorska, M. Musiol, B. Nowak, J. Pajak, S. Labuzek, M. Kowalczuk and G. Adamus, Int. Biodeterior. Biodegrad., 2015, 101, 32 CrossRef.
- C. Wellenreuther, A. Wolf and N. Zander, Clean. Eng. Technol., 2022, 6, 100411 CrossRef.
- (a) P. Zhao, C. Rao, F. Gu, N. Sharmin and J. Fu, J. Cleaner Prod., 2018, 197, 1046–1055 CrossRef CAS; (b) V. C. Agbakoba, N. Webb, E. Jegede, R. Phillips, S. P. Hlangothi and M. J. John, Macromol. Mater. Eng., 2023, 309, 2300276 CrossRef.
- (a) P. McKeown and M. D. Jones, Sustainable Chem., 2020, 1, 1–22 CrossRef; (b) X. Li, J. Wang, T. Zhang, S. Yang, M. Sun, X. Qian, T. Wang and Y. Zhao, Chem. Eng. Sci., 2023, 276, 118729 CrossRef CAS.
- S. D. Mürtz, M. S. Lehnertz, J. Kümper, E. Häger, A. Markus, T. Becker, S. Herres-Pawlis and R. Palkovits, Green Chem., 2024, 26, 6423 RSC.
- (a) L. Cederholm, J. Wohlert, P. Olsen, M. Hakkarainen and K. Odelius, Angew. Chem., Int. Ed., 2022, 61, e202204531 CrossRef CAS; (b) T. M. McGuire, A. Buchard and C. Williams, J. Am. Chem. Soc., 2023, 145, 19840 CrossRef CAS PubMed.
- R. L. Yang, G. Q. Xu, B. Z. Dong, H. B. Hou and Q. G. Wang, Macromolecules, 2022, 55, 1726 CrossRef CAS.
- Y. Miao, Y. Zhao, J. Gao, J. Wang and T. Zhang, J. Am. Chem. Soc., 2024, 146, 4842 CrossRef CAS.
- (a) F. G. Terrade, J. van Krieken, B. J. V. Verkuijl and E. Bouwman, ChemSusChem, 2017, 10, 1904 CrossRef CAS; (b) Y. C. Jiao, M. Wang and D. Ma, Chin. J. Chem., 2023, 41, 2071 CrossRef CAS.
- (a) S. Westhues, J. Idel and J. Klankermayer, Sci. Adv., 2018, 4, eaat9669 CrossRef CAS PubMed; (b) A. C. Fernandes, ChemSusChem, 2021, 14, 4228 CrossRef CAS PubMed; (c) M. Kobylarski, L. J. Donnelly, J. C. Berthet and T. Cantat, Green Chem., 2022, 24, 6810 RSC.
- (a) C. T. Bowmer, R. N. Hooftman, A. O. Hanstveit, P. W. Venderbosch and N. van der Hoeven, Chemosphere, 1998, 37, 1317–1333 CrossRef CAS; (b) E. Zuriaga, L. Lomba, C. B. García and M. S. Valero, Green Chem., 2023, 25, 7344 RSC.
- (a) S. Aparicio and R. Alcalde, Green Chem., 2009, 11, 65 RSC; (b) C. S. M. Pereira, V. M. T. M. Silva and A. E. Rodrigues, Green Chem., 2011, 13, 2658 RSC; (c) S. Planer, A. Jana and K. Grela, ChemSusChem, 2019, 12, 4655 CrossRef CAS; (d) A. V. Dolzhenko, Sustainable Chem. Pharm., 2020, 18, 100322 CrossRef; (e) L. Yang and J. P. Wan, Green Chem., 2020, 22, 3074 RSC.
- (a) R. De Clercq, M. Dusselier, E. Makshina and B. F. Sels, Angew. Chem., Int. Ed., 2018, 57, 3074–3078 CrossRef CAS PubMed; (b) R. De Clercq, M. Dusselier, C. Poleunis, D. P. Debecker, L. Giebeler, S. Oswald, E. Makshina and B. F. Sels, ACS Catal., 2018, 8, 8130 CrossRef CAS.
- (a) J. Payne and M. D. Jones, ChemSusChem, 2021, 14, 4041–4070 CrossRef CAS PubMed; (b) R. A. Clark and M. P. Shaver, Chem. Rev., 2024, 124, 2617–2650 CrossRef CAS PubMed; (c) C. Shi, E. C. Quinn, W. T. Diment and E. Y. Chen, Chem. Rev., 2024, 124, 4393 CrossRef CAS PubMed.
- (a) C. Alberti, N. Damps, R. R. R. Meißner and S. Enthaler, ChemistrySelect, 2019, 4, 6845–6848 CrossRef CAS; (b) E. Cheung, C. Alberti and S. Enthaler, ChemistryOpen, 2020, 9, 1224 CrossRef CAS.
- (a) F. A. Leibfarth, N. Moreno, A. P. Hawker and J. D. Shand, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4814 CrossRef CAS; (b) L. A. Román-Ramírez, P. McKeown, M. D. Jones and J. Wood, ACS Catal., 2018, 9, 409 CrossRef; (c) L. A. Román-Ramírez, P. McKeown, C. Shah, J. Abraham, M. D. Jones and J. Wood, Ind. Eng. Chem. Res., 2020, 59, 11149 CrossRef; (d) J. M. Payne, G. Kociok-Köhn, E. A. C. Emanuelsson and M. D. Jones, Macromolecules, 2021, 54, 8453 CrossRef CAS.
- (a) X. Y. Song, X. J. Zhang, H. Wang, F. S. Liu, S. T. Yu and S. W. Liu, Polym. Degrad. Stab., 2013, 98, 2760 CrossRef CAS; (b) R. Petrus, D. Bykowski and P. Sobota, ACS Catal., 2016, 6, 5222 CrossRef CAS; (c) J. Payne, P. McKeown, M. F. Mahon, E. A. C. Emanuelsson and M. D. Jones, Polym. Chem., 2020, 11, 2381 RSC; (d) R. L. Yang, G. Q. Xu, C. D. Lv, B. Z. Dong, L. Zhou and Q. G. Wang, ACS Sustainable Chem. Eng., 2020, 8, 18347 CrossRef CAS.
- (a) P. T. Anastas and J. C. Warner, Green Chemistry Theory and Practice, Oxford University Press, New York, 1998 Search PubMed; (b) F. A. Etzkorn, Green Chemistry: Principles and Case Studies, The Royal Society of Chemistry, 2019 Search PubMed.
- H. W. Lee, K. Yoo, L. Borchardt and J. G. Kim, Green Chem., 2024, 26, 2087 RSC.
- J. Zhou, T. G. Hsu and J. Wang, Angew. Chem., Int. Ed., 2023, 62, e202300768 CrossRef CAS PubMed.
- For general reviews discussing the sustainability aspects of mechanosynthesis, see: (a) M. Pérez-Venegas and E. Juaristi, ACS Sustainable Chem. Eng., 2020, 8, 8881 CrossRef; (b) K. J. Ardila-Fierro and J. G. Hernández, ChemSusChem, 2021, 14, 2145–2162 CrossRef CAS PubMed; (c) C. Espro and D. Rodríguez-Padrón, Curr. Opin. Green Sustainable Chem., 2021, 30, 100478 CrossRef CAS; (d) N. Fantozzi, J. N. Volle, A. Porcheddu, D. Virieux, F. García and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680 RSC; (e) E. Juaristi and C. G. Avila-Ortiz, Synthesis, 2023, 55, 2439 CrossRef CAS.
- (a) D. J. am Ende, S. R. Anderson and J. S. Salan, Org. Process Res. Dev., 2014, 18, 331 CrossRef CAS; (b) K. Nagapudi, E. Y. Umanzor and C. Masui, Int. J. Pharm., 2017, 521, 337 CrossRef CAS; (c) A. A. L. Michalchuk, K. S. Hope, S. R. Kennedy, M. V. Blanco, E. V. Boldyreva and C. R. Pulham, Chem. Commun., 2018, 54, 4033 RSC; (d) H. M. Titi, J. L. Do, A. J. Howarth, K. Nagapudi and T. Friščić, Chem. Sci., 2020, 11, 7578 RSC.
- (a) L. Gonnet, C. B. Lennox, J. L. Do, I. Malvestiti, S. G. Koenig, K. Nagapudi and T. Friščić, Angew. Chem., Int. Ed., 2022, 61, e202115030 CrossRef CAS; (b) C. B. Lennox, T. H. Borchers, L. Gonnet, C. J. Barrett, S. G. Koenig, K. Nagapudi and T. Friščić, Chem. Sci., 2023, 14, 7475 RSC; (c) J. F. Reynes, V. Isoni and F. Garcia, Angew. Chem., Int. Ed., 2023, 62, e202300819 CrossRef CAS; (d) M. Wohlgemuth, S. Schmidt, M. Mayer, W. Pickhardt, S. Grätz and L. Borchardt, Chem. – Eur. J., 2023, 29, e202301714 CrossRef CAS; (e) A. Nanni, D. Kong, C. Zhu and M. Rueping, Green Chem., 2024, 26, 8341 RSC.
- For a high-yielding, scalable protocol for the synthesis of TBD that utilizes inexpensive substrates, see: S. Usachev and A. Gridnev, Synth. Commun., 2011, 41, 3683 CrossRef CAS as well as the ESI.†.
- For more general discussion on the catalytic activity of TBD, see: (a) E. Fritz-Langhals, Org. Process Res. Dev., 2022, 26, 3015 CrossRef CAS; (b) J. G. Kim, G. S. Lee and A. Lee, J. Polym. Sci., 2024, 64, 42 CrossRef.
- N. Toshikj, J.-J. Robin and S. Blanquer, Eur. Polym. J., 2020, 127, 109599 CrossRef CAS.
- S. Sato, D. Gondo, T. Wada, S. Kanehashi and K. Nagai, J. Appl. Polym. Sci., 2012, 129, 1607 CrossRef.
- M. A. Elsawy, K.-H. Kim, J.-W. Park and A. Deep, Renewable Sustainable Energy Rev., 2017, 79, 1346 CrossRef CAS.
- N. Fantozzi, J.-N. Volle, A. Porcheddu, D. Virieux, F. García and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc04623d |
| This journal is © The Royal Society of Chemistry 2025 |



