Multi-color perovskite nanowire lasers through kinetically controlled solution growth followed by gas-phase halide exchange†
Abstract
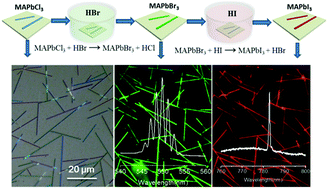
Integration of multi-color semiconductor nanowire lasers (NWLs) on a silicon substrate is a very challenging task, owing to both the material lattice mismatch and the incompatible growth temperature. Recently, organic–inorganic perovskite (CH3NH3PbX3; X = Cl, Br, I) NWLs have been developed using a surface-initiated solution-growth method, which, however, requires post-synthesis transfer of nanowires from a growth substrate to a silicon wafer for device fabrication. Herein, we report multi-color perovskite nanowire lasers on arbitrary substrates (silicon or quartz substrates) through kinetically controlled growth followed by gas-phase halide exchange. First, we developed an antisolvent–vapor-diffusion induced crystallization method to kinetically direct the growth of CH3NH3PbCl3 towards single-crystal nanowires rather than the crystal habit of plate-like morphology. The ratio of nanowires to square microplates was adjusted to be as high as 97% : 3%. Then we introduced a gas-phase halide-anion-exchange reaction to convert chloride nanowires into bromide and iodide ones upon exposure to the vapor of HX (X = Br, I), while preserving both the high crystallinity and the nanowire morphology. Upon optical excitation, Fabry–Perot lasing around 550 and 785 nm occurs from CH3NH3PbBr3 and CH3NH3PbI3 nanowires with an onset of 9.8 and 9.2 μJ cm−2, respectively, with a maximum quality factor of 1260.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...