Near-infrared electrochemiluminescence from non-toxic CuInS2 nanocrystals†
Abstract
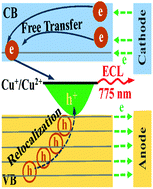
Copper indium sulfide (CIS) nanocrystals (NCs) are a promising solution to the toxicity issue of Cd- and Pb-based NCs. Herein, electrochemical redox-induced radiative charge transfer in p-type CIS NCs was explored for the first time by electrochemiluminescence (ECL). The CIS NCs displayed a weak reductive process for injecting electrons into the conduction band (CB) and four strong oxidative processes for injecting holes into the valence band (VB). Potential-resolved ECL demonstrated that the electrochemically injected CB electrons were stable in the CIS NCs and could recombine with Cu2+ defects (preexisting in the CIS NCs and/or generated via electrochemically oxidizing the CIS NCs) for radiative charge transfer in the CIS NCs. Annihilation ECL confirmed that all the highly mobile VB holes generated via electrochemical oxidation at different potentials could be rapidly re-localized by Cu+ to form Cu2+ defects and then couple with electrochemically injected CB electrons for near-infrared ECL of the same excited states. Coreactant ECL demonstrated that simultaneously injecting VB holes and CB electrons into CIS NCs could improve their radiative charge transfer efficiency for efficient ECL. The CIS NCs are promising electrochemiluminophores; ECL provides an effective alternative for investigating radiative charge transfer in CIS NCs upon photoexcitation.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...