Speciation and reactivity of heptavalent technetium in strong acids†
Abstract
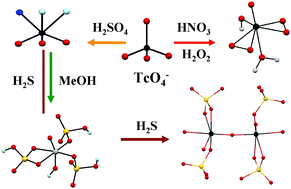
Technetium is the workhorse of the radiopharmaceutical imaging agents; it is also an important byproduct of the nuclear industry. Studies of the chemistry of Tc in strong acids are relevant to nuclear applications, environmental remediation and fundamental chemistry. Nitric acid is used at the industrial level for spent fuel reprocessing while H2SO4 and HClO4 are used at the laboratory scale for Tc separation from Mo or U. During reprocessing activities, radiolysis products from nitric acid (e.g., H2O2) and from organics extracting agents (e.g., alcohols) are formed and these might interact with Tc(VII). An understanding of Tc(VII) chemistry in the presence of H2O2 and/or organics in acidic solution is important to predict its behavior in separation processes. Concerning environmental remediation, sulfur has been proposed to immobilize “Tc2S7”. Technetium heptasulfide can be obtained from the reaction of Tc(VII) with H2S gas in acidic solutions. A better understanding of “Tc2S7” formation could give information to predict its formation and behavior in the environment. Under oxidizing conditions, the aqueous chemistry of Tc(VII) is dominated by TcO4−. In high acid concentrations, pertechnetic acid can be formed and control the reactivity of Tc. Speciation data on Tc(VII) in concentrated acids are still parse, and the structure and reactivity of pertechnetic acid are unknown. Here, the speciation of Tc(VII) in sulfuric, nitric and perchloric acids and its reactivity with H2O2, methanol and H2S is reviewed. Experimental results and density functional calculations show the formation of TcO3(OH)(H2O)2 in concentrated H2SO4 and HClO4. In 12–13 M H2SO4, Tc(V) species and Tc(IV) polymeric species were respectively detected in the presence of methanol and H2S. Finally, peroxo pertechnetic acid was identified in nitric and sulfuric acid in the presence of H2O2.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...
