Recent advances in nanophotosensitizers for overcoming tumor hypoxia in photodynamic therapy
Dongjie
Li
a,
Sa
Wang
a,
Chuang
Zhang
a,
Yueyun
Fan
a,
Fang
Fang
 *a,
Menglin
Li
*b and
Jinfeng
Zhang
*a,
Menglin
Li
*b and
Jinfeng
Zhang
 *a
*a
aSchool of Life Science, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: f.fang@nus.edu.sg; jfzhang@bit.edu.cn
bSchool of Physical Science and Engineering, Beijing Jiaotong University, Beijing 100091, P. R. China. E-mail: menglinli90@bit.edu.cn
First published on 1st December 2025
Abstract
Photodynamic therapy (PDT) has garnered considerable attention due to its remarkable spatiotemporal selectivity, minimal invasiveness, and low potential for drug resistance, making it a widely utilized therapeutic modality for various tumors in clinical practice. However, the hypoxic tumor microenvironment (TME), resulting from accelerated tumor cell proliferation and inadequate oxygen (O2) supply, significantly impedes the therapeutic efficacy of PDT. Furthermore, the O2 consumption during PDT exacerbates tumor hypoxia, which in turn accelerates tumor progression and contributes to suboptimal therapeutic outcomes. To mitigate this challenge, recent advancements in nanotechnology have facilitated the development of nano-photosensitizers (nano-PSs) capable of alleviating hypoxic TME through a variety of strategies. This review provides an overview of recent advancements in PDT strategies aimed at overcoming tumor hypoxia, which encompass: (1) alleviating hypoxia, (2) utilizing hypoxia, (3) regulating the hypoxic TME, and (4) designing type-I PSs. Through a review of recent advancements, this work seeks to offer insights into the design of nano-PSs that can mitigate hypoxia-related limitations in PDT, while also highlighting future opportunities and challenges for clinical translation.
1. Introduction
Photodynamic therapy (PDT), a non-invasive therapeutic modality characterized by high spatiotemporal selectivity, exceptional efficacy, and minimal drug resistance, has been widely implemented in the clinical treatment of tumors for over four decades.1–4 PDT is widely recognized for its reliance on the synergistic interaction of three key components: a photosensitizer (PS), light of a specific wavelength, and molecular oxygen (O2).5–8 Upon light activation, PSs generate reactive oxygen species (ROS) via both Type-I and Type-II photochemical pathways. The resulting oxidative stress induces tumor cell death, disrupts tumor vasculature, and stimulates antitumor immune responses, establishing PDT as a powerful cancer treatment (Fig. 1).9–11 In the Type-I pathway, the excited triplet state of the PS undergoes electron or hydrogen transfer with cellular substrates, resulting in the generation of highly reactive radical species. These radicals promptly interact with water or molecular O2, leading to the formation of secondary ROS, including hydrogen peroxide (H2O2), hydroxyl radicals (˙OH), and superoxide anions (O2˙−).12–14 Notably, in the Type-II pathway, which is considered to be dominant in clinically approved PSs, triplet–triplet energy transfer between the T1 state and molecular O2 generates cytotoxic singlet oxygen (1O2). Therefore, O2 is crucial for the antitumor efficacy of Type-II PDT, as it directly governs the generation of cytotoxic 1O2.15–17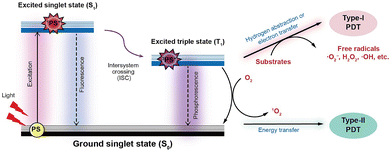 | ||
| Fig. 1 Schematic illustration of the Jablonski diagram depicting type I and type II photochemical pathways in PDT. | ||
Despite significant progress in PDT research, its clinical translation remains hindered, primarily due to the challenges imposed by the hypoxic tumor microenvironment (TME).18 Hypoxia, a defining characteristic of solid tumors resulting from rapid growth, significantly impedes the efficacy of Type-II PDT.19 Correspondingly, rapid tumor proliferation and aberrant vasculature hinder O2 delivery, which is further exacerbated by decreased hemoglobin (Hb) levels, ultimately limiting the generation of cytotoxic 1O2.20 Furthermore, abundant clinical evidence demonstrates that hypoxia compromises genomic stability and modifies proteomic profiles in tumor cells, with stabilization of hypoxia-inducible factor-1 (HIF-1) driving transcriptional programs that promote glycolytic reprogramming, angiogenesis, and extracellular matrix (ECM) remodeling, thereby facilitating tumor progression and enabling adaptation within the hostile microenvironment.21 Therefore, the development of novel strategies to overcome the limitations imposed by hypoxia in PDT presents substantial potential for advancing tumor treatment.
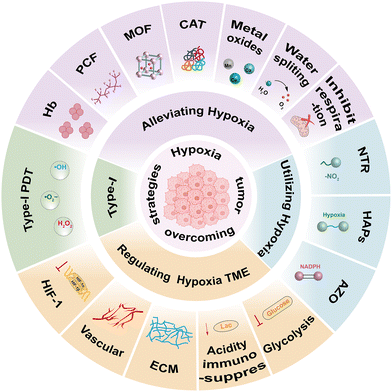
Fortunately, recent advancements in nanotechnology have enabled the development of next-generation nano-photosensitizers (nano-PSs). These systems not only incorporate traditional PSs but also integrate functional components specifically designed to remodel the hypoxic TME.22–24 By rationally combining PSs with O2 carriers, catalytic agents, metal oxides, hypoxia-responsive materials or metabolic modulators, researchers have developed multifunctional nano-assemblies. These systems are precisely engineered to overcome O2 limitations and enhance the efficacy of PDT under tumor-specific conditions.25 To date, strategies for designing nano-PSs can be classified into four interconnected strategies: (1) alleviating hypoxia. These strategies primarily focus on exogenous O2 delivery, endogenous O2 generation, inhibition of tumor cellular respiration to enhance tumor oxygenation and, reprogramming of energy metabolism to alleviate hypoxic TME. (2) Utilizing hypoxia. This category primarily pertains to hypoxia-responsive nano-PSs, which selectively activate therapeutics within the hypoxic TME via distinct bioreduction mechanisms. (3) Regulating the hypoxic TME. This category encompasses nano-PSs that disrupt glycolysis, adjusting acidity, degrade the tumor ECM, normalize tumor blood vessels and modulate hypoxia-inducible factor-1 (HIF-1) expression. (4) Designing type-I nano-PSs. This category primarily focuses on the use of nano-PSs to generate free radicals, such as hydroxyl radicals (˙OH), superoxide anions (O2˙−), lipid hydroxyl radicals (LOO˙), carbon radicals (˙C), and others (Fig. 2). Therefore, this review concentrates on strategies for designing nano-PSs to address the challenges posed by hypoxia in the TME, examining current obstacles, and exploring future research directions and opportunities for nano-PSs in hypoxia-targeted tumor therapy and clinical applications.
2. Alleviating hypoxia
2.1. Exogenous O2 delivery
Direct delivery of exogenous O2 into the hypoxic TME represents one of the most widely adopted strategies to alleviate hypoxia and enhance the therapeutic efficacy of PDT.26 In recent years, they have received attention in drug delivery, overcoming hypoxia, improving PDT, and other fields. Nano-PSs can be designed to either carry O2 directly or be combined with O2-carrying systems, such as hemoglobin (Hb), perfluorocarbons (PFCs), and metal–organic frameworks (MOFs).27–29 These strategies improve intratumoral O2 delivery, reduce systemic toxicity and side effects, and ultimately potentiate the antitumor effects of PDT.Hb is a natural O2 carrier that binds and releases O2 efficiently via the Bohr effect.30 But it easily undergoes autooxidation in circulation, reducing its O2 capacity and causing toxicity.31 To overcome these limitations, Hb is co-assembled with PSs into nano-PSs to prevent oxidation, extend circulation time, and improve O2 delivery efficiency. For instance, Li et al. developed a balloon-like nano-PS (Ce6-Hb-FA@Lip, CHFL) that utilizes Hb as a natural O2 carrier co-encapsulated within folate-modified liposomes to enhance PDT (Fig. 3a).32 In this system, Hb retains its intrinsic O2-binding capacity while benefiting from the vesicle structure, which incorporates cholesterol and PEG to improve O2 affinity, membrane permeability, and circulation stability. And the system achieved substantial O2 generation, reaching ∼5 mg L−1 within 180 s, thereby significantly enhancing the efficacy of PDT (Fig. 3b). This design effectively overcomes the typical drawbacks of free Hb, such as rapid oxidation, poor stability, and associated toxicity.
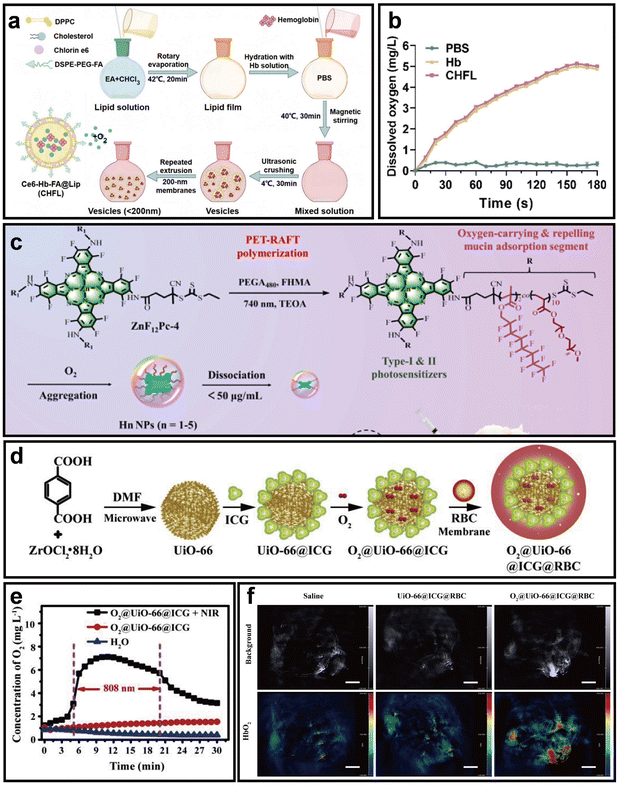 | ||
| Fig. 3 Representative exogenous O2-delivering nano-PSs. (a) Schematic of CHFL preparation via film hydration. (b) Time-dependent dissolved O2 changes in hypoxic solution after adding CHFL. Reprinted with permission from ref. 32. Copyright 2024, Elsevier. (c) Design and mechanism of fluorinated phthalocyanine micelles enabling Type I/II PDT in hypoxic tumors. Reprinted with permission from ref. 34. Copyright 2024, Elsevier. (d) Schematic of O2@UiO-66@ICG@RBC preparation. (e) O2 release of O2@UiO-66@ICG under 808 nm irradiation. (f) In vivo photoacoustic imaging showing tumor oxygenation after treatment with different formulations. Scale bar = 3 mm. Reprinted with permission from ref. 37. Copyright 2018, Elsevier. | ||
In addition to Hb, PFCs possess high O2 solubility and biocompatibility.33 Thus, co-delivering PSs and O2 using PFCs offers a promising way to enhance the efficacy of PDT. To address the challenge of limited O2 availability in the hypoxic TME, Zhang et al. developed dually fluorinated unimolecular nano-PSs (Hn NPs) that use PFCs as efficient O2 carriers, co-encapsulated with fluorinated phthalocyanine for tumor-targeted PDT (Fig. 3c).34 PFCs offer high O2-carrying capacity, overcoming stability and solubility issues of conventional carriers. Upon NIR light irradiation, PFCs release O2, enhancing 1O2 production and alleviating tumor hypoxia, thereby inducing tumor cell death.
Furthermore, MOFs with high surface area and tunable porosity serve as ideal nanocarriers for gas storage and delivery.35 Owing to their versatile and biodegradable structures, MOFs have been widely used for O2 delivery to relieve the hypoxic TME.36 To verify this concept, Gao et al. developed a biomimetic O2-evolving nano-PS containing a zirconium-based MOF (UiO-66@O2@ICG@RBC) for exogenous O2 delivery (Fig. 3d).37 In this design, UiO-66 served as a high-capacity O2 carrier and was functionalized with ICG as a PS. To prolong systemic circulation and evade immune clearance, the nano-PS was further camouflaged with red blood cell (RBC) membranes. Upon 808 nm laser irradiation, the photothermal effect of ICG triggered rapid O2 release from UiO-66, alleviating tumor hypoxia (Fig. 3e). The restored oxygenation was confirmed via photoacoustic imaging (Fig. 3f). Enhanced O2 generation demonstrate significantly improved the efficacy of PDT under hypoxic TME.
Exogenous O2 delivery systems provide an effective means to alleviate tumor hypoxia and enhance the efficacy of PDT. Hb-based nano-PSs offer biomimetic O2 transport with improved stability, PFCs ensure high O2 solubility and rapid release, while MOFs combine large storage capacity with multifunctional design flexibility. Together, these strategies demonstrate how nanotechnology enables precise O2 regulation within the TME, thereby improving ROS generation and therapeutic outcomes.
2.2. O2 generation in Situ
Although nano-PSs with Hb, PFCs or other carriers can deliver O2 to tumors, their low efficiency and potential ROS toxicity limit their ability to relieve hypoxia.38In situ O2 generation provides a more efficient way to relieve hypoxia than exogenous delivery, avoiding transport loss.39–41A common strategy uses catalase (CAT) to decompose tumor H2O2, generating O2 to relieve hypoxia. CAT, a natural enzyme, efficiently converts excess H2O2 (100 µM to 1 mM) in TME into O2 to mitigate hypoxia.42 For example, Huang et al. engineered ultra-acid-sensitive nano-PS (HSA/CAT-PEPA) by co-assembling CAT and human serum albumin (HSA) with a pH-responsive polymer (PEPA), aiming to reverse tumor hypoxia (Fig. 4a).43 Within the acidic TME, HSA/CAT-PEPA disassemble to facilitate lysosomal escape and deep tissue penetration, ensuring efficient release of both O2 and Ce6. CAT retains over 90% enzymatic activity and converts endogenous H2O2 into O2, thereby alleviating hypoxia. HSA confers thermal and proteolytic stability to CAT, while also prolonging systemic circulation. This rational design enables targeted O2 self-supply and PS delivery with minimal off-target effects, offering a potent strategy for overcoming hypoxia-associated resistance in PDT.
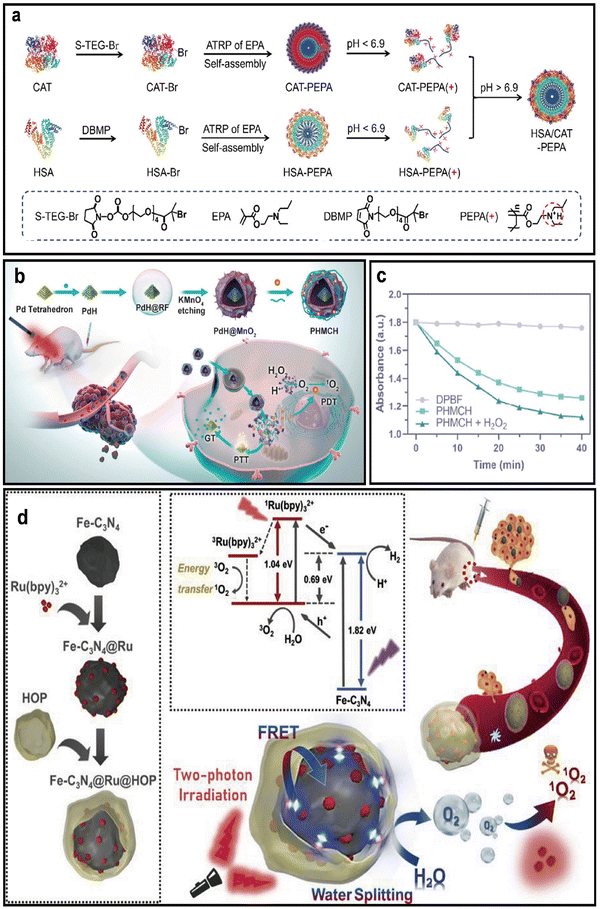 | ||
| Fig. 4 Representative endogenous O2-generating nano-PSs. (a) Synthesis and pH-responsive behavior of CAT-PEPA and HSA-PEPA. Reprinted with permission from ref. 43. Copyright 2024, American Chemical Society. (b) Schematic of the synthesis and O2/PTT/PDT synergistic mechanism of PHMCH. (c) DPBF absorbance decay at 410 nm in PHMCH with/without H2O2 under 650 nm light. Reprinted with permission from ref. 45. Copyright 2022, American Chemical Society. (d) Two-photon excited O2-evolving nano-PS (FCRH) for efficient PDT against hypoxic tumors, showing electron localization between Fe-C3N4 and Ru(bpy)32+. Reprinted with permission from ref. 48. Copyright 2019, Elsevier. | ||
Another endogenous delivery strategy involves metal oxides that enhance O2 generation via chemical reactions.44 To confirm this concept, Wang et al. developed a multifunctional nano-PS (PHMCH), composed of hydrogen-loaded palladium hydride (PdH) tetrahedrons, a MnO2 nanoshell, the PS Ce6, and a HA coating for tumor targeting (Fig. 4b).45 Within this system, MnO2 functions as a catalase mimic, decomposing H2O2 under acidic TME to generate O2 as follow:
| MnO2 + H2O2 + 2H+ → Mn2+ + 2H2O + O2↑ |
Dissolved O2 measurements confirmed that PHMCH rapidly increased O2 levels in solution upon addition of H2O2, demonstrating robust catalytic activity essential for alleviating hypoxia (Fig. 4c).
In addition to the above two strategies, water-splitting technology is an interested strategy for endogenous O2 delivery and has gained attention for its potential in solar energy storage and clean hydrogen production.46 Its involves three key components: an energy input, a catalyst, and water. In this process, photoexcited electron–hole pairs drive redox reactions, where electrons reduce water to generate H2, while holes oxidize water to produce O2 and ˙OH. The water splitting process can be simplified as follows:
| 2H2O → O2 + 4H+ + 4e− |
| 4H+ + 4e− → 2H2 |
These half-reactions represent the overall photocatalytic process, where water is oxidized to O2 and reduced to hydrogen under appropriate catalytic and energy conditions.47 According to its unique mechanism, Li et al. developed a two-photon excited nano-PS (Fe-C3N4@Ru@HOP, FCRH) composed of Fe-C3N4, a PS (Ru(bpy)32+), and a hyperbranched PEGylated copolymer (HOP) (Fig. 4d).48 This nano-PS enables in situ endogenous O2 generation through two-photon-activated water splitting, where Ru(bpy)32+ injects electrons into Fe-C3N4 to enhance photocatalysis and promote O2 evolution. Notably, under two-photon irradiation (800 nm), FCRH raised dissolved O2 levels from ∼0.2 to 3.26 mg L−1 in 10 minutes, significantly surpassing controls. Hypoxia alleviation was further validated in vitro using ROS-ID fluorescence imaging, which showed a marked reduction in red hypoxia signals and increased intracellular 1O2 production, ultimately enhancing PDT-induced cytotoxicity in hypoxic TME.
In situ O2 generation offers a superior approach to relieve tumor hypoxia compared with exogenous O2 delivery, as it enables continuous O2 production within the hypoxic TME and avoids diffusion loss. CAT and MnO2-based nano-PSs efficiently decompose endogenous H2O2, while advanced photocatalytic systems such as Fe-C3N4@Ru@HOP achieve deep-tissue O2 evolution through two-photon excitation. These designs significantly enhance ROS production under hypoxic conditions. However, their catalytic durability, biosafety, and energy conversion efficiency remain major concerns for in vivo applications. Future research should emphasize optimizing catalytic kinetics, improving structural stability, and enhancing biocompatibility to achieve controllable, sustained O2 generation and promote the clinical translation of endogenous O2-supplying nano-PSs for effective hypoxia-targeted PDT.
2.3. Inhibit respiration
To alleviate tumor hypoxia, exogenous O2 delivery and endogenous O2 generation are key strategies. However, their effects are frequently transient due to limited O2 loading, leakage and low generation efficiency.49,50 It is noteworthy that inhibiting cellular aerobic respiration by damaging mitochondria is a canonical strategy to reduce O2 consumption. This approach alleviates tumor hypoxia and enhances the efficacy of O2-dependent therapies.51 Several FDA-approved drugs reduce O2 consumption by targeting components of the mitochondrial electron transport chain, thereby alleviating hypoxic TME. For instance, Huang et al. developed a multifunctional nano-PS (SNP@Ato), which integrates ATO with a PS (TPP-CD4) to enhance the efficacy of PDT by inhibiting mitochondrial respiration (Fig. 5a).52 ATO inhibits mitochondrial complex III respiration, reducing O2 consumption. This inhibition preserves O2 for 1O2 generation. Thus, hypoxia was alleviated, indicating effective tumor oxygenation and enhanced therapeutic outcomes.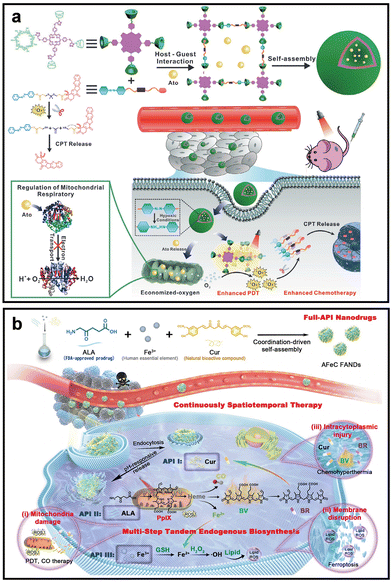 | ||
| Fig. 5 Representative nano-PSs inhibiting mitochondrial respiration to alleviate tumor hypoxia. (a) Schematic of SNP@Ato preparation and its combined PDT/CDT mechanism. Reprinted with permission from ref. 52. Copyright 2025, John Wiley & Sons. (b) Schematic of AFeC FANDs-triggered CO biosynthesis for PDT. Reprinted with permission from ref. 54. Copyright 2023, Springer Nature. | ||
Similarly, gaseous small molecules have been reported to suppress cellular O2 consumption by inhibiting the mitochondrial respiratory chain, thereby reducing O2 utilization and alleviating hypoxic TME.53 To validate the efficacy of gaseous molecules, our group designed a full-active pharmaceutical ingredient nano-PS (AFeC FANDs) that utilizes endogenous biosynthesis to generate CO from 5-aminolevulinic acid (ALA) via the PpIX–heme metabolic pathway (Fig. 5b).54 The biosynthesized CO selectively inhibits mitochondrial respiration by disrupting complex IV activity, thereby reducing O2 consumption in tumor cells. This CO-mediated suppression of cellular O2 utilization effectively alleviates hypoxia, as demonstrated by enhanced ROS fluorescence signals in tumor models, resulting in reinforced antitumor efficacy under hypoxic TME.
Inhibiting mitochondrial respiration offers an effective and sustainable strategy to alleviate tumor hypoxia by directly reducing O2 consumption rather than relying on external O2 supply. By targeting key sites of the electron transport chain, agents such as ATO or CO selectively suppress mitochondrial activity, thereby preserving O2 for ROS generation during PDT. This mechanism not only improves O2 utilization efficiency but also enhances therapeutic outcomes under hypoxic conditions. Compared with O2-delivery systems, respiration inhibition provides longer-lasting hypoxia relief and greater metabolic stability. Nevertheless, potential mitochondrial toxicity and off-target effects require careful evaluation. Future efforts should emphasize tumor-specific delivery, controlled drug activation, and integration with multifunctional nano-PS platforms to achieve precise, safe, and durable regulation of tumor O2 metabolism for optimized PDT efficacy.
3. Utilizing hypoxia
In addition to ameliorating tumor hypoxia, the characteristics of tumor hypoxia can also be exploited to overcome the hypoxia TME. hypoxia-responsive nano-PSs are the potential candidates for constructing smart systems that exhibit functions after receiving hypoxic stimulation.55 The current hypoxia-responsive nano-PSs systems mainly rely on the design with nitroreductase (NTR) compounds, hypoxia-activated prodrugs (HAPs) and azobenzene (AZO)-based NPs.56NTR, a flavin-dependent enzyme overexpressed in hypoxic tumors, utilizes NAD(P)H to reduce nitroaromatic or nitroimidazole groups into amines.57 This bioreduction process serves as a hypoxia-responsive trigger for fluorescence activation or controlled drug release, and has been widely applied in the design of hypoxia-responsive nanomaterials for imaging, drug delivery, and prodrug activation.58 Based on this principle, Zhu et al. designed a NTR-activatable and self-immobilizing PS CyNT-F encapsulated within micelles nano-PS (CyNT-F@PDPA).59 The nitrobenzene moiety on CyNT-F is selectively reduced by overexpressed NTR in hypoxic tumor cells, triggering the generation of reactive quinone methide, which covalently binds to intracellular proteins. This covalent immobilization not only ensures long-term retention of the PS at the tumor site but also enhances ROS generation (Fig. 6a). This strategy achieved specific activation of CyNT-F and effective ROS production in low-O2 conditions, thereby demonstrating superior PDT efficacy and tumor growth inhibition under a hypoxic TME compared to noncovalent controls.
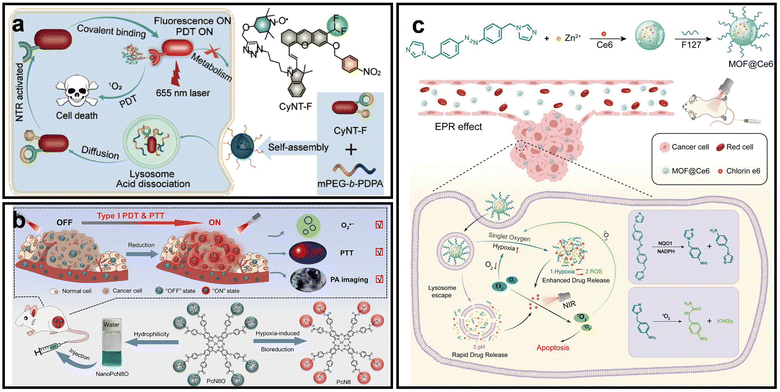 | ||
| Fig. 6 Representative utilizing nano-PSs to relieve hypoxia. (a) Schematic of NTR-activated self-immobilizing PS for PDT of hypoxic tumors. Reprinted with permission from ref. 59. Copyright 2024, American Chemical Society. (b) Schematic of hypoxia-triggered bioreduction of PcN8O for PDT and PTT with controlled ON–OFF switching. Reprinted with permission from ref. 62. Copyright 2025, John Wiley & Sons. (c) Schematic of hypoxia/ROS/pH triple-responsive MOF nanocarriers for enhanced PDT. Reprinted with permission from ref. 64. Copyright 2023, John Wiley & Sons. | ||
The hypoxic TME enables tumor-targeted therapy using HAPs, which are selectively reduced by intracellular oxidoreductases into cytotoxic intermediates that induce DNA damage or inhibit key enzymes.60 Moreover, N-oxide is extensively studied as a hypoxia responsive structure, more than 30 types of N-oxide compounds, including aromatic and aliphatic N-oxides, have been developed as HAPs.61 Recently, Zhao et al. developed a hypoxia-activated nano-PS (NanoPcN8O) based on a hydrophilic zinc(II) phthalocyanine derivative bearing N-oxide groups, which enables selective activation in hypoxic tumor regions (Fig. 6b).62 Under low O2 conditions, NanoPcN8O is bioreduced by CYP450 enzymes and NADPH to NanoPcN8, restoring photoactivity and turning on PDT and PTT effects. The bioreduction was validated by DLS, 1H NMR, and absorption spectra, while ROS generation under hypoxia was confirmed using DCFH-DA. This design enables highly specific and efficient PDT in hypoxic tumors with minimal off-target toxicity.
AZO-based nano-PSs are hypoxia-responsive carriers whose backbones undergo reductive cleavage under hypoxia conditions, enabling stimulus-triggered drug release.63 In 2023, Chen et al. designed a triple-responsive nano-PS (MOF@Ce6) incorporating AZO-containing imidazole ligands and Ce6 (Fig. 6c).64 This nano-PS responds to hypoxia, ROS, and pH, ensuring on-demand release of Ce6 in the TME. Under hypoxic conditions, azoreductase reduces the AZO groups, triggering nanocarrier disassembly and the depletion of antioxidants like GSH, which further enhances ROS production. The pH-responsive imidazole ionization facilitates lysosomal escape and efficient Ce6 release in acidic environments. In vivo studies confirmed the enhanced cytotoxicity and superior tumor inhibition compared to non-responsive systems.
Hypoxia-responsive nano-PSs represent a promising strategy that transforms the challenge of tumor hypoxia into a therapeutic advantage. By exploiting hypoxia-specific enzymatic or reductive reactions, these systems achieve precise activation and controlled therapeutic release exclusively within low-O2 regions, minimizing off-target effects. NTR-based designs enable selective fluorescence activation and long-term PS retention, while hypoxia-activated prodrugs such as N-oxide derivatives restore photoactivity through enzymatic bioreduction. In parallel, AZO-linked frameworks provide multi-responsive capabilities, coupling hypoxia-triggered cleavage with pH and ROS responsiveness for synergistic therapeutic amplification. Collectively, these smart nano-PSs significantly enhance treatment selectivity and efficacy under hypoxic conditions. Nonetheless, challenges remain in ensuring rapid activation kinetics, structural stability, and clinical scalability. Future work should prioritize integrating multi-stimuli responsiveness, optimizing pharmacokinetics, and achieving reproducible activation thresholds to fully realize the clinical potential of hypoxia-triggered PDT.
4. Regulating the hypoxic TME
To enhance the therapeutic efficacy of nano-PSs against hypoxic tumors, various strategies have been developed to engineer nanoparticle-based delivery systems that modify the hypoxic TME.65 The TME is characterized by low O2 levels, dysregulated glycolysis, abnormal vasculature, dense and stiff ECM, acidic pH, and elevated interstitial fluid pressure (IFP).66–68 These factors collectively impair O2 diffusion, limit PS penetration and suppress immune responses, thereby reducing the efficacy of PDT and prompting the development of strategies to alleviate hypoxia and enhance therapeutic outcomes.4.1. Interfering with glycolysis
It is well known that normal cells rely on mitochondrial oxidative phosphorylation (OXPHOS) for ATP production, while tumor cells primarily depend on aerobic glycolysis, even in O2-rich environments, due to the Warburg effect.69 Since tumor cells depend heavily on glycolysis for energy, nano-PSs loaded with glycolysis inhibitors can disrupt glucose metabolism signaling, effectively starving tumor cells and suppressing their proliferation.70 With the help of glycolysis inhibitors, Lei et al. constructed a pH-sensitive covalent-organic polymer (COP) nano-PS (TVW) integrating porphyrin as a PS and vitamin K3 (VK3) as a glycolysis inhibitor to alleviate tumor hypoxia (Fig. 7a).71 VK3 inhibits pyruvate kinase activity, thereby reducing ATP production and O2 consumption in tumor cells. This O2-preserving effect was confirmed by decreased intracellular ATP levels and O2 consumption rate (Fig. 7b). Consequently, TVW significantly elevated ROS generation under laser irradiation, validating that glycolysis inhibition is an effective strategy to relieve hypoxia and potentiate O2-dependent PDT. | ||
| Fig. 7 Representative nano-PSs alleviating hypoxia by interfering glycolysis. (a) Schematic of TVW preparation and its mechanism for enhancing photodynamic immunotherapy by relieving hypoxia and immunosuppression. (b) ATP levels and O2 consumption rates in Hepa1–6 cells after various treatments. Reprinted with permission from ref. 71. Copyright 2025, John Wiley & Sons. (c) Schematic of C&S/Fe@S–S-OSCLMs design and hypoxia relief via glycolysis inhibition. Reprinted with permission from ref. 72. Copyright 2023, Elsevier. | ||
A complementary strategy, Su et al. developed a redox-responsive nano-PS (C&S/Fe@S–S-OSCLMs) co-delivering Ce6 and Salvianolic acid B loaded with iron (Salvianolic acid B/Fe, Sal-B/Fe) to suppress the Warburg effect and enhance PDT (Fig. 7c).72 Sal-B/Fe reduced glucose uptake and glycolytic activity, thereby decreasing intracellular O2 consumption. As a result, intracellular O2 levels increased significantly under hypoxic conditions, as evidenced by decreased [Ru(dpp)3]2+ fluorescence. This elevated O2 availability enhanced 1O2 generation, promoting mitochondrial damage and tumor cell apoptosis.
Inhibiting glycolysis provides an effective metabolic strategy to alleviate tumor hypoxia and enhance PDT by reducing O2 consumption at its metabolic source. By targeting key enzymes involved in aerobic glycolysis such as pyruvate kinase, glycolysis inhibitors like vitamin K3 disrupt ATP generation and suppress excessive O2 utilization, thereby preserving O2 for ROS formation during PDT. Similarly, agents such as Salvianolic acid B with iron further suppress glucose metabolism, increasing intracellular O2 levels and amplifying 1O2 production. These combined effects of metabolic inhibition and enhanced oxidative stress promote mitochondrial damage and induce tumor cell apoptosis. Compared with conventional O2 delivery methods, glycolysis inhibition offers a more sustainable way to relieve hypoxia by reprogramming tumor energy metabolism. However, ensuring metabolic selectivity and minimizing systemic interference remain important challenges. Future studies should focus on integrating precise metabolic regulation with multifunctional nano-PSs to achieve durable hypoxia control and improved the efficacy of PDT.
4.2. Adjusting acidity
Due to anaerobic glycolysis, hypoxic tumor cells accumulate high levels of lactate and hydrogen ions, leading to acidification of the TME with intra- and extracellular pH values ranging from 5.5 to 7.4, lower than the physiological pH of 7.4.73 This acidic environment further exacerbates tumor hypoxia by impairing O2 diffusion and reducing the stability and activity of O2-dependent therapeutic agents.74 In response to this characteristic, Zhang et al. designed a chiral nano-PS (Zn-UCMB) composed of upconversion NPs, a porphyrin-based MOF, and a Zn complex to synergistically enhance PDT through nonoxygen-dependent lactate depletion.75 Upon reaching the mildly acidic TME, the Zn complex is released and selectively binds L-lactate, lowering lactate levels without consuming O2. This strategy reprograms the immunosuppressive TME, and promotes M2 to M1 macrophage polarization (Fig. 8a). Simultaneously, the iron porphyrin MOF catalyzes H2O2 decomposition, generating O2, thus relieving hypoxia and boosting 1O2 production.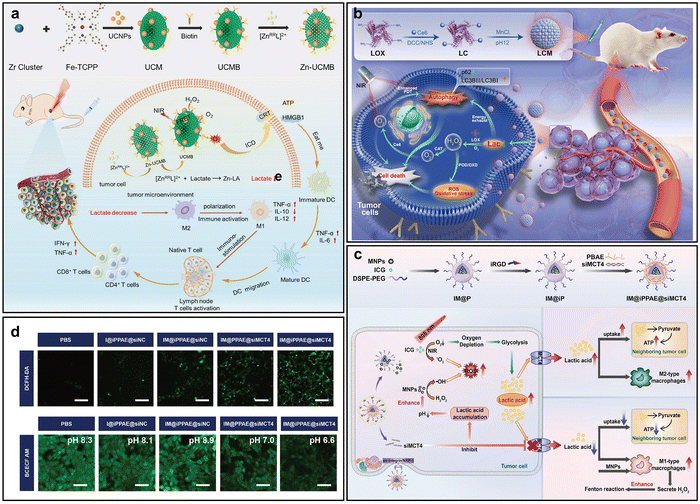 | ||
| Fig. 8 Representative nano-PSs alleviating hypoxia by adjusting acidity. (a) Schematic of Zn-UCMB preparation and mechanism for enhancing PDT through lactate reduction, O2 production, and 1O2 generation. Reprinted with permission from ref. 75. Copyright 2025, Elsevier. (b) Schematic of LCM for lactate depletion and combination CDT/PDT therapy. Reprinted with permission from ref. 76. Copyright 2025, American Chemical Society. (c) Schematic of IM@iPPAE@siMCT4 synthesis and therapeutic mechanism. (d) Fluorescence imaging of 4T1 cells stained with BCECF AM and DCFH-DA after treatment and 808 nm laser irradiation. Scale bar = 50 µm. Reprinted with permission from ref. 77. Copyright 2025, John Wiley & Sons. | ||
In addition to consuming lactate through reactions, degrading lactate by oxidase is also a promising strategy. For example, Wang et al. constructed an artificial peroxisome-based nano-PS (LOX-Ce6-Mn, LCM) that synergistically enhances PDT by depleting lactate and generating O2 (Fig. 8b).76 Lactate oxidase catalyzes the oxidation of lactate within tumor cells, producing hydrogen peroxide, which is subsequently decomposed by the CAT-like activity of Mn components to generate O2. This cascade not only reduces lactate-induced immunosuppression but also alleviates tumor hypoxia, boosting 1O2 generation.
Adjusting lactate through siRNA is also an interesting strategy, Dou et al. designed a multifunctional nano-PS (IM@iPPAE@siMCT4) co-delivering ICG, magnetic NPs (MNPs), and siRNA targeting monocarboxylate transporter 4 (siMCT4) to enhance PDT (Fig. 8c).77 Upon light irradiation, ICG-induced PDT consumes O2, triggering lactate accumulation. siMCT4 downregulates MCT4 expression, inhibiting lactate efflux and leading to intracellular acidification. This pH shift sensitizes the Fenton reaction catalyzed by MNPs and promotes ˙OH generation. At the same time, reduced extracellular lactate levels relieve immunosuppression and impede tumor metabolic symbiosis. Intracellular pH reduction and elevated ROS levels were confirmed using BCECF-AM and DCFH-DA probes, respectively (Fig. 8d). This work highlights lactate metabolism remodeling as an effective strategy to relieve hypoxia and potentiate PDT.
Regulating lactate metabolism offers an effective approach to relieve tumor hypoxia and improve PDT by reprogramming the acidic TME. Lactate accumulation in hypoxic tumors not only impairs O2 diffusion but also promotes immune evasion and metabolic imbalance. Strategies such as lactate binding, enzymatic degradation, and gene silencing effectively reduce lactate levels and restore O2 availability. Systems like Zn-UCMB and LOX-Ce6-Mn achieve simultaneous lactate depletion and O2 generation, while siMCT4-mediated inhibition of lactate transport further enhances ROS production and sensitizes tumors to PDT. These approaches collectively alleviate acidosis, boost ROS generation. Compared with conventional O2 supplementation, lactate regulation provides a more sustainable route to normalize the TME. Future research should focus on developing targeted, biocompatible, and multifunctional nano-PSs that integrate metabolic modulation for enhanced therapeutic outcomes.
4.3. Destructing the tumor ECM
ECM, primarily composed of collagens, hyaluronic acid, and proteoglycans, forms a dense and rigid 3D network that plays a critical role in tumor initiation, metastasis, and drug resistance.78 This fibrotic ECM elevates IFP, compressing blood vessels and hindering the delivery of O2 and therapeutic agents, thereby limiting the effectiveness of PDT.79 As a result, disrupting the tumor ECM has emerged as a promising strategy to enhance PDT, with approaches focusing on either small molecules or proteolytic enzymes to degrade the ECM and improve drug and O2 delivery to tumor cells.80 To deal with this issue, Wang et al. designed a covalent organic framework-based nano-PS (PCPP) by encapsulating the antifibrotic drug pirfenidone (PFD) into COFTTA-DHTA and decorating it with PLGA-PEG, aiming to deplete tumor ECM and alleviate hypoxia (Fig. 9a).81 By downregulating ECM components such as hyaluronic acid (HA) and collagen I, PCPP reduced solid stress, thereby improving O2 perfusion within the tumor (Fig. 9b). Hypoxia alleviation was demonstrated by decreased fluorescence of the hypoxia-responsive Cy7-NO2 probe in vivo (Fig. 9c). This remodeling of the TME facilitated deeper penetration and higher accumulation of subsequently administered NM-PPIX nanomicelles, significantly boosting ROS generation and enhancing PDT-induced tumor suppression.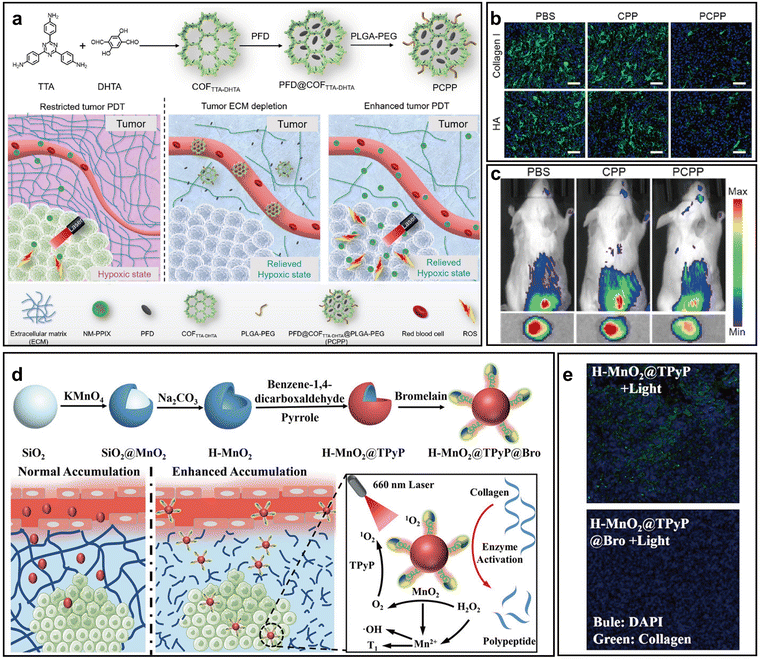 | ||
| Fig. 9 .Representative nano-PSs for hypoxia alleviation via ECM degradation. (a) Schematic of PCPP-mediated ECM depletion using PFD to improve tumor oxygenation and enhance NM-PPIX uptake and efficacy of PDT. (b) Immunostaining of collagen I and HA in tumors after different treatments. Scale bar: 50 µm. (c) In vivo fluorescence imaging using Cy7-NO2 to assess tumor hypoxia. Reprinted with permission from ref. 81. Copyright 2020, Elsevier. (d) Schematic of H-MnO2@TPyP@Bro synthesis and Bro-mediated ECM degradation for enhanced nanoparticle accumulation. (e) Tumor collagen I staining after various treatments. Reprinted with permission from ref. 82. Copyright 2022, John Wiley & Sons. | ||
Similarly, enzymes also can effectively destroy ECM barrier. In 2022, Zhu et al. designed a multifunctional nano-PS (H-MnO2@TPyP@Bro) integrating MnO2, porphyrin, and the proteolytic enzyme bromelain (Bro) to address the dual barriers of hypoxia and dense ECM in tumors (Fig. 9d).82 In this system, Bro enzymatically degraded collagen fibers within the ECM, alleviating the physical barrier and lowering IFP, which facilitated deeper nano-PS penetration into tumor tissue. The ECM degradation was validated in vivo through immunofluorescence staining of tumor slices, showing significantly reduced collagen I levels (Fig. 9e).
In summary, disrupting the dense ECM represents an effective strategy to relieve tumor hypoxia. Recent studies demonstrate that both pharmacological and enzymatic approaches can effectively remodel this barrier. Small-molecule antifibrotic agents, such as pirfenidone-loaded COF-based nanoplatforms, downregulate ECM components to reduce solid stress and improve intratumoral oxygenation. Meanwhile, enzyme-assisted systems like bromelain-modified MnO2 nanostructures degrade collagen fibers, facilitating nanoparticle penetration and O2 delivery. These strategies not only alleviate hypoxia but also enhance ROS generation and tumor suppression, highlighting ECM remodeling as a promising route for optimizing PDT outcomes in solid tumors.
4.4. Normalizing tumor blood vessels
Tumor vasculature exhibits abnormal structure and function, including high tortuosity, uneven distribution, vascular leakage and reduced blood flow, which limit O2 and drug delivery and contribute to tumor hypoxia, ultimately weakening the efficacy of PDT.83 Vascular normalization has emerged as a promising strategy to restore vessel integrity, enhance perfusion and improve oxygenation, thereby enhancing treatment outcomes.84 Current approaches involve thermal stimulation, as well as the use of NO donors and EGFR inhibitors. For instance, Feng et al. developed a NIR-activatable liposomal nano-PS (DiR-hCe6-liposome) composed of hexylamine-conjugated chlorin e6 (hCe6) as the PS and DiR as the photothermal agent, both co-encapsulated within PEGylated liposomes (Fig. 10a).85 Upon irradiation with a 785 nm laser, DiR produced mild heat, raising the tumor temperature to around 45 °C. This mild photothermal effect promoted tumor vascular normalization and enhanced blood perfusion, which facilitated O2 delivery and alleviated tumor hypoxia. The reduction in hypoxia was confirmed through vitro immunofluorescence staining using pimonidazole as a hypoxia marker. The result confirms that the photothermal modulation of tumor vasculature by DiR-hCe6-liposome effectively improves oxygenation, providing a more favorable microenvironment for enhancing PDT.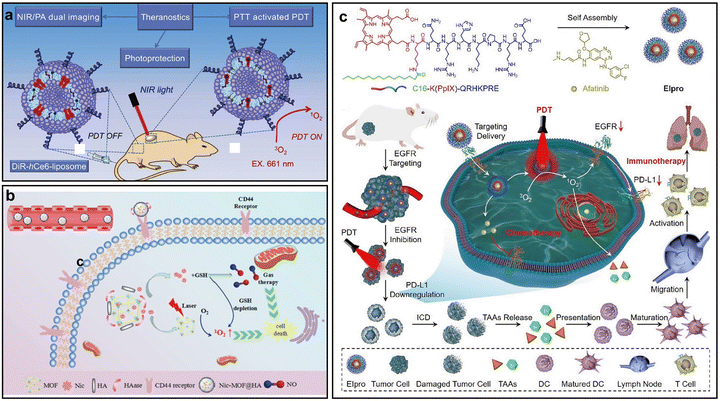 | ||
| Fig. 10 Representative nano-PSs alleviating hypoxia byn normalizing tumor blood vessels. (a) Schematic of the DiR-hCe6-liposome composition and its application in NIR light-activated, skin-protective synergistic phototherapy. Reprinted with permission from ref. 85. Copyright 2017, Elsevier. (b) Illustration of the NO-generating Nic-MOF@HA nano-PS for hypoxia relief. Reprinted with permission from ref. 86. Copyright 2022, Elsevier. (c) Schematic of the EIpro system, showing EGFR-targeted delivery, PDT-induced EGFR degradation, and the resulting improvement in therapeutic efficacy. Reprinted with permission from ref. 87. Copyright 2024, Elsevier. | ||
Furthermore, Xia et al. reported a GSH-responsive nano-PS (Nic-MOF@HA) designed to modulating the TME (Fig. 10b).86 In this system, nicorandil, an NO donor, is released in response to elevated GSH levels within tumors, triggering the local generation of NO. The produced NO exerts a vasodilatory effect, leading to the normalization of aberrant tumor vasculature and consequently improving intratumoral perfusion and O2 delivery. This work exemplifies a rational strategy wherein NO-mediated vascular modulation is employed to remodel the TME, effectively overcoming hypoxic resistance and maximizing therapeutic response. In 2024, Wei et al. developed a nano-PS (EIpro) designed to target and degrade EGFR. The system combines a PS PpIX with an EGFR-targeting peptide (Fig. 10c).87 Upon light activation, the PS generates ROS that degrade EGFR. The degradation of EGFR promotes vascular normalization within the tumor, improving blood flow and O2 delivery, which alleviates tumor hypoxia.
Normalizing abnormal tumor blood vessels is an effective way to improve O2 delivery and strengthen the therapeutic impact of PDT. Recent research shows that controlled thermal stimulation, NO release, and receptor-targeted regulation can effectively restore vascular integrity. Mild photothermal heating helps reopen constricted vessels and enhance blood flow, while NO donors such as nicorandil induce vasodilation and promote more uniform O2 distribution. In addition, molecular regulation that reduces abnormal signaling, such as EGFR degradation, contributes to vessel normalization and improved perfusion. These strategies collectively rebuild a functional vascular network that supports continuous O2 supply and deeper drug penetration. By transforming the hypoxic TME into a more O2-rich and responsive state, vascular normalization has become a key strategy to enhance the overall effectiveness of PDT.
4.5. Modulating HIF-1 expression
HIF-1 is a heterodimer composed of an O2-sensitive α-subunit (HIF-1α) and a constitutive β-subunit (HIF-1β); under normoxic conditions, HIF-1α is hydroxylated by prolyl hydroxylase domain proteins (PHD) and factor inhibiting HIF (FIH), and subsequently degraded via the Von Hippel-Lindau (VHL) pathway.88 However, under the hypoxic TME, this degradation is inhibited, allowing HIF-1α to accumulate, translocate to the nucleus, dimerize with HIF-1β, and activate genes that promote glucose metabolism, proliferation, angiogenesis, and tumor progression.89 Therefore, inhibiting HIF-1 expression and its downstream signaling offers a promising strategy to relieve tumor hypoxia and synergistically enhance PDT efficacy.In recent months, Zhou et al. designed a GSH-responsive peptide dendritic nano-PS (CGP) co-loaded with Ce6 and genistein (Gen) in nasopharyngeal carcinoma (Fig. 11a).90 Gen was incorporated into the nano-PS to inhibit the HIF-1α signaling pathway, which plays a critical role in tumor adaptation to hypoxia. Upon treatment, Gen significantly downregulated HIF-1α expression, thereby reducing the anaerobic glycolysis pathway. PDT-induced ROS further promoted the release of Gen from the nanogel, enhancing the therapeutic efficacy. Experimental results showed a dose-dependent reduction in HIF-1α expression in treated HK1 cells, leading to improved tumor oxygenation and enhanced PDT outcomes (Fig. 11b).
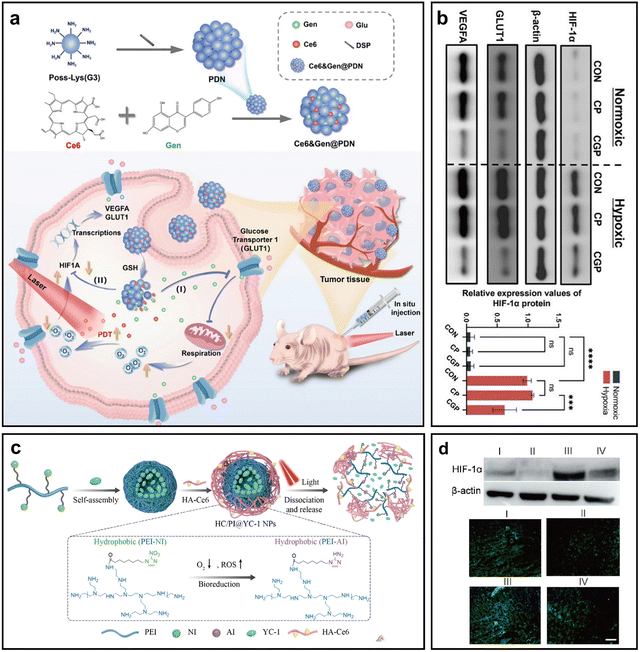 | ||
| Fig. 11 Representative nano-PSs alleviating hypoxia by modulating HIF-1 expression. (a) Preparation and anti-tumor mechanism of CGP: Schematic of CGP composition and synthesis. (b) The expression level of HIF-1α, GLUT1, and VEGFA protein by WB. Reprinted with permission from ref. 90. Copyright 2025, Springer Nature. (c) Schematic of a self-rectifiable, hypoxia-assisted nano-PS for synergistic cancer therapy. (d) Immunoblot and immunofluorescence analysis of HIF-1α expression in tumors harvested from mice 12 hours post-irradiation (groups I–IV, 10 mg YC-1 kg−1, 1-hour post-injection). Scale bar = 100 µm. Reprinted with permission from ref. 91. Copyright 2022, American Chemical Society. | ||
In 2022, Wang et al. designed a self-rectifiable nano-PS (HC/PI@YC-1 NPs) co-loaded with the PS Ce6 and the HIF-1α inhibitor YC-1 (Fig. 11c).91 Upon laser irradiation, PDT-induced O2 consumption aggravated tumor hypoxia, which in turn triggered nanoparticle dissociation and YC-1 release. YC-1 effectively downregulated HIF-1α expression, mitigating hypoxia-driven resistance and promoting sustained ROS generation during PDT. Immunofluorescence and immunoblotting analyses confirmed a marked reduction in intratumoral HIF-1α levels, validating the hypoxia-relieving effect of YC-1 and its synergistic role in enhancing O2-dependent PDT (Fig. 11d).
Targeting HIF-1 provides an effective strategy to overcome tumor hypoxia and improve the efficacy of PDT. Recent advances demonstrate that integrating HIF-1 inhibitors with smart nanoplatforms can simultaneously suppress hypoxia signaling and amplify PDT effects. Systems incorporating agents such as genistein or YC-1 not only downregulate HIF-1α expression but also disrupt metabolic reprogramming and enhance ROS generation. These dual-action designs relieve O2 deficiency and sustain therapeutic activity even under fluctuating O2 levels. Future research should focus on optimizing the stability, selectivity, and biocompatibility of HIF-1 targeted nanomedicines, while exploring combination therapies that integrate hypoxia regulation with immune activation or metabolic modulation to achieve durable and precise tumor control.
5. Type-I nano-PSs
Type-I PDT has recently gained attention as an effective strategy to overcome the limitations of hypoxic TME.92–94 In contrast to conventional Type-II PDT, Type-I pathways can be activated under O2-deficient conditions, enabling sustained therapeutic efficacy even in severely hypoxic tumors.95 In Type-I PDT, triplet PSs generate highly reactive free radicals through hydrogen abstraction or electron transfer, which quickly interact with H2O or O2 to form H2O2, O2˙−, and ˙OH.96 Additional radicals such as ˙C and LOO˙ further contribute to oxidative stress, causing severe mitochondrial damage, nuclear DNA fragmentation, and lipid peroxidation.97–99 Since the concept of Type-I PDT was introduced, a wide range of Type-I PSs have been developed, including metal oxide-based, supramolecular and hybrid nanomaterials, as well as organic small molecules, each offering unique advantages for ROS generation under hypoxic conditions.For metal oxide, Luo et al. designed a photoactivated nano-PS (1-NBS@CeO2) by co-assembling a type I PS, coupled peptide and ultrasmall cerium oxide nanozymes to amplify oxidative stress for PDT in hypoxic tumors (Fig. 12a).100 CeO2, endowed with SOD-like and POD-like activities, catalyzed the conversion of photogenerated O2˙− into highly toxic ˙OH through a cascade reaction. This strategy bypassed O2 dependence and significantly enhanced ROS generation under hypoxic conditions. In vitro studies confirmed elevated ROS levels, GSH depletion, and mitochondrial dysfunction in A375 cells, leading to apoptosis (Fig. 12b). The system demonstrated superior antitumor efficacy in vivo, highlighting the potential of CeO2-mediated cascade amplification to boost Type I PDT in O2-deficient tumors.
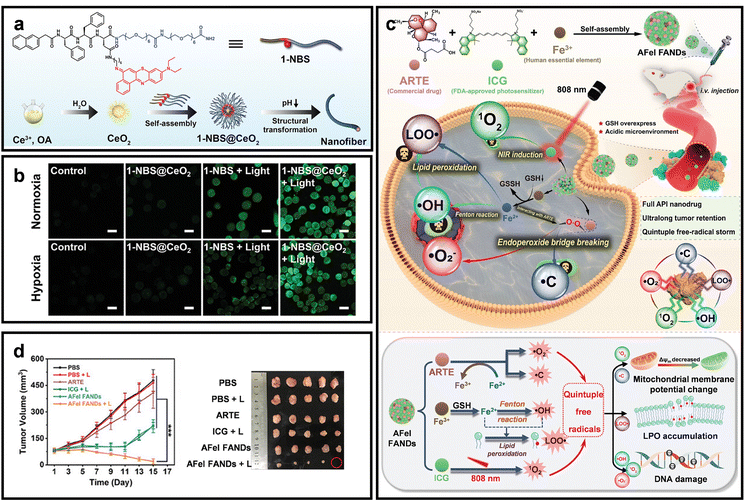 | ||
| Fig. 12 Representative O2-independent Type-I nano-PSs for enhanced the efficacy of hypoxic tumors. (a) Schematic of 1-NBS@CeO2 nano-PS design for cascade oxidative stress and Type-I PDT. (b) Intracellular ROS imaging in A375 cells under normoxia and hypoxia. Scale bar: 20 µm. Reprinted with permission from ref. 100. Copyright 2024, John Wiley & Sons. (c) Design and mechanism of AFeI FANDs generating multiple free radicals for multisite tumor damage. (d) Tumor volume and size in mice following different treatments. Reprinted with permission from ref. 101. Copyright 2024, John Wiley & Sons. | ||
In 2024, our group developed a full-API nanodrug (AFeI FANDs) composed of artesunate, Fe3+, and ICG, designed to overcome the O2-dependency of traditional PDT (Fig. 12c).101 In the acidic TME, AFeI FANDs decompose to release all active components, producing five types of free radicals, including O2˙−, ˙C, ˙OH, LOO˙, and 1O2, without relying on O2. These radicals induce mitochondrial damage, DNA fragmentation, and lipid peroxidation, achieving robust anticancer effects. Importantly, intracellular hypoxia relief was demonstrated through DCFH-DA fluorescence, confirming high ROS output even under hypoxia. In vivo studies also confirmed the enhanced the efficacy of PDT under hypoxic TME (Fig. 12d).
Additionally, Yao et al. designed a novel PS (EBSe) by structurally modifying methylene blue with selenium substitution and ethylation to enhance triplet yield, ROS generation, and cellular uptake (Fig. 13a).102 Upon NIR irradiation, EBSe exhibited self-adaptive photocatalytic behavior, generating type I ROS (O2˙−, ˙OH), ensuring efficient phototoxicity regardless of O2 availability. EBSe localized in lysosomes, and upon light activation, translocated to the nucleus, inducing severe organelle damage. Compared to traditional PSs, EBSe showed over 2500-fold higher phototoxicity under hypoxia and significantly boosted antitumor efficacy (Fig. 13b). This work presents a promising molecular strategy for overcoming hypoxia-associated resistance and amplifying PDT through radical pathways.
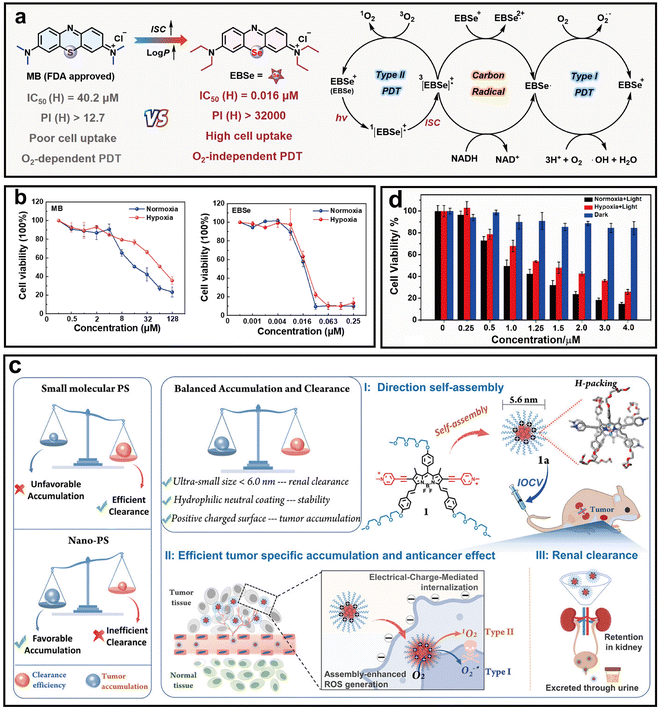 | ||
| Fig. 13 Representative O2-independent Type-I nano-PSs for enhanced the efficacy of hypoxic tumors. (a) Schematic of EBSe design and adaptive photocatalytic PDT mechanism. (b) Cell viability of PAN02 cells treated with MB or EBSe under normoxic and hypoxic conditions. Reprinted with permission from ref. 102. Copyright 2025, American Chemical Society. (c) Schematic of 1a with enhanced ROS generation, tumor targeting, and renal clearance. (d) Cell viability of HeLa cells treated with 1a. Reprinted with permission from ref. 103. Copyright 2023, John Wiley & Sons. | ||
Interestingly, Zhang et al. designed a renal-clearable ultra-small nano-PS (1a) by self-assembling a BODIPY derivative functionalized with pyridinium groups and triethylene glycol chains (Fig. 13c).103 The amphiphilic structure facilitated the formation of stable 5.6 nm NPs with high tumor-targeting efficiency and minimal off-target accumulation. Upon light irradiation, 1a exhibited significantly enhanced ROS production through type I mechanisms. Notably, the O2˙− generation dominated under hypoxic conditions, ensuring PDT efficacy even in O2-deficient tumors. In vitro cytotoxicity assays confirmed that 1a retained strong phototoxicity under hypoxia, highlighting its potential to overcome tumor hypoxia and enhance PDT outcomes (Fig. 13d).
In conclusion, type-I PDT provides a promising solution to the O2 dependence that limits traditional PDT in hypoxic tumors. By generating free radicals through electron or hydrogen transfer, Type-I PSs maintain strong cytotoxicity even under severe hypoxia. Recent studies have developed diverse nanoplatforms and molecular systems to amplify these reactions. Metal-oxide nanozymes, such as CeO2-based composites, catalyze cascade redox processes to enhance radical production. Hybrid systems like AFeI nanodrugs integrate multiple active components to generate various reactive species simultaneously, while small-molecule PSs such as EBSe and BODIPY derivatives achieve efficient radical generation through structural modification. These strategies collectively enable robust oxidative damage, mitochondrial dysfunction, and apoptosis in O2-poor environments. Future work should focus on optimizing radical selectivity, improving biocompatibility, and integrating imaging or targeting functions to achieve precise, safe, and clinically translatable Type-I PDT platforms.
6. Conclusion and outlook
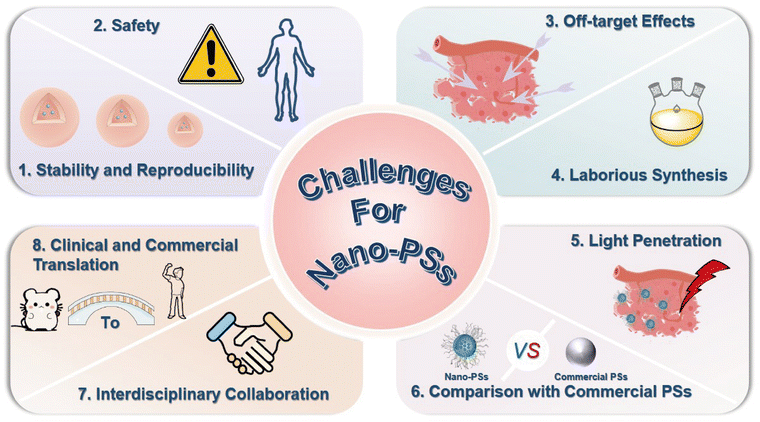
PDT is a promising non-invasive cancer treatment characterized by high selectivity and minimal side effects, yet its efficacy is often hindered by tumor hypoxia. Recent nanotechnological advances have addressed this challenge through strategies such as alleviating or utilizing hypoxia, regulating the TME, and designing Type-I nano-PSs. Nano-PSs preserve their structural integrity within the cellular environment through the cooperative stabilization of multiple weak non-covalent forces. Hydrophobic interactions, π–π stacking, hydrogen bonding, and electrostatic attraction act together to form a significant energy barrier that prevents premature disassembly. This stability is further reinforced by kinetic factors as the compact nanostructure limits solvent access and protects sensitive components from ionic or enzymatic disruption. Consequently, the assembly remains locked during circulation and cellular uptake. When exposed to tumor-specific stimuli such as acidic pH or redox imbalance, these weak interactions are selectively disrupted, leading to controlled disassembly and thereby enhancing the efficiency of PDT.Although multifunctional nano-PSs exhibit great potential for overcoming tumor hypoxia, most remain at the preclinical stage. To bridge the gap toward clinical application, several major challenges must be addressed, as outlined below (Fig. 14).
(1) Stability and reproducibility
The long-term stability of nano-PSs under cellular and physiological conditions is a critical determinant of therapeutic efficacy. Many nano-PSs suffer from photobleaching or structural degradation during irradiation or circulation. Future studies should focus on improving photostability and chemical robustness through molecular engineering, core–shell protection, and encapsulation strategies to maintain consistent performance during treatment.Reproducibility across batches remains an essential prerequisite for clinical reliability. Variations in synthesis conditions, precursor quality, and environmental parameters often lead to inconsistent physicochemical properties. Establishing standardized preparation protocols, quality control systems, and scalable synthesis routes will be vital to achieving uniform product performance.
(2) Safety
While nano-PSs enhance tumor selectivity and reduce systemic exposure compared with free PSs, safety concerns remain. Nanoparticles that are not efficiently cleared may accumulate in major organs such as the liver and spleen, leading to chronic toxicity or inflammation. Comprehensive pharmacokinetic and toxicological studies should evaluate biodistribution, metabolism, and excretion profiles to clarify their long-term safety. The design of biodegradable or self-eliminating nanocarriers that can degrade under enzymatic or redox conditions may effectively mitigate toxicity. Using naturally derived materials such as lipids, proteins, or polysaccharides can further improve biosafety. Establishing unified evaluation frameworks and long-term monitoring will provide a solid foundation for clinical translation.(3) Off-target effects
Reducing phototoxic damage to healthy tissues remains one of the main challenges in PDT. Even with improved tumor targeting, non-specific accumulation, and uncontrolled irradiation can still cause collateral injury. The development of stimuli-responsive nano-PSs that can be activated by pH changes, enzymatic activity, or hypoxic conditions provides a promising solution. Functionalization with tumor-targeting ligands such as folic acid, peptides, or antibodies enhances cellular recognition and uptake. Moreover, integrating imaging guidance with precise control of light exposure can improve spatial accuracy. Combining these strategies allows PDT to evolve into a more selective and controllable therapy with minimal off-target effects.(4) Laborious synthesis
Many advanced nano-PSs require complex synthesis routes that involve multiple steps, low yields, or toxic reagents, which restrict large-scale production and increase cost. Simplifying synthesis while maintaining functionality is therefore a key goal. Modular fabrication, self-assembly, and biomimetic templating provide effective methods to improve efficiency and reproducibility. Green chemistry approaches that avoid hazardous solvents and reduce environmental impact are also highly desirable. The use of automated or continuous-flow systems can ensure better uniformity between batches and support future industrial manufacturing. Simplified yet stable nano-PS systems will be critical for moving from laboratory-scale experiments to clinical and commercial applications.(5) Light penetration
Limited light penetration depth continues to constrain PDT, making it more effective for superficial tumors. Expanding light activation into the NIR region, especially the NIR-II window, can improve tissue penetration due to lower scattering and absorption. Developing PSs that respond to longer wavelengths will extend treatment to deep-seated tumors. Combining PDT with other energy-based therapies such as photothermal, sonodynamic, or X-ray activation may further enhance therapeutic depth and flexibility. These hybrid approaches can allow precise control of energy delivery while maintaining the selectivity of PDT, enabling more effective treatment for tumors located deep within tissues.(6) Comparison with commercial PSs
Although many innovative nano-PSs have been reported, most still fall short of the performance achieved by clinically approved PSs such as Photofrin, Verteporfin, and Talaporfin sodium. These commercial agents exhibit well-characterized pharmacokinetics, stability, and established regulatory approval. In contrast, many new nano-PSs face challenges related to stability, reproducibility, and incomplete safety validation. Systematic comparison with existing clinical benchmarks is therefore necessary to identify performance gaps and guide further optimization. Such comparative studies can help determine the critical parameters required for clinical translation and provide a clearer roadmap for bringing emerging nano-PS systems to practical use.(7) Interdisciplinary collaboration
The advancement of PDT depends on close collaboration among researchers from chemistry, materials science, biology, and medicine. Chemists contribute to the molecular design of PSs and nanocarriers, while biologists provide insights into cell interactions and immune responses. Collaboration with clinicians ensures that these technologies address real therapeutic needs. Computational modeling and machine learning can accelerate the prediction of structure–activity relationships and improve material optimization. Meanwhile, advanced imaging technologies enable real-time visualization of biodistribution and ROS dynamics. Strengthening interdisciplinary cooperation will help bridge fundamental discoveries with clinical practice and foster the development of next-generation multifunctional nano-PSs.(8) Clinical and commercial translation
Although numerous nano-PSs have achieved promising preclinical outcomes, few have progressed to clinical trials. The transition from laboratory research to clinical application requires standardized evaluation protocols, large-animal verification, and regulatory compliance. Early collaboration between academic researchers, industrial partners, and regulatory experts can accelerate optimization and ensure adherence to manufacturing and safety standards. Developing scalable production processes with consistent quality control will be essential for commercialization. Furthermore, demonstrating clear advantages over existing therapies in terms of safety, efficacy, and cost-effectiveness will determine the ultimate success of nano-PSs in clinical and industrial settings.In summary, future research should focus on achieving a balance between stability, safety, efficiency, and scalability through rational design and interdisciplinary innovation. With continuous advancements in synthetic strategies, optical technologies, and biomedical evaluation, nano-PSs are expected to move closer toward clinical realization and broader therapeutic applications.
Author contributions
DL: writing – original draft. SW: writing – review and editing. CZ: writing – review and editing. YF: writing – review and editing. FF: writing – review and editing. ML: writing – review and editing. JZ: funding acquisition, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Data availability
Data for this study are available in the main article.Acknowledgements
This work was funded by the Beijing Natural Science Foundation (No. 7252289) and the National Natural Science Foundation of China (No. 32371442). J. Z. would like to thank Biological & Medical Engineering Core Facilities (Beijing Institute of Technology) for providing advanced equipment.Notes and references
- S. Wang, C. Zhang, F. Fang, Y. Fan, J. Yang and J. Zhang, J. Mater. Chem. B, 2023, 11, 8315–8326 RSC.
- Y. Wan, L. Fu, C. Li, J. Lin and P. Huang, Adv. Mater., 2021, 33, 2103978 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhang, G. Zhang, J. Wu, L. Wang, Z. Dong, Y. Zheng, Q. Huang, M. Zou, R. Liao, F. Wang and P. Liang, Coord. Chem. Rev., 2024, 518, 216069 CrossRef CAS.
- L. Fang, Z. Chen, J. Dai, Y. Pan, Y. Tu, Q. Meng, Y. Diao, S. Yang, W. Guo, L. Li, J. Liu, H. Wen, K. Hua, L. Hang, J. Fang, X. Meng, P. Ma and G. Jiang, Adv. Sci., 2025, 12, 2409157 CrossRef CAS PubMed.
- X. Li, Y.-H. Jeon, N. Kwon, J.-G. Park, T. Guo, H.-R. Kim, J.-D. Huang, D.-S. Lee and J. Yoon, Biomaterials, 2021, 266, 120430 CrossRef CAS PubMed.
- Y. Zhang, J. Wang, T. Zhang, D. Tang, H. Yang, S. Guo, Y. Fan, C. Sun, H. Xiao, Y. Huang and Y. Weng, Exploration, 2025, 20240047 CrossRef CAS PubMed.
- Q. Zhang, X. Wang, J. Chen, J. Wu, M. Zhou, R. Xia, W. Wang, X. Zheng and Z. Xie, Chem. Commun., 2024, 60, 13641–13652 RSC.
- C. Zhang, X. Hu, L. Jin, L. Lin, H. Lin, Z. Yang and W. Huang, Adv. Healthcare Mater., 2023, 12, 2300530 CrossRef CAS PubMed.
- Y. Cai, T. Chai, W. Nguyen, J. Liu, E. Xiao, X. Ran, Y. Ran, D. Du, W. Chen and X. Chen, Signal Transduct. Target Ther., 2025, 10, 115 CrossRef PubMed.
- T. Wu, W.-T. Dou, C. Yang, L. Zhou, F. Wang, L. He, X. Qian and L. Xu, Coord. Chem. Rev., 2025, 541, 216839 CrossRef CAS.
- E. Nestoros, A. Sharma, E. Kim, J. S. Kim and M. Vendrell, Nat. Rev. Chem., 2024, 9, 46–60 CrossRef PubMed.
- Y. Zhao, Y. Xu, X. Zhang, Z. Chen, H. Kim, X. Li and J. Yoon, Angew. Chem., Int. Ed., 2025, 64, e202506412 CrossRef CAS PubMed.
- K. Teng, L. Niu, J. Li, D. Zhang and Q. Yang, Angew. Chem., Int. Ed., 2025, e202509416 CAS.
- D. Chen, Q. Xu, W. Wang, J. Shao, W. Huang and X. Dong, Small, 2021, 17, 2006742 CrossRef CAS PubMed.
- F. Yang, F. Chen, S. Li, S. Wang, L. Peng, S. Deng, F. Zhang, U. Jeschke, Y. Wang and C. Luo, Coord. Chem. Rev., 2025, 516, 163962 CAS.
- Y. Pan, L. Liu, X. Mou and Y. Cai, ACS Nano, 2023, 17, 20875–20924 CrossRef PubMed.
- L. Yao, S. Xie, Y. Liu, L. Mengqi, J. Xia and B. Lu, Chem. Commun., 2024, 60, 14012–14021 RSC.
- C. Chen, C. Wu, J. Yu, X. Zhu, Y. Wu, J. Liu and Y. Zhang, Coord. Chem. Rev., 2022, 461, 214495 CrossRef CAS.
- J. Zou, L. Li, J. Zhu, X. Li, Z. Yang, W. Huang and X. Chen, Adv. Mater., 2021, 33, 2103627 CrossRef CAS PubMed.
- W. Zeng, P. Liu, W. Pan, S. R. Singh and Y. Wei, Cancer Lett., 2015, 356, 263–267 CrossRef CAS PubMed.
- X. Zheng, W. Sun, M. Ju, J. Wu, H. Huang and B. Shen, Biomater. Sci., 2022, 10, 4681–4693 RSC.
- B. Wang, S. Hu, Y. Teng, J. Chen, H. Wang, Y. Xu, K. Wang, J. Xu, Y. Cheng and X. Gao, Signal Transduction Targeted Ther., 2024, 9, 200 CrossRef PubMed.
- J. I. Garcia-Peiro, M. Sancho-Albero, S. Miguel, A. Mosseri, F. Hornos, R. Contreras-Montoya, J. L. Hueso and J. Santamaria, Adv. Funct. Mater., 2025, e02999 CrossRef.
- Z. Zhang, B. Li, C. Zhang, L. Li, L. Cui, P. Zhao, Y. Zhang, J. Song, D. Zhang, C. Wei, Y. Zhang, L. Liu and B. Zhao, Adv. Compos. Hybrid Mater., 2025, 8, 228 CrossRef CAS.
- L. Huang, S. Zhao, J. Wu, L. Yu, N. Singh, K. Yang, M. Lan, P. Wang and J. S. Kim, Coord. Chem. Rev., 2021, 438, 213888 CrossRef CAS.
- W. Tang, Z. Yang, L. He, L. Deng, P. Fathi, S. Zhu, L. Li, B. Shen, Z. Wang, O. Jacobson, J. Song, J. Zou, P. Hu, M. Wang, J. Mu, Y. Cheng, Y. Ma, L. Tang, W. Fan and X. Chen, Nat. Commun., 2021, 12, 523 CrossRef CAS PubMed.
- Y. Wang, S. Luo, Y. Wu, P. Tang, J. Liu, Z. Liu, S. Shen, H. Ren and D. Wu, ACS Nano, 2020, 14, 17046–17062 CrossRef CAS PubMed.
- X. Guo, J. Qu, C. Zhu, W. Li, L. Luo, J. Yang, X. Yin, Q. Li, Y. Du, D. Chen, Y. Qiu, Y. Lou and J. You, Drug Delivery, 2018, 25, 585–599 CrossRef CAS PubMed.
- S. Wang, F. Yuan, K. Chen, G. Chen, K. Tu, H. Wang and L.-Q. Wang, Biomacromolecules, 2015, 16, 2693–2700 CrossRef CAS PubMed.
- Z. Luo, M. Zheng, P. Zhao, Z. Chen, F. Siu, P. Gong, G. Gao, Z. Sheng, C. Zheng, Y. Ma and L. Cai, Sci. Rep., 2016, 6, 23393 CrossRef CAS PubMed.
- W. Liu, T. Liu, M. Zou, W. Yu, C. Li, Z. He, M. Zhang, M. Liu, Z. Li, J. Feng and X. Zhang, Adv. Mater., 2018, 30, 1802006 CrossRef PubMed.
- Z. Li, Y. Ji, Y. Su, Z. Zhou, X. Yang, Y. Huang, M. Yan and L. Shen, Chem. Eng. J., 2024, 500, 157406 CrossRef CAS.
- J. Ruiz-Cabello, B. P. Barnett, P. A. Bottomley and J. W. M. Bulte, NMR Biomed., 2011, 24, 114–129 CrossRef CAS PubMed.
- S. Zhang, N. Yang, S. Sun, H. Zhao, W. Wang, J. Nie, Z. Pei, W. He, L. Zhang, L. Cheng and Z. Cheng, Acta Biomater., 2025, 193, 406–416 CrossRef CAS PubMed.
- G. Li, H. Kobayashi, J. M. Taylor, R. Ikeda, Y. Kubota, K. Kato, M. Takata, T. Yamamoto, S. Toh, S. Matsumura and H. Kitagawa, Nat. Mater., 2014, 13, 802–806 CrossRef CAS PubMed.
- C. G. Piscopo, F. Trapani, A. Polyzoidis, M. Schwarzer, A. Pace and S. Loebbecke, New J. Chem., 2016, 40, 8220–8224 RSC.
- S. Gao, P. Zheng, Z. Li, X. Feng, W. Yan, S. Chen, W. Guo, D. Liu, X. Yang, S. Wang, X.-J. Liang and J. Zhang, Biomaterials, 2018, 178, 83–94 CrossRef CAS PubMed.
- T. Zuo, X. Li, X. Ma, Y. Zhang, X. Li, X. Fan, M. Gao, D. Xia and H. Cheng, Front. Bioeng. Biotechnol., 2024, 12, 1383930 CrossRef PubMed.
- Y. Zhang, J. Zhang, Q. Jia, J. Ge and P. Wang, Mater. Chem. Front., 2021, 5, 4474–4501 RSC.
- N. Yang, W. Xiao, X. Song, W. Wang and X. Dong, Nano-Micro Lett., 2020, 12, 15 CrossRef CAS PubMed.
- L. Yu, Z. Liu, W. Xu, K. Jin, J. Liu, X. Zhu, Y. Zhang and Y. Wu, Acta Pharm. Sin. B, 2024, 14, 1111–1131 CrossRef CAS PubMed.
- H. Cheng, J. Zhu, S. Li, J. Zeng, Q. Lei, K. Chen, C. Zhang and X. Zhang, Adv. Funct. Mater., 2016, 26, 7847–7860 CrossRef CAS.
- W. Huang, L. Zhang, J. Sun, Y. Sun, L. Gong, S. Ge, Y. Zheng, W. Gao and X. Wei, J. Am. Chem. Soc., 2024, 146, 7543–7554 CrossRef CAS PubMed.
- D. Lee, S. Kwon, S. Jang, E. Park, Y. Lee and H. Koo, Bioact. Mater., 2022, 8, 20–34 CAS.
- W. Wang, C. Chen, Y. Ying, S. Lv, Y. Wang, X. Zhang, Z. Cai, W. Gu, Z. Li, G. Jiang and F. Gao, ACS Nano, 2022, 16, 5597–5614 CrossRef CAS PubMed.
- K. Yan, Y. Zhang, C. Mu, Q. Xu, X. Jing, D. Wang, D. Dang, L. Meng and J. Ma, Theranostics, 2020, 10, 7287–7318 CrossRef CAS PubMed.
- G. C. Dismukes, V. V. Klimov, S. V. Baranov, Yu. N. Kozlov, J. DasGupta and A. Tyryshkin, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 2170–2175 CrossRef CAS PubMed.
- R.-Q. Li, C. Zhang, B.-R. Xie, W.-Y. Yu, W.-X. Qiu, H. Cheng and X.-Z. Zhang, Biomaterials, 2019, 194, 84–93 CrossRef CAS PubMed.
- M. Li, Y. Shao, J. H. Kim, Z. Pu, X. Zhao, H. Huang, T. Xiong, Y. Kang, G. Li, K. Shao, J. Fan, J. W. Foley, J. S. Kim and X. Peng, J. Am. Chem. Soc., 2020, 142, 5380–5388 CrossRef CAS PubMed.
- Y. Fan, T. Zhou, P. Cui, Y. He, X. Chang, L. Xing and H. Jiang, Adv. Funct. Mater., 2019, 29, 1806708 CrossRef.
- S. Liang, Y. Liu, H. Zhu, G. Liao, W. Zhu and L. Zhang, Exploration, 2024, 4, 20230163 CrossRef CAS PubMed.
- B. Huang, M. Zhu, Z. Cui, S. Chen, G. Huang, J. Tian and W. Zhang, Small, 2025, 21, 2402956 CrossRef CAS PubMed.
- J. Zhang, X. Cao, H. Wen, Q. T. H. Shubhra, X. Hu, X. He and X. Cai, Coord. Chem. Rev., 2025, 539, 216746 CrossRef CAS.
- F. Fang, S. Wang, Y. Song, M. Sun, W.-C. Chen, D. Zhao and J. Zhang, Nat. Commun., 2023, 14, 1660 CrossRef CAS PubMed.
- A.-D. Guo, D. Wei, H.-J. Nie, H. Hu, C. Peng, S.-T. Li, K.-N. Yan, B.-S. Zhou, L. Feng, C. Fang, M. Tan, R. Huang and X.-H. Chen, Nat. Commun., 2020, 11, 5472 CrossRef CAS PubMed.
- Y. Li, J. Jeon and J. H. Park, Cancer Lett., 2020, 490, 31–43 CrossRef CAS PubMed.
- B. D. Palmer, P. Van Zijl, W. A. Denny and W. R. Wilson, J. Med. Chem., 1995, 38, 1229–1241 CrossRef CAS PubMed.
- F. Xue, C. Li, Y. Kuang, L. Shi, J. Chen, S. Chen, M. Ma, X. Wang and H. Chen, Sens. Actuators, B, 2022, 369, 132311 CrossRef CAS.
- Z. Zhu, Y. Feng, Q. Tian, J. Li, C. Liu, Y. Cheng, S. Zhang, Y. Dang, J. Gao, Y. Lai, F. Zhang, H. Yu, W. Zhang and Z. Xu, JACS Au, 2024, 4, 4032–4042 CrossRef CAS PubMed.
- D. Kang, S. T. Cheung, A. Wong-Rolle and J. Kim, ACS Cent. Sci., 2021, 7, 631–640 CrossRef CAS PubMed.
- Y. Ge, X. Lai, J. Li, R. Yu, Z. Zhuang, G. Sun, X. Cui, N. Zhang, L. Zhao, P. Upadhyaya and R. Zhong, Future Med. Chem., 2019, 11, 269–284 CrossRef CAS PubMed.
- Y. Zhao, Y. Xu, X. Zhang, Z. Chen, H. Kim, X. Li and J. Yoon, Angew. Chem., Int. Ed., 2025, e202506412 CAS.
- X. Liu, Z. Wu, C. Guo, H. Guo, Y. Su, Q. Chen, C. Sun, Q. Liu, D. Chen and H. Mu, Drug Delivery, 2022, 29, 138–148 CrossRef CAS PubMed.
- W. Chen, H. He, P. Jiao, L. Han, J. Li, X. Wang and X. Guo, Adv. Healthcare Mater., 2023, 12, 2301785 CrossRef CAS PubMed.
- Q. Yu, X. Li, J. Wang, L. Guo, L. Huang and W. Gao, Small, 2024, 20, 2305708 CrossRef CAS PubMed.
- J. Du, T. Shi, S. Long, P. Chen, W. Sun, J. Fan and X. Peng, Coord. Chem. Rev., 2021, 427, 213604 CrossRef CAS.
- A. Gulzar, J. Xu, C. Wang, F. He, D. Yang, S. Gai, P. Yang, J. Lin, D. Jin and B. Xing, Nano Today, 2019, 26, 16–56 CrossRef CAS.
- K. Moloudi, H. Abrahamse and B. P. George, WIREs Nanomed. Nanobiotechnol., 2024, 16, e1937 CrossRef CAS PubMed.
- L. Fu, C. Qi, Y. Hu, J. Lin and P. Huang, Adv. Mater., 2019, 31, 1808325 CrossRef PubMed.
- T. A. Muluh, X. Shu and Y. Ying, Biomed. Pharmacother., 2023, 162, 114658 CrossRef CAS PubMed.
- L. Lei, W. Dai, J. Zhao, A. Jiang, H. Peng, Q. Jin, X. Li and Z. Tang, Adv. Sci., 2025, e04860 CrossRef CAS PubMed.
- Y. Su, K. Lu, Y. Huang, J. Zhang, X. Sun, J. Peng, Y. Zhou and L. Zhao, Biomaterials, 2023, 294, 122017 CrossRef CAS PubMed.
- Z. Chen, M. Liu, M. Zhang, S. Wang, L. Xu, C. Li, F. Gao, B. Xie, Z. Zhong and X. Zhang, Adv. Funct. Mater., 2018, 28, 1803498 CrossRef.
- Y. Zhou, F. Tong, W. Gu, S. He, X. Yang, J. Li, Y.-D. Gao and H. Gao, Acta Pharm. Sin. B, 2022, 12, 1416–1431 CrossRef CAS PubMed.
- X. Zhang, J. Bai, S. Sun, Y. Li, X. Li, G. Meng, W. Cheng, Y. Yin, Z. Wang and B. Wang, Biomaterials, 2025, 319, 123203 CrossRef CAS PubMed.
- H. Wang, X. Huang, R. Gao, K. Li, D. Li, Z. Xu, Z. Ling, C. Pan, L. Gao and H. Chen, ACS Appl. Mater. Interfaces, 2025, 17, 7275–7290 CrossRef CAS PubMed.
- R. Dou, L. Wang, J. Zhang, X. Cai, J. Tang, X. Liu, Y. Hu and J. Chen, Small, 2025, 21, 2409052 CrossRef CAS PubMed.
- K. C. Valkenburg, A. E. De Groot and K. J. Pienta, Nat. Rev. Clin. Oncol., 2018, 15, 366–381 CrossRef PubMed.
- Y. Liu, X. Wu, F. Chen, H. Li, T. Wang, N. Liu, K. Sun, G. Zhou and K. Tao, Biomaterials, 2022, 289, 121813 CrossRef CAS PubMed.
- J. Chen, S. Li, X. Liu, S. Liu, C. Xiao, Z. Zhang, S. Li, Z. Li and X. Yang, Nanoscale, 2021, 13, 9989–10001 RSC.
- S.-B. Wang, Z.-X. Chen, F. Gao, C. Zhang, M.-Z. Zou, J.-J. Ye, X. Zeng and X.-Z. Zhang, Biomaterials, 2020, 234, 119772 CrossRef CAS PubMed.
- X. Zhu, M. Wang, H. Wang, Y. Ding, Y. Liu, Z. Fu, D. Lin, C. Lu and X. Tu, Small, 2022, 18, 2204951 CrossRef CAS PubMed.
- M. Potente, H. Gerhardt and P. Carmeliet, Cell, 2011, 146, 873–887 CrossRef CAS PubMed.
- X. Sun, N. Ni, Y. Ma, Y. Wang and D. T. Leong, Small, 2020, 16, 2003000 CrossRef CAS PubMed.
- L. Feng, D. Tao, Z. Dong, Q. Chen, Y. Chao, Z. Liu and M. Chen, Biomaterials, 2017, 127, 13–24 CrossRef CAS PubMed.
- M. Xia, Y. Yan, H. Pu, X. Du, J. Liang, Y. Sun, J. Zheng and Y. Yuan, Chem. Eng. J., 2022, 442, 136295 CrossRef CAS.
- Y. Wei, Z. Chen, C. Huang, H. Cheng, X. Jiang and S. Li, Chem. Eng. J., 2024, 488, 150822 CrossRef CAS.
- G. N. Masoud and W. Li, Acta Pharm. Sin. B, 2015, 5, 378–389 CrossRef PubMed.
- V. Petrova, M. Annicchiarico-Petruzzelli, G. Melino and I. Amelio, Oncogenesis, 2018, 7, 10 CrossRef PubMed.
- Q. Zhou, Q. T. H. Shubhra, P. Lai, J. Shi, C. Fang, Q. Guo, W. Li, R. Chen, X. Shen, L. Huang, X. Cai and S. Lin, Adv. Compos. Hybrid Mater., 2025, 8, 145 CrossRef CAS.
- Y. Wang, J. Huo, S. Li, R. Huang, D. Fan, H. Cheng, B. Wan, Y. Du, H. He and G. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 10092–10101 CrossRef CAS PubMed.
- B. Lu, Z. Zhang, Y. Huang, Y. Zhang, J. Wang, Y. Ding, Y. Wang and Y. Yao, Chem. Commun., 2022, 58, 10353–10356 RSC.
- Y. Wang, J. Li, Y. Zhang, Y. Nan and X. Zhou, Chem. Commun., 2022, 58, 7797–7800 RSC.
- T. Xiong, Y. Chen, M. Li, X. Chen and X. Peng, Small, 2025, 21, 2501911 CrossRef CAS PubMed.
- M. Yang, L. Zhou, Y.-Y. Zhao, K. M. K. Swamy, A. Chen and J. Yoon, Sci. Bull., 2025, 70, 1203–1206 CrossRef CAS PubMed.
- X. Du, S. Huang, Z. Lin, G. Chen, Y. Jiang and H. Zhang, Chem. Commun., 2025, 61, 7236–7252 RSC.
- S. Kuang, L. Sun, X. Zhang, X. Liao, T. W. Rees, L. Zeng, Y. Chen, X. Zhang, L. Ji and H. Chao, Angew. Chem., Int. Ed., 2020, 59, 20697–20703 CrossRef CAS PubMed.
- H. Bayır, S. Dixon, Y. Tyurina, J. Kellum and V. Kagan, Nat. Rev. Nephrol., 2023, 19, 315–336 CrossRef PubMed.
- X. Liu, Y. Yang, M. Ling, R. Sun, M. Zhu, J. Chen, M. Yu, Z. Peng, Z. Yu and X. Liu, Adv. Funct. Mater., 2021, 31, 2101709 CrossRef CAS.
- X. Luo, Q. Jiao, S. Pei, S. Zhou, Y. Zheng, W. Shao, K. Xu and W. Zhong, Adv. Healthcare Mater., 2024, 13, 2401787 CrossRef CAS PubMed.
- C. Zhang, D. Zhao, F. Fang, L. Zhu, W. Li, S. Wang, Y. Fan, J. Yang, Y. Liu and J. Zhang, Adv. Funct. Mater., 2025, 35, 2401840 CrossRef CAS.
- S. Yao, F. Xu, Y. Wang, J. Shang, S. Li, X. Xu, Z. Liu, W. He, Z. Guo and Y. Chen, J. Am. Chem. Soc., 2025, 147, 11132–11144 CrossRef CAS PubMed.
- D. Zhang, K. Teng, L. Zhao, L. Niu and Q. Yang, Adv. Mater., 2023, 35, 2209789 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |