New curcumin-derived ligands and their affinity towards Ga3+, Fe3+ and Cu2+: spectroscopic studies on complex formation and stability in solution†
Abstract
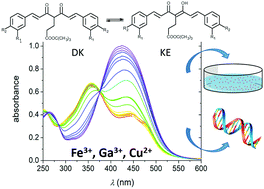
The metal complexing ability in solution of four substituted curcumin (CUR)-derived ligands K3T, originated by the insertion of the –CH2CH2COOtBu branch on the central atom of the diketonic moiety of CUR and related derivatives with variable meta and para substituents (OH, OMe, H, OCOCH3) on the peripheral aromatic rings, is examined. These molecules can act as new chelators with biological properties comparable to those of CUR but with improved stability. In fact, curcuminoids represent new perspectives for the development of novel therapeutic agents for several diseases including Alzheimer's disease. CUR showed neuroprotective properties, and a probable mechanism of its action is related to the complexation ability towards endogenous metal ions Fe3+ and Cu2+. K3T derivatives retain the solvent-dependent diketo–ketoenol tautomerism, with the prevalence of the diketonic form in aqueous solution. They show enhanced stability in simulated physiological conditions (phosphate buffered solution at pH = 7.4) compared to CUR, together with similar or even higher anti-proliferative activity against human colon carcinoma cells HCT116. The addition of the metal ion causes dissociation of the enolic proton creating chelate complexes and shift of the tautomeric equilibrium toward the keto–enol species. The formation of metal complexes was followed and confirmed by both NMR (using Ga3+ as a diamagnetic probe for Fe3+) and UV-visible spectroscopy. All the ligands showed high affinity for Fe3+ and Ga3+, forming M : L 1 : 2 species. In view of therapeutic applications, notable is the good affinity of K3T31, i.e. the ligand bearing only OH groups in para positions of the aromatic rings, for Cu2+, and the ability of the Cu : K3T31 1 : 1 complex to bind to DNA.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...