Facile synthesis of net-like Fe3O4/MWCNTs decorated by SnO2 nanoparticles as a highly efficient microwave absorber
Lei Wang,
Honglong Xing *,
Zhenfeng Liu,
Ziyao Shen,
Xiang Sun and
Guocai Xu
*,
Zhenfeng Liu,
Ziyao Shen,
Xiang Sun and
Guocai Xu
School of Chemical Engineering, Anhui University of Science and Technology, Huainan, Anhui Province 232001, P. R. China. E-mail: austxhl@163.com; Tel: +86-554-6668497
First published on 4th October 2016
Abstract
Net-like SnO2/Fe3O4/MWCNTs were prepared through a simple two-step hydrothermal process. The as-synthesized composites were characterized by X-ray diffraction, vibrating sample magnetometer, transmission electron microscopy (TEM), and vector network analyses. In the process of Fe3O4 ripening, Fe3O4 particles play a role of bridge to connect MWCNTs to form a real net-like structure. TEM images indicate that Fe3O4 microspheres have size ranging from 100 to 200 nm, which can be seen as a scaffold for binding MWCNTs. The electromagnetic parameter results show that SnO2 nanocrystals were introduced into the system for tuning the parameters of SnO2/Fe3O4/MWCNTs composites to improve impedance match and SnO2 nanocrystals can supply space charge and interfacial polarization. The SnO2/Fe3O4/MWCNTs composites exhibit highly efficient microwave absorption capacity within the tested frequency range (2–18 GHz). The optimal reflection loss of electromagnetic waves is −42.0 dB at 10.9 GHz with an absorber thickness of 1.9 mm. The composites are potential excellent microwave absorbers with a special net-like structure for synergistic interaction between Fe3O4 microspheres with MWCNTs and semiconductors for tuning of electromagnetic parameters. The fabricated SnO2/Fe3O4/MWCNTs composites are promising materials for high-performance microwave absorption and satisfy the highly efficient absorption capability, thin thickness, and light weight requirements for electromagnetic absorption.
1 Introduction
Magnetic–dielectric composites have been increasingly used for microwave absorption (MA). Composite materials not only retain the inherent properties of their components but also provide enhanced functionality and multifunctional properties because of the interaction among their components.1–4 As a class of one-dimensional carbon nanostructure, carbon nanotubes (CNTs) have received much attention due to their unique chemical and physical properties; these materials are suitable for various applications, including coatings and films,5 batteries,6–8 environment and energy storage,9 and biotechnology.10–12 Studies have investigated the application of MWCNTs in MA; these composites include ZnO@MWCNTs,13 WMCNTs/Fe3O4 (ref. 14 and 15), SnO2@MWCNTs.16 Ferroferric oxide, a conventional microwave absorbing material, has also been investigated as a promoter for MA enhancement because of its excellent magnetic properties.17–21Dielectric and magnetic loss materials are combined to obtain an excellent microwave absorber.22–36 Wang et al.14 report a hybrid material consisting of magnetite (Fe3O4) nanocrystals with a core size of 8 nm grown on multiwalled carbon nanotubes (MWCNTs). The composites exhibit a surprisingly high-performance capability for MA with minimum reflection loss reaching −41.61 dB at a thickness of 3.4 mm. Cao et al.15 fabricated a novel dielectric–magnetic nanostructure by hybridizing 3D Fe3O4 nanocrystals and multi-walled carbon nanotubes minimum RL values of the composites with a thickness of 6.8 mm reach −23.0 dB and −52.8 dB at 4.08 GHz and 12.8 GHz, respectively. Besides, they fabricated grape-like Fe3O4-MWCNTs through co-precipitation. It could be seen that the samples with 3.2 mm possess the best MA performance.37 Huang et al.38 prepared boron and nitrogen doped carbon nanotubes/Fe3O4 composite. Nano-scale Fe3O4 particles with the diameter about 30 nm are uniformly distributed on the nanotubes and the composite have a minimum reflection loss is −51.2 dB at 11.7 GHz with a thickness of 5 mm. Du et al.39 prepared core–shell Fe3O4@C composites through in situ polymerization of phenolic resin followed by high-temperature carbonization. Fe3O4@C shows dielectric loss, interfacial loss, and improved matching impedance after carbon coating. Hence, the capacity of these composites for MA can be significantly improved by assigning appropriate impedance from the combination of dielectric and magnetic losses. MWCNTs were decorated with Fe3O4 nanoparticles with a size distribution of 7–18 nm using the wet chemical method, which could be used as the microwave absorber.40 As mentioned above, Fe3O4 nanocrystals and carbon composites have been invested in microwave absorber because they combine magnetic loss ability and dielectric loss ability effectively, which is contribute to improve MA. In comparison with the Fe3O4 nanocrystals decorate the MWCNTs, there are few correlative literatures report Fe3O4 microspheres combine with MWCNTs.
In this study, we demonstrate the synthesis of Fe3O4 microspheres decorate the MWCNTs with a net-like structure through the hydrothermal process. SnO2 nanocrystals were introduced into the system though the other hydrothermal process. Fig. 1 shows the synthesis process of SnO2/Fe3O4/MWCNTs composites.
Small Fe3O4 nanoparticles were adhered to the surface of MWCNTs and assembled to obtain large Fe3O4 microspheres with size ranging from 100 nm to 200 nm. In the process of Fe3O4 ripening, Fe3O4 particles play a role of bridge to connect MWCNTs to form a real net-like structure. SnO2 nanocrystals were then introduced to the system to tune the complex permittivity of Fe3O4/MWCNTs composites and improved impedance match. This study aims to fabricate high-performance microwave absorbers and discuss the synergistic interaction between magnetic microspheres and MWCNTs for MA.
2 Experimental sections
2.1 Materials
MWCNTs were provided by Chengdu Organic Chemicals Co., Ltd. (China). Raw MWCNTs (∼1 g) were refluxed at 120 °C for 12 h in the HNO3 solution (65–68 wt%). Ethylene glycol, sodium acetate trihydrate (CH3COONa·3H2O), iron(III) nitrate nonahydrate [Fe(NO3)3·9H2O], and tin(IV) chloride pentahydrate (SnCl4·5H2O) were purchased from Sinopharm Chemical Reagent Co., Ltd. Ammonium water was supplied by Yantai Shuangshuang Chemical Factory. All of the chemical regents used in this work were analytically pure and were not subjected to further purification.2.2 Synthesis of Fe3O4/WMCNTs
Fe3O4/WMCNTs were fabricated by a simple hydrothermal process. Briefly, the as-treated MWCNTs (80 mg) were dispersed in 70 mL of ethylene glycol solution and then added with Fe(NO3)3·9H2O (1, 2, 3 and 4 mmol) and CH3COONa·3H2O. The mixture was sonicated for 2 h. The mixed solution was then transferred into a Teflon-lined stainless steel autoclave with a capacity of 100 mL and heated at 200 °C for 20 h. The resulting sample was collected by washing with distilled water several times and drying in an oven at 60 °C.2.3 Synthesis of SnO2/Fe3O4/WMCNTs
SnO2/Fe3O4/MWCNTs composites were prepared by a hydrothermal process. All of the as-prepared Fe3O4/MWCNTs products were dispersed in 70 mL of distilled water and then added with 4 mmol SnCl4·5H20. The mixture suspension was sonicated for 2 h. Next, NH3·H2O (25 wt%) was added drop-wise into the reaction mixture to adjust the pH equal to 10. The mixed solution was transferred into a Teflon-lined stainless steel autoclave and heated at 160 °C for 18 h. The final products were washed by distilled water several times until pH = 7 and dried in an oven at 60 °C. The SnO2/Fe3O4/MWCNTs composites with different mole ratio of Fe3O4 and SnO2 nanocrystals (δFe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δSnO2) were obtained and labeled as S-1 (1
δSnO2) were obtained and labeled as S-1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), S-2 (2
4), S-2 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), S-3 (3
4), S-3 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), and S-4 (1
4), and S-4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The SnO2/MWCNTs composite was prepared with the same method.
1). The SnO2/MWCNTs composite was prepared with the same method.
2.4 Characterization
The X-ray powder diffraction (XRD) patterns of the SnO2/Fe3O4/MWCNTs were tested on PANayltical Empyrean powder X-ray diffractometer using monochromatic Cu–Kα radiation (λ = 0.154178 nm, 40 kV, 40 mA) and a scanning speed of 4° min−1. Magnetic properties were determined using HH-20 vibrating sample magnetometer (VSM). TEM images were recorded on a FEI Tecnai G20 TEM system. The sample for microwave measurement was prepared by uniformly blending SnO2/Fe3O4/MWCNTs absorbents with paraffin wax at a mass fraction of 70% and manufactured into a coaxial ring (3.04 mm inner diameter, 7.00 mm outer diameter). The complex permittivity and permeability of the SnO2/Fe3O4/MWCNTs were measured by a vector network analyzer (VNA, AV3629D) through transmission/reflection method.3 Results and discussion
3.1 Formation mechanism of SnO2/Fe3O4/MWCNTs
The synthesis of SnO2/Fe3O4/MWCNTs is shown in Fig. 1. After 2 h of ultrasonic treatment, acid-treated MWCNTs and Fe(NO3)3·9H2O were dispersed in ethylene glycol solution (Fig. 1a). The suspension was added with sodium acetate trihydrate and heated at 200 °C. Fe3+ first formed Fe(OH)3 under weak base environment and further reduced to Fe3O4 nanoparticles in the absence of ethylene glycol. Binding of Fe3O4 could be induced by magnetic dipole–dipole attraction (Fig. 1c).41 After that, the Fe3O4/MWCNTs composites were dispersed in distilled water through ultrasonic treatment (Fig. 1d). These functional groups and defects provide a location for Sn4+, which forms nuclei and converts into SnO2 nanoparticles. Electrostatic interactions play a key role in this process (Fig. 1e).Fig. 2a–c show the SEM images of the Fe3O4/MWCNTs composites without the addition of SnO2, the synthesized Fe3O4 with size of 100–200 nm in the Fe3O4/MWCNTs composites, which partly are cross-linked with the CNTs. The surface of MWCNTs looks smooth because of the small Fe3O4 particles self-assembled into microspheres. With addition of 4 mmol SnO2, the obtained SnO2/Fe3O4/MWCNTs sample consists of net-like Fe3O4/MWCNTs and the SnO2 crystals. At the same time, the surfaces of MWCNTs looks rough due to the surfaces of MWCNTs were decorated by the SnO2 nanoparticles. Further increasing the Fe3+ amount to 2 mmol (Fig. 3b), 3 mmol (Fig. 3c), and 4 mmol (Fig. 1d), the more Fe3O4 microspheres were obtained, but the size have a slight decrease and the microsphere stricture was damaged to a certain extent. Clearly, the less the added Fe3+, the complete stricture of Fe3O4 microspheres formed. Thus, it is possible to tune the network structure or morphology by varying the content of Fe3+.
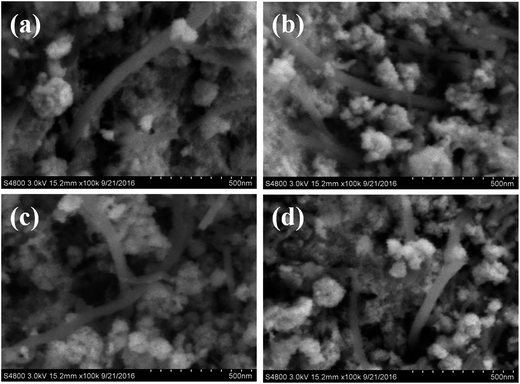 | ||
| Fig. 3 SEM images of SnO2/Fe3O4/MWCNTs obtained at different amounts of Fe3+ added: (a) 1 mmol, (b) 2 mmol, (c) 3 mmol, and (d) 4 mmol. | ||
3.2 Characterization of SnO2/Fe3O4/MWCNTs
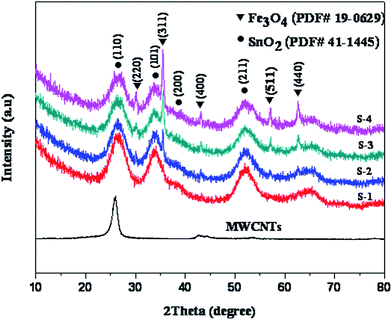
Fig. 4 shows the crystal structure of the final SnO2/Fe3O4/MWCNTs samples identified using XRD patterns. MWCNTs have its peaks, which appeared at 26.1° and 42°. The peaks at 26.6°, 33.9°, 37.9°, and 51.8° are assigned to the (110), (101), (200), and (211) planes of tetragonal rutile SnO2 (JCPDS no. 41-1445), with lattice parameters of a = b = 4.738 nm and c = 3.187 nm. Notably, there are no obvious diffraction peaks for the Fe3O4 in S1 sample, which might be duo to the relatively low content in the SnO2/Fe3O4/MWCNTs system and covered by the peaks of SnO2. The diffraction peaks of Fe3O4 gradually emerged as the content of Fe3+ added to the system was increased. Diffraction peaks at 30.1°, 35.4°, 43.0°, 56.9°, and 62.5° represent the Bragg reflection from the (220), (311), (400), (511), and (440) planes of the cubic spinel crystal structure of Fe3O4 (JCPDS no. 19-0629), with lattice parameters of a = b = c = 8.396 nm. The diffraction peaks of MWCNTs were covered by the peaks of SnO2 and Fe3O4. Based on calculation using Scherrer's formula, the average crystallite sizes of SnO2 and Fe3O4 are about 2 and 20 nm, respectively. No peaks corresponding to impurities were detected; hence, the composites are composed of SnO2, Fe3O4, and MWCNTs.The magnetization of four SnO2/Fe3O4/MWCNTs composites was measured at room temperature by a vibrating sample magnetometer (HH-20). Hysteresis loops of sample S-1 with a small coercivity (Hc) 18.2 emu g−1 indicated the presence of the soft ferromagnetic behavior.42 The values of saturation magnetization (Ms) increased from 1.83 to 15.59 emu g−1 in Fig. 5. These results demonstrated that the composites are ferromagnetic and thus may be used to absorb microwave after introducing Fe3O4 particles.
The TEM images in Fig. 6a shows that Fe3O4 microspheres are cross-linked by MWCNTs or decorated on the MWCNTs. The Fe3O4 microspheres with size of 100–200 nm are regarded as scaffold for binding MWCNTs to form a real net-like structure. After introducing the SnO2 nanocrystals, there is a clearly decrease about the size of Fe3O4 microspheres. Fig. 6c shows that Fe3O4 nanoparticles decorated on the MWCNTs and SnO2 nanocrystals uniformly covered the surface of the nanotubes, thereby allowing incident electromagnetic waves to enter into the interior of the composites. Fig. 6d shows the HRTEM image of SnO2/Fe3O4/MWCNTs. The Fe3O4 microspheres consist of nanocrystals with size of 16–22 nm. Fig. 6e presents the diffraction profile generated by the inserted SAED pattern and confirms the structure of SnO2 and Fe3O4. The results of EDS analysis confirmed the presence of C, O, Fe, and Sn in the composites. The valence states of Fe and Sn were determined through XPS analysis. Fig. 7a shows the binding energies of Fe 2p at 711.6 and 724.7 eV, but no satellite peak was observed at 720 eV; this findings and the XRD result can demonstrate that the microspheres are Fe3O4. For the spectrum of Sn in Fig. 7b, the peaks of Sn were found at 487.5 and 495.8 eV, which are assigned to the lattice of Sn4+ ions in tin oxide.
 | ||
| Fig. 6 (a) TEM images of Fe3O4/MWCNTs; (b and c) TEM images, (d) HRTEM image, (e) SAED pattern, and (f) EDS pattern of SnO2/Fe3O4/MWCNTs sample with Fe3+ contents 1 mmol. | ||
 | ||
| Fig. 7 XPS spectra: (a) Fe 2p and (b) Sn 3d spectra of SnO2/Fe3O4/MWCNTs sample with Fe3+ contents 1 mmol. | ||
3.3 Microwave absorbing properties
Generally, the electromagnetic wave absorption properties of absorbers were evaluated by reflection loss (RL), which is calculated using relative complex permittivity and permeability according to transmit line theory. RL is defined in the following equation:14,46,47
RL (dB) = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10|(Zin − Z0)/(Zin + Z0)| log10|(Zin − Z0)/(Zin + Z0)|
| (1) |
 | (2) |
The complex permittivity (εr = ε′ − jε′′) and complex permeability (μr = μ′ − jμ′′) of SnO2/Fe3O4/MWCNTs composites were observed in 2–18 GHz. The real permittivity (ε′) and real permeability (μ′) represent the storage ability of microwave energy, whereas the imaginary permittivity (ε′′) and imaginary permeability (μ′′) indicate the dissipation capacity of microwave energy.23,33,43 Fig. 8a shows that the real permittivity (ε′) value decreased with increasing frequency in the investigated region. Sample S-1 exhibited maximum changes, particularly ε′ decreased from 17.6 to 12.2. The ε′ values of sample S-1 are the highest among the four SnO2/Fe3O4/MWCNTs samples; in other words, this sample features high energy storage and polarization.43 Fig. 8b presents the imaginary part (ε′′) of the four SnO2/Fe3O4/MWCNTs composites, which show a similar trend between different samples. S-1 exhibits the highest ε′′ value, which indicating its superior dielectric loss. The curves of the samples present a complicated frequency-dependence behavior. For example, the ε′′ value of sample S-1 first decrease from 5.7 at 2 GHz to 4.4 at 7.1 GHz with a slight peak at 5.4 GHz, then increase gradually within 7.1–18 GHz with multiple peaks. This finding indicates the presence of multiple resonances behavior, which is related to highly conductivity and skin effects, electronic spin, and charge polarization because of point effects and polarized centers.26,44,45 It mostly comes from the synergistic influence of Fe3O4 microspheres, SnO2 nanocrystal, and the interfaces between Fe3O4 and dielectric MWCNTs. Moreover according to the free electron theory, ε′′ ≈ 1/πε0ρf, which ρ is the resistivity. The conductivity of S-1 samples is higher than others samples, the Fe3O4 microspheres can connect with MWCNTs to form a conductivity work, which will induce conduction.
Fig. 8c and d show the μ′ and μ′′, respectively, of all SnO2/Fe3O4/MWCNTs composites within 2–18 GHz. Both the μ′ and μ′′ of different Fe3+ loading concentrations indicate a similar decreasing trend with increasing frequency. For example, the μ′ value of sample S-1 decreased from 1.17 to 1.00 with several small fluctuant peaks. The μ′′ value increased from 0.15 at 2 GHz to a maximum of about 0.25 at 2.5 GHz, sharply decreased to 0 at 9 GHz, and maintained negative within 9–18 GHz. In the band 2–8 GHz and the band 14–18 GHz, all the samples exhibit two distinct peaks. Theoretically, the probable reasons of these peaks contribute to domain wall resonance, eddy current resonance and natural resonance.
The simulated RL curves of the Fe3O4/MWCNTs, SnO2/MWCNTs and SnO2/Fe3O4/MWCNTs composites are shown in Fig. 9. The Fe3O4 microspheres decorate MWCNTs have a very poor performance in the MA, because the majority of the surface of MWCNTs is naked to space (Fig. 4a) and most incident EM wave will be reflected on the surface due to high surface resistance. For SnO2/MWCNTs composites exhibit a low EM wave ability and the optimal RL of EM wave is −16.5 dB at 5.04 GHz with an absorber thickness of 3.5 mm. The poor EM wave absorption performance may cause by the relatively low characteristic impedance and signal dielectric loss action. The impedance properties of the composites have greatly improved after introducing the SnO2 nanoparticles into the system (Fig. 8c), which is beneficial for microwave penetration into the system and the SnO2/Fe3O4/MWCNTs composites exhibit an effective absorption ability. For example, S-1 exhibited significantly enhanced electromagnetic wave absorption compared with the other three SnO2/Fe3O4/MWCNTs in 2–18 GHz. Absorber thickness of 1.9 mm and mass fraction of Fe3O4 is 12.9%, the relevant Zin/Z0 values of sample S1 almost close to 1. Therefore, the EM wave can incident in the interior of absorber to be attenuated rather than reflect at the surfaces. So the S-1 sample have an optimal RL of electromagnetic wave is −42.0 dB at 10.9 GHz, the effective bandwidth (RL ≤ −10 dB) could reach 2.8 GHz (9.4–12.2 GHz) and when the thickness of the absorber is 1.5 mm, the microwave absorber shows the maximum effective absorption bandwidth of 3.8 GHz (12.4–16.2 GHz). The testing data indicate that the thickness of absorber can be easily adjusted to meet the need of absorption toward different frequency microwaves. Table 1 gives the information of other SnO2/Fe3O4/MWCNTs composites.
 | ||
| Fig. 9 Frequency dependence of simulated reflection loss value of Fe3O4/MWCNTs (a), SnO2/MWCNTs (b) and SnO2/Fe3O4/MWCNTs composites: (c) S-1, (d) S-2, (e) S-3, and (f) S-4 samples. | ||
| Sample | Mass fraction of Fe3O4 (%) | RL(Min) (dB) | f (GHz) (RLMin) | Thickness (mm) |
|---|---|---|---|---|
| a WFe3O4 = (MSnO2/Fe3O4/MWCNTs − MSnO2 − M MWCNTs)/(MSnO2/Fe3O4/MWCNTs + Mparaffin). | ||||
| MWCNTs/Fe3O4 | 65.2 | −3.2 | ||
| MWCNTs/SnO2 | −19.1 | 7.68 | 2.5 | |
| S-1 | 12.9 | −42.0 | 10.9 | 1.9 |
| S-2 | 15.5 | −22.2 | 6.7 | 3.5 |
| S-3 | 18.6 | −21.7 | 4.6 | 4.5 |
| S-4 | 22.4 | −38.9 | 4.2 | 5.0 |
In Fig. 10b, the maximum absorption peak shifted from high frequency to low frequency with increasing absorber thickness. According to the quarter-wavelength match principle, the relationship between absorber thickness (tm) and peak frequency (fm) can be described by the following equation:18,38,52
 | (3) |
 | ||
| Fig. 10 (a) RL curves for sample 1, (b) relationship between simulation thickness and peak frequency, (c) the relationship between Zin/Z0 and frequency. | ||
Fig. 10b shows the variations in the RL curve of the SnO2/Fe3O4/MWCNTs (S-1) with different thicknesses at 2–18 GHz. When the matching thickness of samples satisfies eqn (3), then microwave will be reflected from various interfaces with opposite phases, resulting in a counter act of one another at the interfaces. Based on the quarter-wavelength condition, absorber thickness (tm) versus peak frequency (fm) for the SnO2/Fe3O4/MWCNTs composites is simulated and displayed in Fig. 10b. The red dots on the λ/4 curve are the match thickness (denoted as texpm) of absorption peaks. The relationship between the experimental match thickness and peak frequency is in good agreement with the simulations using the quarter-wavelength principle for SnO2/Fe3O4/MWCNTs composites. In Fig. 10c, the frequency dependence of Zin/Z0 for composite S-1 is almost close to 1 at the absorber thickness of 1.9 mm, therefore the EM wave can easily permeate into the absorber to be attenuated and the composite exhibit maximum reflection loss at the 10.6 GHz. It can conclude that S-1 has a better MA property at identical coating thickness (1.9 mm).
SnO2/Fe3O4/MWCNTs composites exhibited the improved MA and satisfied the highly efficient absorption capability, thin thickness, and light weight requirements for electromagnetic absorption. Microwave absorption properties may be ascribed to several factors, such as impedance match, dielectric loss, magnetic loss, and interface relaxation. According to Debye theory, ε′′ represents dielectric loss, which consists of polarization loss and conductivity losses. The ε′′ is mainly enhanced by the conductivity of the SnO2/Fe3O4/MWCNTs as well as polarization at the Fe3O4-MWCNTs, Fe3O4–Fe3O4, and SnO2-MWCNTs interfaces (Fig. 11a). The plenty of interfaces in SnO2/Fe3O4/MWCNTs composites offer more chances of multi-reflection and diffusion scattering to form microwave dissipation paths. The specific structure of SnO2/Fe3O4/MWCNTs could build a conductive network and increases the conductive pathways between Fe3O4 and MWCNTs. In this heterostructure, Fe3O4 microspheres play at least two roles in ameliorating MA properties. First, the Fe3O4 microspheres can provide a microwave magnetic loss action as a soft ferromagnetic material. The natural resonance and the eddy current effect play a pivotal role in magnetic loss process. Natural resonance generally appears at frequency below 8.2 GHz (Fig. 8d). At same time, the steady values of C0 (C0 = μ′′(μ′)−2f−1) for samples with different Fe3O4 concentrations at 10–18 GHz, which suggests that the composites have an obvious eddy current loss.18,20,37,43 Second, the Fe3O4 microspheres are considered as a scaffold for binding two individual MWCNTs to form a real network. This makes it possible for electron hopping and migrating, which is benefit for MA (Fig. 11b).
In Table 2, we listed the reflection loss properties of some Fe3O4 based composites. The poor impedance matching properties caused the unbalancing of the complex permittivity and the permeability. No matter Fe3O4 nanospheres or Fe3O4 micro-spheres, the single Fe3O4 cannot show the efficient MA. Besides, Fe3O4 presents several limitations, including poor impedance matching, large thickness, and high loading content, which restrict their practical applications. When Fe3O4 combine with some dielectric loss materials, such as MWCNTs, SnO2 and PANI, those Fe3O4 based composites got an efficient MA with a higher impedance matching and the synergy effect between dielectric and magnetic loss materials. But the large thickness of those materials will limit their practical applications. In this work, SnO2 nanocrystals were introduced to the system, which not only tune the complex permittivity and complex permeability of SnO2/Fe3O4/MWCNTs composites to improve the impedance match, but also supply more space charge polarization and interfacial polarization. The optimal reflection loss (RL) of electromagnetic wave is −42.0 dB with the absorber thickness of only 1.9 mm. This is an attractive candidate for high performance MA materials, which satisfies the current requirements of electromagnetic absorbing materials, which include high-efficiency absorption capability, thin thickness and light weight.
4 Conclusions
Net-like SnO2/Fe3O4/MWCNTs were prepared through hydrothermal process. Fe3O4 microspheres decorated on the MWCNTs can connect MWCNTs form a conductive network and provide a microwave magnetic loss action as a soft ferromagnetic material. SnO2 nanocrystal can not only tune the electromagnetic parameters to improve the impedance match, but also supply more space charge polarization and interfacial polarization. When the mass fraction of Fe3O4 is 12.9% with an absorber thickness of only 1.9 mm the SnO2/Fe3O4/MWCNTs composites shown an optimal microwave reflection loss is −42.0 dB at 10.9 GHz. The excellent electromagnetic absorption capacity is attributed to the well impendence match, dielectric and magnetic loss, and special structure.Conflict of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the Natural Science Foundation of China (Grant No. 51477002; 51173002).References
- H. W. Wang, Z. J. Xu, H. Yi, H. G. Wei, Z. H. Guo and X. F. Wang, Nano Energy, 2014, 7, 86–96 CrossRef CAS.
- K. Mujeeb, N. T. Muhammad, F. A. Syed, U. K. Hadayat, H. S. M. Rafiq, A. Al-warthan Abdulrahman and T. Wolfgang, J. Mater. Chem. A, 2015, 3, 18753–18808 Search PubMed.
- K. M. Neelesh, M. Vijay and N. K. Jain, Biomaterials, 2013, 10, 032 Search PubMed.
- P. B. Liu, Y. Huang and X. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 12355–12360 CAS.
- F. L. Michael Volder De, H. T. Sameh, H. B. Ray and H. A. John, Science, 2013, 339, 535–539 CrossRef PubMed.
- D. Y. Ren, H. Jiang, Y. J. Hu, L. Zhang and C. Z. Li, RSC Adv., 2014, 4, 40368–40372 RSC.
- S. L. Yang, C. Y. Cao, G. Li, Y. B. Sun, P. P. Huang, F. F. Wei and W. G. Song, Nano Res., 2015, 8, 1339–1347 CrossRef CAS.
- L. Xu, Y. J. Hu, H. X. Zhang, H. Jiang and C. Z. Li, ACS Sustainable Chem. Eng., 2016, 4, 4251–4255 CrossRef CAS.
- L. M. Dai, D. W. Chang, J. B. Baek and W. Lu, Small, 2012, 8, 1130–1166 CrossRef CAS PubMed.
- E. Birgit, M. S. Jan and M. S. Timothy, Angew. Chem., Int. Ed., 2012, 51, 5752–5756 CrossRef PubMed.
- F. L. Tian, T. T. Yue, Y. Li and X. R. Zhang, Sci. China: Chem., 2014, 57, 1662–1671 CrossRef CAS.
- B. Alberto, K. Kostas and P. Maurizio, Chem. Commun., 2011, 47, 10182–10188 RSC.
- M. M. Lu, W. Q. Cao, H. L. Shi, X. Y. Fang, J. Yang, Z. L. Hou, H. B. Jin, W. Z. Wang, J. Yuan and M. S. Cao, J. Mater. Chem. A, 2014, 2, 10540–10547 CAS.
- Z. J. Wang, L. N. Wu, J. G. Zhou, W. Cai, B. Z. Shen and Z. H. Jiang, J. Phys. Chem. C, 2013, 117, 5446–5452 CAS.
- Y. H. Chen, Z. H. Huang, M. M. Lu, J. Y. Cao, D. Q. Zhang and M. S. Cao, J. Mater. Chem. A, 2015, 3, 12621–12625 CAS.
- L. Lin, H. L. Xing, R. W. Shu, L. Wang, X. L. Ji, D. X. Tan and Y. Gan, RSC Adv., 2015, 5, 94539–94550 RSC.
- C. Y. Liang, C. Y. Liu, H. Wang, L. N. Wu, Z. H. Jiang, Y. J. Xu, B. Z. Shen and Z. J. Wang, J. Mater. Chem. A, 2014, 2, 16397–16402 CAS.
- F. B. Meng, W. Wei, X. G. Chen, X. L. Xu, M. Jiang, L. Jun, Y. Wang and Z. W. Zhou, Phys. Chem. Chem. Phys., 2016, 18, 2510–2516 RSC.
- C. K. Cui, Y. C. Du, T. H. Li, X. Y. Zheng, X. H. Wang, X. J. Han and P. Xu, J. Phys. Chem. B, 2012, 116, 9523–9531 CrossRef CAS PubMed.
- H. L. Lv, G. B. Ji, W. Liu, H. Q. Zhang and Y. W. Du, J. Mater. Chem. C, 2015, 3, 10232–10241 RSC.
- L. B. Cui, Y. Liu, X. H. Wu, Z. Q. Hu, Z. J. Shi and H. J. Li, RSC Adv., 2015, 5, 75817–75822 RSC.
- D. P. Sun, Q. Zou, Y. P. Wang, Y. J. Wang, W. Jiang and F. S. Li, Nanoscale, 2014, 6, 6557–6562 RSC.
- W. C. Zhou, X. J. Hu, X. X. Bai, S. Y. Zhou, C. H. Sun, J. Yan and P. Chen, ACS Appl. Mater. Interfaces, 2011, 3, 3839–3845 CAS.
- Q. L. He, T. T. Yuan, X. Zhang, X. R. Yan, J. Guo, D. W. Ding, A. K. Mojammel, P. Y. David, K. Airat, Z. P. Luo, J. R. Liu, T. D. Shen, X. Y. Liu, S. Y. Wei and Z. H. Guo, J. Phys. Chem. C, 2014, 118, 24784–24796 CAS.
- J. Xiang, J. L. Li, X. H. Zhang, Q. Ye, J. H. Xua and X. Q. Shen, J. Mater. Chem. A, 2014, 2, 16905–16914 CAS.
- B. Zhao, W. Y. Zhao, G. Shao, B. B. Fan and R. Zhang, Dalton Trans., 2015, 44, 15984–15993 RSC.
- F. B. Meng, W. Wei, J. J. Chen, X. G. Chen, X. L. Xu, M. Jiang, Y. Wang, J. Lu and Z. W. Zhou, RSC Adv., 2015, 5, 101121–101126 RSC.
- J. J. Jiang, D. Li, D. Y. Geng, J. An, J. He, W. Liu and Z. D. Zhang, Nanoscale, 2014, 6, 3967–3971 RSC.
- Y. B. Zhang, P. Wang, Y. Wang, L. Qiao, T. Wang and F. S. Li, J. Mater. Chem. C, 2015, 3, 10813–10818 RSC.
- Y. L. Ren, H. Y. Wu, M. M. Lu, Y. J. Chen, C. L. Zhu, P. Gao, M. S. Cao, C. Y. Li and Q. Y. Ouyang, ACS Appl. Mater. Interfaces, 2012, 4, 6436–6442 CAS.
- G. H. Pan, J. Zhu, S. L. Ma, G. B. Sun and X. J. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 12716–12724 CAS.
- L. Wang, Y. Huang, X. Sun, H. J. Huang, P. B. Liu, M. Zong and Y. Wang, Nanoscale, 2014, 6, 3157–3164 RSC.
- L. N. Wang, X. L. Jia, Y. F. Li, F. Yang, L. Q. Zhang, L. P. Liu, X. Ren and H. T. Yang, J. Mater. Chem. A, 2014, 2, 14940–14946 CAS.
- F. S. Wen, F. Zhang and Z. Y. Liu, J. Phys. Chem. C, 2011, 115, 14025–14030 CAS.
- G. P. Wan, G. Z. Wang, X. Q. Huang, H. N. Zhao, X. Y. Li, K. Wang, L. Yu, X. G. Peng and Y. Qin, Dalton Trans., 2015, 44, 18804–18809 RSC.
- G. Wang, Z. Gao, S. Tang, C. Chen, F. Duan, S. Zhao, S. Lin, Y. Feng, L. Zhou and Y. Qin, ACS Nano, 2012, 6, 11009–11017 CrossRef CAS PubMed.
- M. M. Lu, M. S. Cao, Y. H. Chen, W. Q. Cao, J. Liu, H. L. Shi, D. Q. Zhang, W. Z. Wang and J. Yuan, ACS Appl. Mater. Interfaces, 2015, 7, 19408–19415 CAS.
- T. Zhang, B. Zhong, J. Q. Yang, X. X. Huang and G. Wen, Ceram. Int., 2015, 41, 8163–8170 CrossRef CAS.
- Y. C. Du, W. W. Liu, R. Qiang, Y. Wang, X. J. Han, J. Ma and P. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 12997–13006 CAS.
- H. Hekmatara, M. Seifi and K. Forooraghi, J. Magn. Magn. Mater., 2013, 346, 186–191 CrossRef CAS.
- L. Guo, F. Liang, X. G. Wen, S. H. Yang, L. He, W. Z. Zheng, C. P. Chen and Q. P. Zhong, Adv. Funct. Mater., 2007, 17, 425–430 CrossRef CAS.
- Q. He, T. Yuan, Z. Luo, D. P. Young, S. Wei and Z. Guo, Chem. Commun., 2013, 49, 2679–2681 RSC.
- B. Zhao, B. B. Fan, G. Shao, W. Y. Zhao and R. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 18815–18823 CAS.
- H. Li, Y. Huang, G. Sun, X. Yan, Y. Yang, J. Wang and Y. Zhang, J. Phys. Chem. C, 2010, 114, 10088–10091 CAS.
- P. C. P. Watts, W. K. Hsu, A. Barnes and B. Chambers, Adv. Mater., 2003, 15, 600–603 CrossRef CAS.
- B. Zhao, B. B. Fan, Y. W. Xu, G. Shao, X. D. Wang, W. Y. Zhao and R. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 26217–26225 CAS.
- T. Xia, Y. H. Cao, A. O. Nathan, M. James, L. Liu and X. B. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 10407–10413 CAS.
- Y. P. Wang, D. P. Sun, G. Z. Liu, Y. J. Wang and W. Jing, J. Electron. Mater., 2015, 44, 2292–2299 CrossRef CAS.
- S. B. Ni, X. L. Sun, X. H. Wang, G. Zhou, F. Yang, J. M. Wang and D. Y. He, Mater. Chem. Phys., 2010, 124, 353–358 CrossRef CAS.
- L. B. Cui, Y. Liu, X. H. Wu, Z. Q. Hu, Z. J. Shi and H. J. Li, RSC Adv., 2015, 5, 75817–75822 RSC.
- X. Jian, B. Wu, Y. F. Wei, S. X. Dou, X. L. Wang, W. D. He and N. Mahmood, ACS Appl. Mater. Interfaces, 2016, 8, 22615–22622 Search PubMed.
- H. L. Lv, G. B. Ji, H. Q. Zhang and Y. W. Du, RSC Adv., 2015, 5, 76836–76843 RSC.
- Y. J. Chen, P. Gao, R. X. Wang, C. L. Zhu, L. J. Wang, M. S. Cao and H. B. Jin, J. Phys. Chem. C, 2009, 113, 10061–10064 CAS.
| This journal is © The Royal Society of Chemistry 2016 |