Ultrafast excited-state dynamics of isocytosine†
Abstract
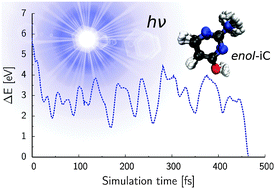
The alternative nucleobase isocytosine has long been considered as a plausible component of hypothetical primordial informational polymers. To examine this hypothesis we investigated the excited-state dynamics of the two most abundant forms of isocytosine in the gas phase (keto and enol). Our surface-hopping nonadiabatic molecular dynamics simulations employing the algebraic diagrammatic construction to the second order [ADC(2)] method for the electronic structure calculations suggest that both tautomers undergo efficient radiationless deactivation to the electronic ground state with time constants which amount to τketo = 182 fs and τenol = 533 fs. The dominant photorelaxation pathways correspond to ring-puckering (ππ* surface) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching/N–H tilting (nπ* surface) for the enol and keto forms respectively. Based on these findings, we infer that isocytosine is a relatively photostable compound in the gas phase and in these terms resembles biologically relevant nucleobases. The estimated S1
O stretching/N–H tilting (nπ* surface) for the enol and keto forms respectively. Based on these findings, we infer that isocytosine is a relatively photostable compound in the gas phase and in these terms resembles biologically relevant nucleobases. The estimated S1 ![[radiolysis arrow - arrow with voltage kink]](https://www.rsc.org/images/entities/char_e116.gif) T1 intersystem crossing rate constant of 8.02 × 1010 s−1 suggests that triplet states might also play an important role in the overall excited-state dynamics of the keto tautomer. The reliability of ADC(2)-based surface-hopping molecular dynamics simulations was tested against multireference quantum-chemical calculations and the potential limitations of the employed ADC(2) approach are briefly discussed.
T1 intersystem crossing rate constant of 8.02 × 1010 s−1 suggests that triplet states might also play an important role in the overall excited-state dynamics of the keto tautomer. The reliability of ADC(2)-based surface-hopping molecular dynamics simulations was tested against multireference quantum-chemical calculations and the potential limitations of the employed ADC(2) approach are briefly discussed.
- This article is part of the themed collection: Prebiotic chemistry and the molecular origins of life


 Please wait while we load your content...
Please wait while we load your content...