1D Co(ii) coordination polymers based on cyclobutyl- and cyclopentyl-substituted zoledronate analogues: synthesis, structural comparison, thermal stability and magnetic properties†
Abstract
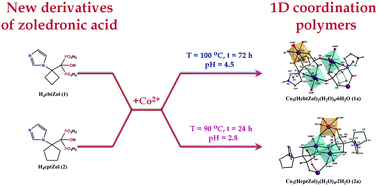
Two novel derivatives of zoledronic acid (1-hydroxy-2-[1-(1H-imidazol-1-yl)cyclobutyl]ethylidene-1,1-diphosphonic acid (H4cbtZol) and 1-hydroxy-2-[1-(1H-imidazol-1-yl)cyclopentyl]ethylidene-1,1-diphosphonic acid (H4cptZol)) were synthesized, crystallized from water solutions as H4cbtZol·H2O (1) and H4cptZol·4H2O (2), and characterized by single-crystal X-ray diffraction. The reactions of H4cbtZol and H4cptZol with acetate or sulphate Co(II) salts, carried out under hydrothermal conditions, afforded Co3(HcbtZol)2(H2O)6·6H2O (1a), being isomorphous with recently reported Co(II)/Ni(II) complexes based on HdmtZol3− and HcppZol3− anions, and Co3(HcptZol)2(H2O)4·2H2O (2a). Both 1a and 2a were characterized by means of X-ray crystallography, IR and NIR-Vis-UV spectroscopic methods. Furthermore, their thermal stabilities and magnetic properties were compared. Compounds 1a and 2a comprise 1D polymeric chains with crystallographically and spectroscopically distinct six-coordinated Co1 and Co2 centers, which differ in architectures. In 1a, the chains feature alternately arranged [Co2(HcbtZol)(H2O)2]2 and {Co1O6} units. The chains of 2a are constructed from dinuclear [Co2(HcptZol)(H2O)2]2 units built up from symmetry related edge-sharing {Co2O6} octahedrons, extended by corner-sharing {Co1O6} octahedrons, which results in higher rigidity imposed on trinuclear units and shorter Co2⋯Co2 and Co1⋯Co2 distances as compared to 1a. The crystal field parameters Dq, Ds, Dt and B of 798 cm−1, 450 cm−1, −75 cm−1 and 857 cm−1 for 1a and 644 cm−1, 509 cm−1, −146 cm−1 and 902 cm−1 for 2a revealed that the {Co2O6} octahedron of 2a exhibits a larger tetragonal distortion as compared to 1a, despite the nearly identical values of the tetragonality T parameter (1.07 for 2a and 1.06 for 1a). These differences are reflected in the thermal and magnetic behaviors of 1a and 2a. In particular, compound 2a is more stable than 1a, retaining thermal stability up to 208 °C. On the other hand, its dehydration is a one-stage process, accompanied by simultaneous destruction of its 1D architecture and immediate elimination and decomposition of ligands. The analysis of magnetic properties revealed the existence of competing interactions, with the predominant participation of antiferromagnetic interactions in 1a (spin-3/2 Heisenberg trimer chain) and ferromagnetic interactions in 2a (spin-1/2 Ising diamond chain).
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...