Analysis of the structures, stabilities and electronic properties of MB16− (M = V, Cr, Mn, Fe, Co, Ni) clusters and assemblies†
Abstract
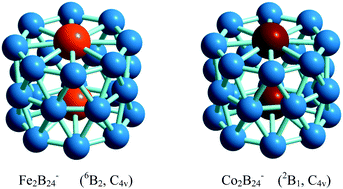
Doping of a cluster has often been used to stabilize and modify the structure and properties of the resulting doped cluster. In the current work, we reported the structures, stabilities and electronic properties of 3d transition metal (V, Cr, Mn, Fe, Co, Ni) atoms doped B16− clusters using CALYPSO searching method and density functional theory. The drum-shaped MB16− are favored for M = V, Cr, Mn, Fe and Co with high symmetry of C2v, C4v, C4v, D8d and D8d symmetry respectively, while M = Ni with Cs symmetry exhibits an open bowl-shaped structure. The infrared spectra, Raman spectra and photoelectron spectra are predicted and can be used to identify the structures of these isomers from experiments. The stabilities are discussed by analyzing the binding energy, substitution reaction, HOMO–LUMO gaps and chemical hardness. In addition, electronic properties are analyzed by calculating the charge transfer, magnetic properties and chemical bonding. Finally, with the assembly of FeB16− and CoB16− drum-shaped clusters, two different Fe2B24− and Co2B24− clusters, which are structurally characterized as tubular forms whose triple rings are composed of three eight-membered boron rings, are identified in a high symmetry C4v, respectively. Subsequently, the magnetic properties are investigated. The present results open a door for the discovery of more drum-shaped assemblies.


 Please wait while we load your content...
Please wait while we load your content...