Long-term chemothermal stability of delithiated NCA in polymer solid-state batteries†
Abstract
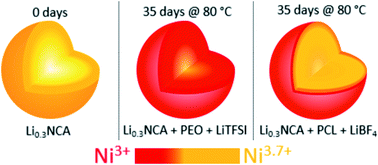
In this study, the long-term chemothermal stability of chemically delithiated Li0.3Ni0.8Co0.15Al0.05O2 (Li0.3NCA) was systematically investigated at relevant operating temperatures of polymer solid-state batteries using ex situ synchrotron-based hard and soft X-ray absorption spectroscopy. The reduction of nickel on the surface, subsurface, and in the bulk of secondary NCA particles was studied and directly related to aging time, temperature, the presence of polymeric electrolyte (poly(ethylene oxide) or polycaprolactone), and lithium salt (lithium tetrafluoroborate or lithium bis(trifluoromethanesulfonyl)imide). Depending on the polymer and/or lithium salt accompanying the delithiated Li0.3NCA, reduction of nickel at the surface, subsurface, and bulk occurs to varying extents, starting at the surface and propagating into the bulk material. Our results indicate how degradation (reduction of nickel) is strongly correlated to temperature, time, and the presence of blended polymer and/or lithium salt in the cathode. The relative stability of the NCA material in cathodes having different polymer and lithium salt combinations identified in the ex situ spectroscopy study is directly demonstrated in solid-state polymer batteries.


 Please wait while we load your content...
Please wait while we load your content...
