The revelation of glucose adsorption mechanisms on hierarchical metal–organic frameworks using a surface plasmon resonance sensor†
Gilang
Gumilar
*abc,
Silvia
Chowdhury
 d,
Ganes
Shukri
d,
Ganes
Shukri
 bc,
Aep
Patah
e,
Nugraha
Nugraha
bc,
Joel
Henzie
bc,
Aep
Patah
e,
Nugraha
Nugraha
bc,
Joel
Henzie
 f,
Isa
Anshori
g,
Yusuf Valentino
Kaneti
d and
Brian
Yuliarto
f,
Isa
Anshori
g,
Yusuf Valentino
Kaneti
d and
Brian
Yuliarto
 *bc
*bc
aFaculty of Vocational Studies, Institut Teknologi Sains Bandung, Central Cikarang, Bekasi 17530, Indonesia. E-mail: gilang.gumilar@itsb.ac.id
bResearch Center for Nanoscience and Nanotechnology (RCNN), Institut Teknologi Bandung, Bandung 40132, Indonesia. E-mail: brian@itb.ac.id
cAdvanced Functional Materials Research Group, Faculty of Industrial Technology, Institut Teknologi Bandung, Bandung 40132, Indonesia
dSchool of Chemical Engineering and Australian Institute for Bioengineering and Nanotechnology (AIBN), The University of Queensland, Brisbane, QLD 4072, Australia
eInorganic & Physical Chemistry Research Division, Institut Teknologi Bandung, Bandung 40132, Indonesia
fInternational Center for Materials Nanoarchitectonics (WPI-MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan
gLab-on-Chip Group, Biomedical Engineering Department, Institut Teknologi Bandung, Bandung 40132, Indonesia
First published on 28th March 2023
Abstract
The gold layer on the surface plasmon resonance (SPR) sensor chip cannot detect small molecules, such as glucose without the use of specific receptors. Metal–organic frameworks (MOFs) are useful in biosensing technologies for capturing and co-localizing enzymes and receptors with the target biomolecule. In many previous studies, the properties of the MOFs were often ignored, with these studies focusing on the selection of appropriate receptors. To take advantage of the unique properties of MOFs in biosensors, one must also consider the technique and transducer used because these aspects will strongly influence the detection mechanism. In this work, we have investigated for the first time, the applications of hierarchical metal-BDC (M-BDC) MOFs for glucose detection using the SPR technique without the use of specific receptors. The underlying interactions and adsorption mechanisms were analyzed using adsorption isotherm and kinetic models. The sensing measurements show that the SPR chips functionalized with M-BDC MOFs exhibit higher sensitivity and lower limit of detection (LOD). Specifically, the sensitivity follows the order of Zr-BDC > Cu-BDC > Mn-BDC > Ni-BDC > bare Au SPR chips with the LOD in the order of Zr-BDC < Mn-BDC < Ni-BDC < Cu-BDC < bare Au SPR chips. The selectivity test results reveal that Zr-BDC exhibits a decent selectivity to glucose in the presence of other interfering compounds, such as ascorbic acid, uric acid, maltose, and urea. These results demonstrate the promising potential of MOFs for SPR biosensing.
1. Introduction
The blood glucose level is one of the most important parameters for monitoring diabetes. Diabetes is commonly caused by the inability of the pancreas to meet the demand for insulin in the body or the ineffective use of insulin by the body. Diabetes mellitus (DM) is divided into two categories, namely, type 1 and type 2. Type 1 diabetes is referred to as insulin-dependent or juvenile/childhood-onset diabetes characterized by a lack of insulin production. Meanwhile, type 2 diabetes, also known as non-insulin-dependent or adult-onset diabetes, is caused by the ineffective use of insulin by the body.1 A high glucose level in the blood or urine may indicate either that the insulin is not used effectively by the body or the pancreas is unable to produce sufficient insulin for the body. The blood glucose level under physiopathological conditions is controlled by the human body to be around 2–30 mM.2,3 According to the World Health Organization (WHO), the number of diabetics significantly rose from 108 million adults in 1980 to 422 million adults in 2014. Furthermore, the International Diabetes Federation (IDF) projected that the number of diabetics would increase to 592 million by 2035.1,4 To minimize the risk of diabetes, it is important to develop an inexpensive sensing technology that can detect and monitor blood glucose levels.There has been growing interest in the development of optical sensors for detecting blood glucose levels. Surface plasmon resonance (SPR) sensing is at the forefront of this research because light can excite free electrons on the surface of a noble metal, such as gold (Au) or silver (Ag), to generate surface plasmon polaritons (SPPs). These SPPs propagate as spatially confined evanescent waves in a narrow region on the top surface of the metal and interact with the surrounding dielectric environment.5–7 Adsorption of the target analyte by the ligand will change the SPP resonance angle (θSP) further, allowing for the real-time monitoring of molecular binding without the need for labeling. SPR sensors have fast responses, high sensitivity, and relatively high specificity depending on the analyte-capturing ligand.8–10 However, they are the most sensitive to large molecular weight biomolecules because the large size generates a greater change in the refractive index. Thus, small molecules like glucose (∼180 Da) are difficult to detect using SPR sensors.8,11
To overcome this challenge, several studies have employed surface functionalization as a strategy to enhance the adsorption of small molecules. For example, polymers,12,13 zinc oxide,9,10 and silica-based glucose-sensitive membranes14 have all been employed to improve glucose detection with SPR. These studies use glucose oxidase (GOx) to enhance surface binding of glucose molecules. However, the enzymatic activity of GOx is highly dependent on pH and temperature and may decrease over time.3,15,16 Besides, in the immobilization process onto the sensor surface, the use of polymers also requires a strong adhesive compound.16 Other studies used boronic acid compounds with high affinity towards glucose, but they were highly dependent on the pH.15,17,18 Glucose/galactose binding proteins (bacteria) have also been used in the detection of glucose by SPR, which led to a good response, even near the minimum concentration of glucose in the human body.11 Unfortunately, the SPR biosensor required labeling and genetic engineering to detect high concentrations of glucose.15
Metal oxides, metal hydroxides, and MOFs have been previously employed in non-enzymatic glucose detection.19–25 MOFs can increase the bonding interactions with the analyte and serve as active sites to drive electrocatalytic reactions. Host–guest interactions because of Lewis acid or base sites in ligands, open metal sites, hydrophobic interactions, and aromatic groups in MOFs can be utilized to increase the selectivity towards the target analyte. Moreover, the large surface area of MOFs provides rich active sites for catalytic processes and the interconnected porosity eases the diffusion of the analyte to access the active sites. The electron donor–acceptor activity on the MOF structure ligands affects the orbital energy levels of the electrochemical devices.26–28 MOFs can greatly enhance glucose adhesion in SPR by coordination with the metal ions and multifunctional organic ligands.29 For example, Hang et al. used the SPR technique to detect glucose using core–shell Au@MIL-100(Fe).30 This study modified the core–shell MOF with 3-aminophenylboronic acid hemisulfate (PBA) which led to high responses toward glucose in the concentration range of 0–40 mM. The study further revealed that the modification of MOF with PBA could enhance the glucose adsorption by increasing the wavelength shift. Another study employed ZIF-8 as a glucose collector in waveguide-based optical fiber biosensors.31 The biosensors were coated with a GOx shell to protect them from harsh environments. The wavelength shift had a linear relationship with the glucose concentration in the range of 1–8 mM with a response coefficient of ∼0.5 nm mM−1. Although this optical fiber biosensor showed promising results for glucose detection, the ZIF-8 was only used as a matrix for encapsulating the GOx, so the MOF did not play a central role in glucose detection.
In a previous report, we employed hierarchical M-BDC MOFs for electrochemical non-enzymatic glucose detection. The results showed that the hierarchical sheet-like Ni-BDC had considerable electrocatalytic activity towards glucose with a sensitivity of 635.9 μA mM−1 cm2 in the concentration range of 0.01–0.8 mM and LOD of 6.68 μM (S/N = 3). Other M-BDC MOFs (M = Cu, Mn, and Zr) did not show any electrochemical response towards glucose, thus indicating the limitations of electrochemical detection.32 Zeng et al. studied several MOFs [ZIF-8, MIL-53(Cr), MIL-96(Al), MIL-100(Cr), MIL-100(Fe), MIL-101(Cr), and UiO-66] for the adsorptive separation of a fructose–glucose mixture. They discovered the presence of strong hydrogen bonding interactions between the hydroxyl groups of glucose and fructose with a Zr metal center in UiO-66 (i.e. Zr-BDC).33 To develop M-BDC MOF-based SPR sensors, we will exploit these interactions and explore the possibility of other functional groups that play a role in glucose binding because the different metal types will affect the open metal sites (OMS) and the available functional groups. Additionally, it is also necessary to study the underlying glucose adsorption mechanisms on the surface of M-BDC MOFs and their interactions in the absence of enzymes or other specific bioreceptors. This will enable the main properties that cause the change of the dielectric constant during the detection to be revealed.
In this work, hierarchical sheet-/plate-like M-BDC (M = Cu, Mn, Ni, and Zr) MOFs were synthesized using our previous method and subsequently hybridized with the Au surface on the SPR chip sensor.32 The SPR measurements for non-enzymatic glucose sensing were carried out using phosphate buffer saline (PBS) at pH 7.4 in the glucose concentration range 0.1–20 mmol L−1. Then, the dynamic responses of the M-BDC MOFs were analyzed by adsorption isotherms and kinetic models to determine the adsorption mechanisms and to obtain the affinity constants. The results reveal that the M-BDC MOF-functionalized SPR sensors can respond to glucose but with different adsorption mechanisms. The functionalization of bare Au SPR chip sensor with M-BDC can enhance glucose adsorption at low concentrations even without using GOx or other receptors. Among all the fabricated SPR sensors, the Zr-BDC-functionalized sensor shows the highest sensitivity towards glucose as it has the highest specific area and adsorption capacity. Therefore, this MOF can facilitate effective glucose diffusion, enhance accessibility to the active sites, and promote a high adsorption rate. Moreover, it also exhibits relatively good selectivity towards glucose in the presence of other interfering compounds, such as uric acid (UA), ascorbic acid (AA), urea (U), and maltose (M).
2. Experimental section
The detailed chemicals, fabrication procedures for hierarchical M-BDC (M = Cu, Mn, Ni, and Zr), and functionalization of SPR sensor chips with M-BDC MOFs are provided in the ESI.†2.1. SPR measurements
The SPR measurements were performed using a NanoSPR 6 device with two-channel systems (NanoSPR LLC, US) and the laser was emitted by the GaAs semiconductor laser with a wavelength of 650 nm through a prism for collimating, polarizing, and coupling processes. The SPR sensorgrams were exhibited in the SPR angular changes and time parameters for each glucose concentration (0.1–20 mmol L−1). A syringe pump was used for injecting the glucose solution into the measurement chamber through a flow cell with a flow rate of 25 μL min−1. The measurement procedures were as follows. First, the baseline was taken by measuring the SPR response for the PBS flows on the chamber in 8 minutes. The glucose solution association onto the M-BDC surface was taken for 8 minutes and the dissociation process (using PBS) took 8 minutes as well. The measurements were carried out in series, that is, after the measurement for one glucose concentration was completed, immediately, the next concentration of glucose was flowed into the chamber until all glucose concentrations were tested.2.2. Adsorption isotherm studies
Adsorption isotherm analysis was carried out to determine the interactions between glucose molecules and M-BDC MOFs during SPR measurements. The obtained parameters from adsorption isotherm models could be used to obtain information on the sorption mechanism, surface properties, and M-BDC affinity towards glucose. The adsorption experiments reported in previous studies typically employ more than one model.34–38 This is typically done to determine the adsorption mechanisms and the interactions between glucose and the adsorbent more precisely. Several reports have compared the two-parameter and three-parameter adsorption isotherm models to understand the adsorption process and the adsorbate–adsorbent interaction.34–38Sarıkaya et al. modified the general adsorption isotherm model by changing the physical quantity of adsorption capacity (q) with the physical quantity of the intensity change (ΔR) directly.34 In the present work, the adsorption capacity (q) was changed with the physical quantity of angular change (Δθ) and the two-parameter adsorption isotherm models used were Langmuir,35 Freundlich,35 Jovanovic,35 Temkin,38 and Dubinin–Radushkevich.38 Meanwhile, the three-parameter adsorption isotherm models used were Langmuir–Freundlich (Sips),35,39 Vieth–Sladek,35 Brouers–Sotolongo,35 Redlich–Peterson,35 and Toth.35 The non-linear regression analysis was performed using Origin Lab software. The equations of the adsorption isotherm models are shown Table S1 (ESI†). The isotherm parameters that were used to determine the accuracy of the theoretical model on the experimental data were chosen based on the correlation coefficient (R2) of the non-linear fitting. If the value of R2 is closer to 1, the model will be used to predict the glucose adsorption mechanism on the M-BDC system used in the experiment. From the selected model, the sensitivity of the M-BDC-functionalized SPR sensor could be obtained from the linear plot of the model and then analyzed using the linear regression method.
2.3. Adsorption kinetic studies
Adsorption kinetic studies were conducted to analyze the adsorption behavior of glucose on different M-BDC MOFs. In this work, we applied the pseudo-first order,35,36 pseudo-second order,35,36 Elovich,36 and Avrami35,36 adsorption kinetic models on our experimental data. Similar to the adsorption isotherm model, the physical quantity q in the equation of the adsorption kinetic model was modified into the change in angle (Δθ) and it was analyzed using the non-linear regression in Origin Lab software. All adsorption kinetic model equations are given in Table S2 (ESI†).2.4. Selectivity, reusability, and leaching tests
The selectivity test was carried out using the optimum M-BDC-functionalized SPR sensor, for non-enzymatic glucose sensing in the presence of interfering compounds, such as uric acid (UA), ascorbic acid (AA), urea (U), and maltose (M). The concentrations of the interfering compounds were adjusted to the highest concentration limit in healthy human blood, i.e., 0.45 mmol L−1 for UA, 0.11 mmol L−1 for AA, 5 mmol L−1 for U, and 0.1 mmol L−1 for M. The concentration of glucose used in the selectivity test was 5 mmol L−1, which was adjusted to the physiopathological conditions of blood glucose level range (2–30 mmol L−1).2 In addition, the selectivity test was also conducted using the same concentration of molecules with closely related structures as glucose, such as fructose (F) and sucrose (S). The selectivity relationship of the interfering compounds with glucose is expressed by the selectivity coefficient equation,34 which is shown in the ESI.† The reusability test was carried out using the optimum M-BDC-functionalized SPR sensor towards 5 mmol L−1 glucose for 5 consecutive measurements (for the reusability calculation, please see the ESI†). The leaching test was performed to investigate the stability of the M-BDC MOFs on the surface of SPR chip sensors. The waste solutions obtained after the SPR measurements were collected for further analysis by ICP-OES (Inductively Coupled Plasma – Optical Emission Spectrometry) to identify the presence or absence of Cu, Ni, Mn, and Zr metals after adsorption.2.5. Characterization
The morphology of the M-BDC MOFs was characterized using a scanning electron microscope (SEM, Hitachi SU-8000) operated at 10 kV. The crystal structure and composition of the M-BDC MOFs were checked by X-ray diffraction (XRD, Rigaku RINT 2500X) with Cu-Kα radiation (λ = 0.15406 nm). The Fourier Transform Infrared (FTIR) spectra of the samples before and after the SPR measurements were collected using a Bruker Alpha in the wavenumber range of 500 to 4000 cm−1 to investigate the interactions between the functional groups of glucose and M-BDC MOFs. X-Ray photoelectron spectroscopy (XPS) was used to investigate the elemental compositions and electronic states of the M-BDC MOFs. In order to calibrate the high-resolution XPS peaks concerning the C 1s peak at 285 eV, CasaXPS software was used. ICP-OES (ICP-OES 715-ES, Varian Inc.) was used to identify the metal content in the waste solutions obtained after the SPR measurements.2.6. Theoretical calculations
First-principles density functional theory (DFT) calculations were performed using Gaussian 09 package code. Generalized Gradient Approximation using the Perdew–Burke–Ernzerhof (GGA-PBE) functional was used to describe the exchange interaction and electronic correlations. We used the 3–21G basis sets for the H, O and C atoms. Furthermore, LANL2DZ was used for the three representative transition metals considered in this study (Zr, Cu and Ni).2.7. Detection of glucose in human serum
First, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 volume ratio of human serum in PBS was used to prepare the glucose solution in human serum (Vhuman
100 volume ratio of human serum in PBS was used to prepare the glucose solution in human serum (Vhuman![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) serum: VPBS). The preparation of glucose solutions with concentrations of 0.1–20 mmol L−1 was subsequently done using this human serum solution as the solvent. The SPR measurement conditions for glucose sensing in blood serum were similar to those described in Section 2.1, except that human serum solution was utilized as the measurement medium.
serum: VPBS). The preparation of glucose solutions with concentrations of 0.1–20 mmol L−1 was subsequently done using this human serum solution as the solvent. The SPR measurement conditions for glucose sensing in blood serum were similar to those described in Section 2.1, except that human serum solution was utilized as the measurement medium.
3. Results and discussion
3.1. Structural and compositional characterizations of M-BDC MOFs
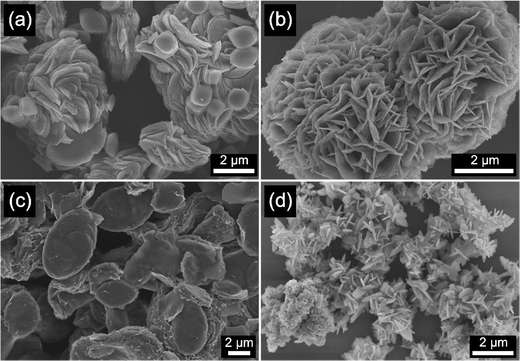
As shown in Fig. 1, all the prepared M-BDC samples exhibit a hierarchical 3D morphology, which is an assembly of two-dimensional (2D) plate- or sheet-like particles. The hierarchical Cu-BDC sample is arranged by the stacking of numerous plate-like particles (Fig. 1a). In comparison, the Mn-BDC sample exhibits a hierarchical flower-like morphology which is an assembly of nanosheets (Fig. 1b). Furthermore, the Ni-BDC sample displays a hierarchical multilayered structure which is an assembly of sheet-like particles (Fig. 1c). Furthermore, the Zr-BDC sample has a well-defined hierarchical morphology which is composed of plate-like particles of 59 nm in thickness (Fig. 1d).The formation of the hierarchical 3D M-BDC MOFs under solvothermal conditions at 135 °C for 24 h is caused by the good solvation of metal ions in solution by acetonitrile which allows for the interaction of metal ions with the deprotonated BDC linker. During the synthesis process, PVP serves as a shape-control agent by decreasing the MOF crystal growth and promoting bind metal cations and metal surfaces through the strong interactions >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → M and forming weak hydrogen bonds with organic molecules.32,40–45
O → M and forming weak hydrogen bonds with organic molecules.32,40–45
XRD patterns of the as-synthesized hierarchical M-BDC MOFs are presented in Fig. 2. The XRD pattern of the hierarchical plate-like Cu-BDC sample matches well with the reference pattern for Cu-BDC with the C2/m space group (CCDC no. 687690),46 with the major peaks at around 10.3°, 12.2°, 17.26°, and 24.86° assigned to (110), (020), (20![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), and (131) planes, respectively (Fig. 2a). The hierarchical sheet-like Mn-BDC product exhibits peaks at around 9.77°, 10.27°, 18.59°, and 20.75° indexed to (11
), and (131) planes, respectively (Fig. 2a). The hierarchical sheet-like Mn-BDC product exhibits peaks at around 9.77°, 10.27°, 18.59°, and 20.75° indexed to (11![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), (20
), (20![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ), (112), and (311) planes of Mn-BDC with the C2/c space group (Fig. 2b). This results matches with the reference pattern for Mn-BDC (CCDC no. 265904) reported by Rosi et al.47 For the hierarchical sheet-like Ni-BDC sample (Fig. 2c), the XRD pattern is in good agreement with the reference pattern for Ni-BDC (CCDC no. 638866) previously reported by Carton and co-workers.48 The major peaks observed at around 9.26° and 20.2° can be assigned to the (100) and (400) planes of Ni-BDC with the P
), (112), and (311) planes of Mn-BDC with the C2/c space group (Fig. 2b). This results matches with the reference pattern for Mn-BDC (CCDC no. 265904) reported by Rosi et al.47 For the hierarchical sheet-like Ni-BDC sample (Fig. 2c), the XRD pattern is in good agreement with the reference pattern for Ni-BDC (CCDC no. 638866) previously reported by Carton and co-workers.48 The major peaks observed at around 9.26° and 20.2° can be assigned to the (100) and (400) planes of Ni-BDC with the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. As seen in Fig. 2d, the XRD pattern of the hierarchical plate-like Zr-BDC sample displays strong peaks at approximately 9.77°, 10.27°, and 20.75° that can be indexed to the (111), (200), and (400) planes of Zr-BDC with the Fm
space group. As seen in Fig. 2d, the XRD pattern of the hierarchical plate-like Zr-BDC sample displays strong peaks at approximately 9.77°, 10.27°, and 20.75° that can be indexed to the (111), (200), and (400) planes of Zr-BDC with the Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group (CCDC no. 733458).49,50
m space group (CCDC no. 733458).49,50
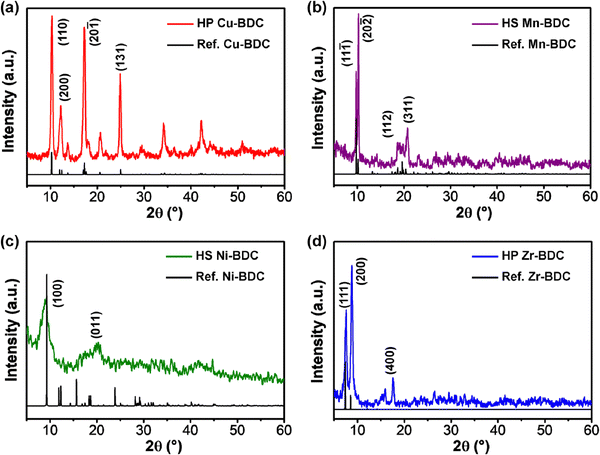 | ||
| Fig. 2 XRD patterns of hierarchical (a) Cu-BDC, (b) Mn-BDC, (c) Ni-BDC, and (d) Zr-BDC. HS = Hierarchical sheet-like and HP = Hierarchical plate-like. | ||
3.2. SPR measurements
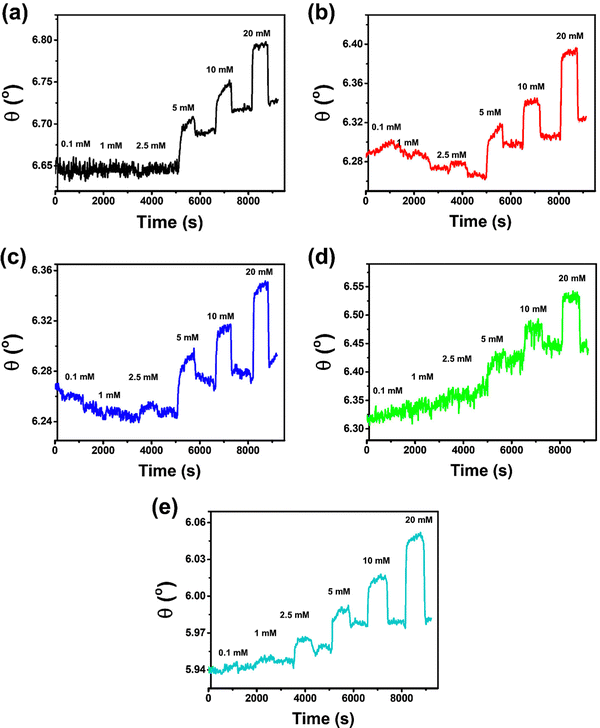
The Cu-BDC-functionalized SPR sensor shows angular changes of 0.0106°, 0.0064°, 0.0072°, 0.0546°, 0.0483°, and 0.0829° upon exposure to 0.1, 1, 2.5, 5, 10, and 20 mmol L−1 of glucose (in PBS solution), respectively (Fig. 3b). However, after the ablution process of 0.1 mmol L−1 glucose from the chip surface with PBS solution, the SPR signal initially decreases until below the baseline and becomes stabilized after running the PBS for the dissociation process at 1 mmol L−1 of glucose. However, beyond this point, the response of the Cu-BDC-based SPR sensor continues to increase with increasing glucose concentration, as normally expected. The initial decrease in the SPR signal of Cu-BDC may be caused by its lower stability in an aqueous environment, leading to some structural decomposition due to the hydrolysis reaction between water molecules and the Cu–O–C group in Cu-BDC.51–53 Similar to the case of Cu-BDC, the response of the Ni-BDC-based SPR sensor initially also decreases at the beginning between 0.1 and 1 mmol L−1 of glucose (Fig. 3c). However, above this concentration, the response continues to increase with the rise in concentration up to 20 mmol L−1. The angular changes towards glucose concentrations of 0.1, 1, 2.5, 5, 10, and 20 mmol L−1 are 0.0021°, 0.0006°, 0.0103°, 0.0494°, 0.0511°, and 0.0727°, respectively, for the Ni-BDC-based SPR sensor. Although the SPR signals of both sensors initially decrease at the beginning, the dynamic responses towards glucose are still distinguishable from the baseline for each glucose concentration and an upward trend in response is subsequently observed for both sensors.
Unlike Cu-BDC and Ni-BDC, the response of the Mn-BDC-functionalized SPR sensor appears to increase continuously with the increase of glucose concentration up to 5 mmol L−1 of glucose (Fig. 3d). This may have occurred because Mn-BDC is less stable in the aqueous environment. Based on the principle of coordination chemistry, the metal–ligand bond in Mn-BDC is less stable so kinetically, to coordinate in solution, the ligand will tend to compete with solvent molecules, such as water.54 Consequently, the dynamic response of the Mn-BDC-based SPR sensor continues to increase even without the addition of glucose because Mn-BDC may bind directly with the water molecules. The angular changes produced by the interaction between Mn-BDC and glucose at concentrations of 0.1, 1, 2.5, 5, 10, and 20 mmol L−1 are 0.0147°, 0.0117°, 0.0203°, 0.0568°, 0.0591°, and 0.0850°, respectively. For the Zr-BDC-functionalized SPR sensor (Fig. 3e), the response that is generated upon exposure to PBS solution at the beginning of measurement appears stable. Then, after 0.1 mmol L−1 of glucose solution is injected, the dynamic response is observed to increase and the dissociation process with the use of PBS solutions, response decreased slightly. The angular changes produced by the Zr-BDC-based SPR sensor at glucose concentrations of 0.1, 1, 2.5, 5, 10, and 20 mmol L−1 are 0.0006°, 0.0076°, 0.0185°, 0.0431°, 0.1177°, and 0.1496°, respectively. These results clearly indicate that the greater the glucose concentration, the greater the response of the Zr-BDC-functionalized SPR sensor.
The detection of 0.1 mmol L−1 glucose by the SPR sensors based on Cu-BDC, Mn-BDC, and Ni-BDC MOFs is somewhat unsatisfactory. At low glucose concentrations (0.1–1 mmol L−1), there may be binding competition between glucose and water molecules in these M-BDC MOFs. Hence, the dynamic responses obtained at such low concentrations may be less reliable. Therefore, the glucose concentration of 0.1 mmol L−1 in the case of Cu-BDC, Mn-BDC, and Ni-BDC was not included in the non-linear regression analysis.
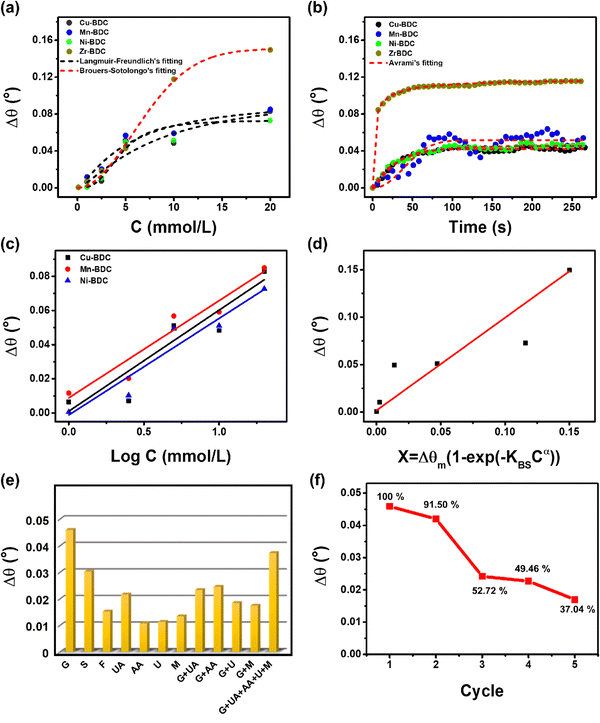
According to the Langmuir–Freundlich adsorption model, the distribution of adsorption energy occurs on a heterogeneous surface where the adsorbate adsorption with high concentration becomes the Langmuir isotherm model and, at low concentration, the adsorption mechanism becomes the Freundlich model.39 The Freundlich model determines the adsorption process with the adsorbate binding mechanism on a multilayer site whereas the Langmuir model assumes binding at a homogeneous binding site.34 The heterogeneous parameter (MLF) values for Cu-BDC, Mn-BDC and Ni-BDC are 1.3251, 1.28131, and 2.95308, respectively, which are greater than 1. This indicates that in these MOFs, the adsorption process occurs by cooperative interactions as described in the adsorption isotherm model. In cooperative interactions, the adsorbate has the ability to bind at one site on the adsorbent, which in turn, affects other binding sites on the same adsorbent (binding of different species on a homogeneous layer).55,56 In comparison, the Zr-BDC-based SPR sensor follows the Brouers–Sotolongo adsorption isotherm model where the interaction has a high heterogeneity and is complex in terms of the sorption energy distribution. The high heterogeneity of the Zr-BDC surface in glucose adsorption can be identified from the value of exponent α, where the greater the value, the higher the heterogeneity.57–59 The obtained exponent α value from the non-linear regression analysis is 1.95791. Since the exponent α > 1, the adsorption process is a slow initial biosorption kinetics process so the active sites would have dissimilar energy.57 Further analyses of the adsorption isotherm data are provided in the ESI.†
The maximum adsorption capacity (Δθm) values of Cu-BDC, Mn-BDC, Ni-BDC, and Zr-BDC are 0.10432 mmol g−1, 0.09717 mmol g−1, 0.07345 mmol g−1, and 0.15067 mmol g−1, respectively. The Δθm value of Zr-BDC is the highest among the samples, due to the higher surface area of this compared to the other M-BDC MOFs. From our previous work, the specific areas of hierarchical Zr-BDC, Mn-BDC, Cu-BDC, and Ni-BDC samples were measured to be 1248.4, 93.7, 90.2, and 34.7 m2 g−1, respectively.32 Therefore, the specific surface area plays a major role in increasing the adsorption capacity.60 The affinity constants for Cu-BDC, Mn-BDC, Ni-BDC and Zr-BDC are 0.12146, 0.18805, 0.22405, and 0.01614 mmol L−1, respectively.
From the non-linear regression results (Table S5, ESI†), it can be concluded that, for all M-BDC MOFs, the highest correlation coefficient is obtained using the Avrami model with R2 values of 0.96849, 0.78500, 0.95603, and 0.98447 for Cu-BDC, Mn-BDC, Ni-BDC, and Zr-BDC, respectively (Fig. 4b). The adsorption processes that take place can be identified by the values of KAV and nAV. In the study of Li et al., nAV was found to be closely related to the process of releasing adsorbates from the adsorbents;29 however, in this study, nAV is associated with the glucose adsorption by M-BDC. If the nAV value is in the range of 0.3–1, the adsorption process that occurs is a diffusion process, while nAV values between 1 and 2 indicate that the adsorption process is similar to a first-order kinetic process.29 However, according to the report of Oladoja et al.,61 if the nAV value is between 1 and 2, it indicates a one-dimensional (1D) crystal growth. Both studies used the same parameters to describe the different functions, i.e., adsorption and crystal growth. Therefore, Oladoja et al.61 stated that 2D crystal growth occurs when the nAV value is in the range of 2–3 and using a similar analogy, the adsorption mechanism is a second-order kinetic process.
The Cu-BDC MOF has an nAV value of 1.28621, hence, the adsorption process is a first-order kinetic process. In contrast, the adsorption of glucose on the surface of Ni-BDC and Zr-BDC occurs through diffusion, as indicated by their nAV values of 0.92541 and 0.3307, respectively. However, for Mn-BDC the value is greater than 2 (2.198); therefore, the adsorption process is a second-order kinetic process. The reaction rate constants (KAV) of all M-BDC MOFs are different with the order being Zr-BDC (0.66681 s−1) > Ni-BDC (0.03987 s−1) > Cu-BDC (0.01291 s−1) > Mn-BDC (1.712 × 10−4 s−1). Based on Avrami's kinetic adsorption parameters, the relationship between KAV and nAV tends to be inversely related, that is, the greater the value of nAV, the smaller the value of KAV. Hence, it can be assumed that the adsorption mechanism determines the adsorption rate constant, KAV. Furthermore, the adsorption of glucose by Zr-BDC occurs by diffusion, so the process is faster compared to first-order (Cu-BDC) and second-order (Mn-BDC) kinetic processes. The second-order kinetic process on Mn-BDC is slower than the glucose adsorption by other M-BDC MOFs because it has the smallest adsorption rate of 1.712 × 10−4 s−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C plot from the Langmuir–Freundlich model (for Cu-BDC, Mn-BDC, and Ni-BDC) and the Δθ vs. X = Δθm [1 − exp(−KBSCα)] plot from the Brouers–Sotolongo model (for Zr-BDC).36,62 However, for the bare Au substrate, as the dynamic response only becomes measurable from an initial concentration of 5 to 20 mmol L−1, it does not follow a specific adsorption model (Fig. S4, ESI†). The calibration curves derived from the dynamic responses of M-BDC-functionalized SPR sensors (M = Cu, Mn, and Ni) towards glucose are given in Fig. 4c, whereas the calibration curve for Zr-BDC is given in Fig. 4d, and the obtained parameters are shown in Table 1.
C plot from the Langmuir–Freundlich model (for Cu-BDC, Mn-BDC, and Ni-BDC) and the Δθ vs. X = Δθm [1 − exp(−KBSCα)] plot from the Brouers–Sotolongo model (for Zr-BDC).36,62 However, for the bare Au substrate, as the dynamic response only becomes measurable from an initial concentration of 5 to 20 mmol L−1, it does not follow a specific adsorption model (Fig. S4, ESI†). The calibration curves derived from the dynamic responses of M-BDC-functionalized SPR sensors (M = Cu, Mn, and Ni) towards glucose are given in Fig. 4c, whereas the calibration curve for Zr-BDC is given in Fig. 4d, and the obtained parameters are shown in Table 1.
| MOFs | Linear equation | Sensitivity | LOD (mmol L−1) (S/N = 3) | Concentration range (mmol L−1) | R 2 |
|---|---|---|---|---|---|
| Bare Au | Δθ = 0.00151C + 0.04655 | 0.00151 | 14.763 | 5–20 | 0.9560 |
| Cu-BDC | Δθ = 0.05914![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C + 0.00107 C + 0.00107 |
0.05914 | 10.383 | 1–20 | 0.91254 |
| Mn-BDC | Δθ = 0.05689![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C + 0.00887 C + 0.00887 |
0.05689 | 4.790 | 1–20 | 0.95886 |
| Ni-BDC | Δθ = 0.05653![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C + 0.00118 C + 0.00118 |
0.05653 | 4.945 | 1–20 | 0.94295 |
| Zr-BDC | Δθ = 0.97702X + 0.00172 | 0.97702 | 4.499 | 0.1–20 | 0.98918 |
The linear regression analysis of Zr-BDC shows that it has a higher sensitivity (0.99702) than other M-BDC MOFs. Therefore, the order of sensitivity from the highest to the lowest is Zr-BDC > Cu-BDC > Mn-BDC > Ni-BDC > bare Au substrate. In terms of LOD, Zr-BDC shows the best LOD followed by Mn-BDC, Ni-BDC, Cu-BDC, and bare Au substrate, respectively. The use of hierarchical M-BDC as an active layer on the bare Au substrate can enhance the glucose adsorption capacity and therefore, the LODs of the M-BDC functionalized sensors are much lower than that of the bare Au substrate, allowing them to detect lower concentrations of glucose. Other than that, these results indicate that the Zr-BDC-functionalized SPR sensor is superior to those functionalized with Cu-BDC, Mn-BDC, and Ni-BDC in terms of both sensitivity and LOD. Therefore, for selectivity and reusability experiments, the hierarchical plate-like Zr-BDC was used as it is the optimum sample.
| Biomolecules | Δθ (°) | K | Biomolecules | Δθ (°) | k |
|---|---|---|---|---|---|
| Δθ is the angular change.k is the selectivity coefficient. | |||||
| G | 0.0459 | — | M | 0.0134 | 3.425 |
| S | 0.0302 | 1.518 | G + UA | 0.0233 | 1.970 |
| F | 0.0152 | 3.016 | G + AA | 0.0245 | 1.873 |
| UA | 0.0216 | 2.125 | G + U | 0.0184 | 2.495 |
| AA | 0.0107 | 4.290 | G + M | 0.0174 | 2.638 |
| U | 0.0113 | 4.062 | G + UA + AA + U + M | 0.0372 | 1.234 |
In addition, selectivity tests were also carried out on molecules with similar structures to glucose (G), namely sucrose (S) and fructose (F) with the same concentration as glucose. As depicted in Fig. S5a (ESI†), the response curves of the Zr-BDC-based SPR sensor returns to the baseline position following the dissociation of fructose and sucrose; however this is not the case for glucose. This suggests the ability of Zr-BDC to bind G more strongly than S and F. In addition, the angular change upon G exposure is more significant than those to both S and F with the order being G > S > F and k F > k S (see Table 2). Moreover, the characteristics of the three curves are different. For S and F exposure, after the initial increase in the angular change to a certain extent, the response eventually becomes saturated. However, for G exposure, the angular change continues to increase until the dissociation process begins. This phenomenon indicates that glucose continues to be bound by the hydroxyl group in Zr-BDC and saturation has not yet occurred. Hence, it can be deduced that the Zr-BDC is more specific for binding G than S and F.
The angular changes of Zr-BDC towards each interfering compound, in descending order, are G (Δθ = 0.0459°) > UA (Δθ = 0.0216°) > M (Δθ = 0.0459°) > U (Δθ = 0.0113°) > AA (Δθ = 0.0107°) with the selectivity coefficient values being k (UA) = 2.125, k (M) = 3.425, k (U) = 4.062, and k (AA) = 0.0107. The selectivity coefficient value indicates the selectivity of Zr-BDC towards glucose against competing molecules. If it has a value of k = 1, then the selectivity is relatively similar, and if the value of k <1, then Zr-BDC is more selective against competing molecules. As shown in Table 2, the competing molecules have k values >1, so Zr-BDC is not selective towards these compounds, and the response towards glucose can still be distinguished from the others.
To ensure the response to glucose can be distinguished from other compounds, selectivity tests were carried out on glucose mixtures with various interfering compounds with the same concentration as glucose. Following the addition of 5 mmol L−1 glucose, the response of Zr-BDC to competing molecules was increased, but the angular changes are still lower than that towards glucose itself (Table 2). The angular changes and the selectivity coefficients towards binary mixtures of G + UA, G + AA, G + U, G + M, and G + UA + AA + U + M are 0.0233° (k = 1.970), 0.0245° (k = 1.873), 0.0184° (k = 2.495), 0.0174° (k = 2.638), and 0.0372° (k =1.234), respectively (Fig. S5b, ESI†). These results reveal that Zr-BDC is more likely to interact with glucose even in the presence of other interfering compounds.
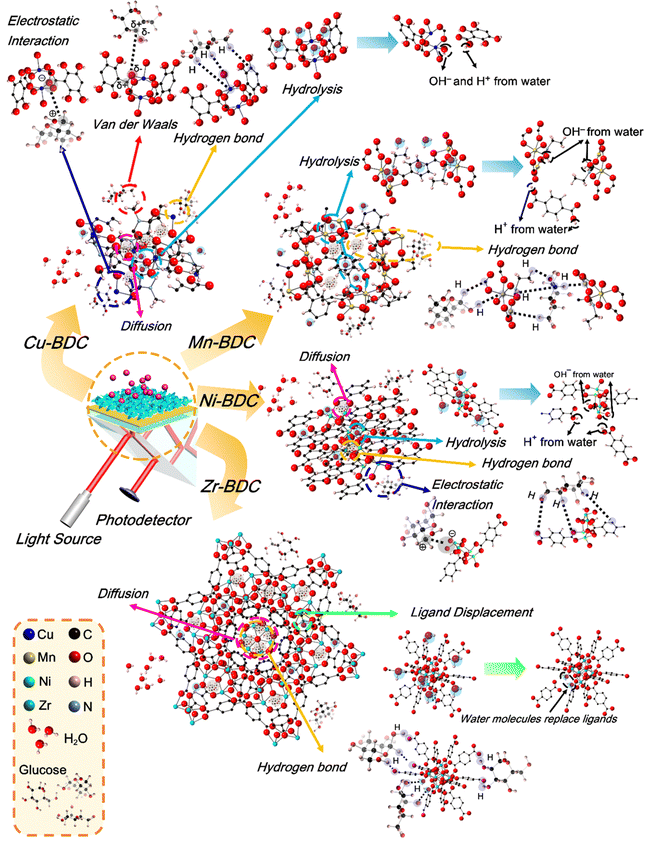
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending vibrations located between 806 and 959 cm−1 and 655–800 cm−1, respectively, and the bending vibration of Cu–O at 599 cm−1 also become more intense after glucose adsorption. Based on the adsorption analysis, the glucose adsorption on Cu-BDC involves cooperative interactions with the first-order kinetic process. The strengthening of the IR spectra indicates that glucose molecules interact cooperatively with these functional groups. Besides, the lack of broadening of these peaks, may indicate the occurrence of physisorption, where the binding process between the adsorbent and the adsorbate involve electrostatic interactions, van der Waals forces, diffusion, or hydrogen bonding.63,64
C bending vibrations located between 806 and 959 cm−1 and 655–800 cm−1, respectively, and the bending vibration of Cu–O at 599 cm−1 also become more intense after glucose adsorption. Based on the adsorption analysis, the glucose adsorption on Cu-BDC involves cooperative interactions with the first-order kinetic process. The strengthening of the IR spectra indicates that glucose molecules interact cooperatively with these functional groups. Besides, the lack of broadening of these peaks, may indicate the occurrence of physisorption, where the binding process between the adsorbent and the adsorbate involve electrostatic interactions, van der Waals forces, diffusion, or hydrogen bonding.63,64
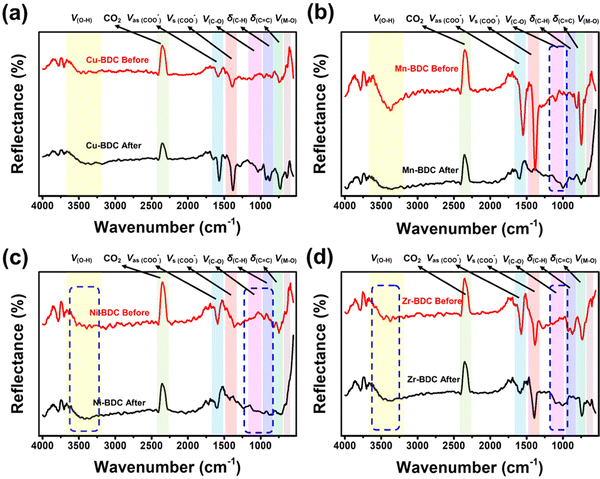 | ||
| Fig. 5 FTIR spectra of M-BDC-functionalized SPR sensors before and after non-enzymatic glucose sensing: (a) Cu-BDC, (b) Mn-BDC, (c) Ni-BDC, and (d) Zr-BDC. | ||
The IR spectra of Mn-BDC before and after glucose adsorption (Fig. 5b) reveal the weakening of the O–H stretching vibration, the asymmetric and symmetric stretching vibrations of carboxyl group, the C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bending vibrations, and Mn–O vibration observed in the original Mn-BDC. However, the C–O stretching vibration at ∼1000 cm−1 is slightly strengthened. The weakening of the IR bands after the glucose adsorption suggests the occurrence of chemisorption where the functional groups chemically react with the adsorbate and/or competing molecules to form chemical bonds.63,65 Zhang and co-workers showed that Mn-BDC could react with water molecules through Mn-carboxyl oxygen and Mn-hydroxyl oxygen bonds, accompanied by weakening of the Mn–O bond.66 The cooperative interaction and second-order kinetic process for glucose adsorption on Mn-BDC may be caused by the simultaneous binding of glucose and water molecules on the active sites, because the molecules used in the adsorption model are assumed to be homogeneous.
O bending vibrations, and Mn–O vibration observed in the original Mn-BDC. However, the C–O stretching vibration at ∼1000 cm−1 is slightly strengthened. The weakening of the IR bands after the glucose adsorption suggests the occurrence of chemisorption where the functional groups chemically react with the adsorbate and/or competing molecules to form chemical bonds.63,65 Zhang and co-workers showed that Mn-BDC could react with water molecules through Mn-carboxyl oxygen and Mn-hydroxyl oxygen bonds, accompanied by weakening of the Mn–O bond.66 The cooperative interaction and second-order kinetic process for glucose adsorption on Mn-BDC may be caused by the simultaneous binding of glucose and water molecules on the active sites, because the molecules used in the adsorption model are assumed to be homogeneous.
In Ni-BDC, several bands are weakened in intensity after the glucose adsorption, namely the asymmetric and symmetrical stretching vibrations of carboxyl groups and the bending vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and Ni–O bonds (Fig. 5c). In contrast, strengthening of the O–H and C–O stretching vibrations and C–H bending vibration (located between 857 and 1147 cm−1) is observed for Ni-BDC after glucose adsorption. This may be attributed to the binding of glucose to Ni-BDC via hydroxyl groups at its terminal and hydroxyl bridges. The rearranged hydrogen bonds during the interaction with Ni-BDC can increase the C–H bending vibration and the C–O stretching vibration.
C and Ni–O bonds (Fig. 5c). In contrast, strengthening of the O–H and C–O stretching vibrations and C–H bending vibration (located between 857 and 1147 cm−1) is observed for Ni-BDC after glucose adsorption. This may be attributed to the binding of glucose to Ni-BDC via hydroxyl groups at its terminal and hydroxyl bridges. The rearranged hydrogen bonds during the interaction with Ni-BDC can increase the C–H bending vibration and the C–O stretching vibration.
The C–O stretching vibration of Cu-BDC, Mn-BDC, and Ni-BDC at around 1000 cm−1 is increased after the glucose adsorption. This increase is due to the effect of M–OH group coordination and the rearrangement of the glucose hydrogen bond network on metal complexation.67 The weakening of the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the IR spectra of Cu-BDC, Mn-BDC, and Ni-BDC indicates the release of electrons from the metal center so that the H+ ion of water donates electrons to O, leading to the formation of the M–OH bond. This process can also increase the stretching vibration of O–H. Unfortunately, the strong polarity of the water molecule through the H and OH groups attached to the M–O bond can also affect the breaking of the metal center-ligand bond so that the degradation or hydrolysis of the M-BDC can occur.68,69 Still, the hydrolysis reaction between water molecules and M-BDC may lead to the breakage of the metal–ligand bond to form the hydroxide anion-metal bond and proton-displaced ligand bond from the water dissociation process. The hydrolysis of water molecules with M-BDC is expressed as follows.70,71
O bonds in the IR spectra of Cu-BDC, Mn-BDC, and Ni-BDC indicates the release of electrons from the metal center so that the H+ ion of water donates electrons to O, leading to the formation of the M–OH bond. This process can also increase the stretching vibration of O–H. Unfortunately, the strong polarity of the water molecule through the H and OH groups attached to the M–O bond can also affect the breaking of the metal center-ligand bond so that the degradation or hydrolysis of the M-BDC can occur.68,69 Still, the hydrolysis reaction between water molecules and M-BDC may lead to the breakage of the metal–ligand bond to form the hydroxide anion-metal bond and proton-displaced ligand bond from the water dissociation process. The hydrolysis of water molecules with M-BDC is expressed as follows.70,71
| Mn+ − Ln− + H2O → Mn+ − (OH)− + HL(n−1)− | (4) |
For Zr-BDC, the glucose adsorption occurs through biosorption and diffusion. Most of the biosorption process is strongly influenced by the isoelectric charge of the adsorbent surface, which causes the surface to have a neutral charge.72 The IR bands between 3272 and 3453 cm−1, 946 and 1128 cm−1, and the IR band at 596 cm−1, belonging to the O–H stretching vibration, C–O stretching vibration, and Zr–O, respectively, become more intense after glucose adsorption (Fig. 5d). The increase in the intensities of the O–H and C–O stretching vibrations may be caused by the binding of glucose to Zr-BDC through the hydroxyl group with the terminal and hydroxyl bridges at the Zr6 node of Zr-BDC via hydrogen bonding.33 Moreover, the increase of the Zr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak may originate from the binding of the C–O group of glucose with the Zr metal center, which provides the active sites due to material defects originating from the release of the BDC linker. However, there is a possible influence of water molecules on the asymmetric stretching vibration of the carboxyl group, as indicated by the weakening of the IR bands between 1523 and 1629 cm−1, and this phenomenon has been previously explained by DeCoste et al.73 The water molecule interpolates a metal–ligand bond of the framework and replaces the ligand to form a hydrated cation, releasing a free ligand. The reaction is expressed by the equation below.70,71
O peak may originate from the binding of the C–O group of glucose with the Zr metal center, which provides the active sites due to material defects originating from the release of the BDC linker. However, there is a possible influence of water molecules on the asymmetric stretching vibration of the carboxyl group, as indicated by the weakening of the IR bands between 1523 and 1629 cm−1, and this phenomenon has been previously explained by DeCoste et al.73 The water molecule interpolates a metal–ligand bond of the framework and replaces the ligand to form a hydrated cation, releasing a free ligand. The reaction is expressed by the equation below.70,71
| Mn+ − Ln− + H2O → Mn+ − (OH2)⋯Ln− | (5) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending vibration (706–798 cm−1) remain relatively unchanged after glucose adsorption.
C bending vibration (706–798 cm−1) remain relatively unchanged after glucose adsorption.
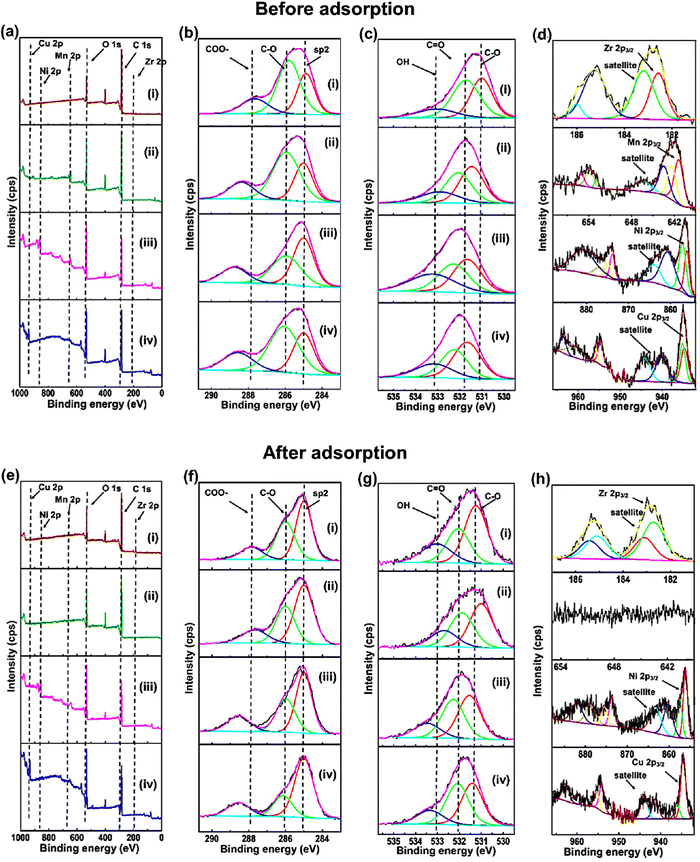
XPS measurements were conducted to obtain more information about the chemical composition and chemical state of the M-BDC MOFs before and after glucose adsorption, as presented in Fig. 6a–d. The XPS survey spectra presented in Fig. 6a(i–iv) show four peaks at around 285.0, 531.5, 330, 642.5, 856.4, and 935.5 eV, which suggests the presence of C 1s, O 1s, Zr 2p, Mn 2p, Ni 2p, and Cu 2p, respectively.74,75 To understand the XPS results in detail, all these peaks have been deconvoluted to identify the chemical bonds (Fig. 6b–d). The high-resolution C 1s XPS spectra of Zr-BDC, Mn-BDC, Ni-BDC, and Cu-BDC in Fig. 6b(i–iv) show a C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peak at 285.00 eV and carbonyl carbon (C–O) at 285.92, 285.94, 285.94 and 286.05 respectively, carboxylate carbon (O–C
C peak at 285.00 eV and carbonyl carbon (C–O) at 285.92, 285.94, 285.94 and 286.05 respectively, carboxylate carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) corresponds to 287.78, 288.36, 288.75 and 288.56 respectively.76 The atomic percentages of these bonds are given in Fig. S7a (ESI†). The high-resolution O 1s XPS spectra of Zr BDC, Mn BDC, Ni BDC, and (iv) Cu-BDC can be fitted with three peaks belonging to C–O, C
O) corresponds to 287.78, 288.36, 288.75 and 288.56 respectively.76 The atomic percentages of these bonds are given in Fig. S7a (ESI†). The high-resolution O 1s XPS spectra of Zr BDC, Mn BDC, Ni BDC, and (iv) Cu-BDC can be fitted with three peaks belonging to C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H species (Fig. 6c) with the corresponding atomic percentages presented in Fig. S7b (ESI†).
O and O–H species (Fig. 6c) with the corresponding atomic percentages presented in Fig. S7b (ESI†).
The deconvoluted Zr 2p spectrum of Zr-BDC reveals two major peaks at 182.71 and 185.10 eV with two strong satellite peaks at 183.10 and 185.41 eV (Fig. 6d(i)), which are indicative of Zr2+. The deconvoluted Mn 2p spectrum of Mn-BDC shows the presence of peaks at 641.36 and 652.93 eV with two strong satellite peaks at 643.46 and 653.87 eV (Fig. 6d(ii)), which indicates the presence of Mn2+. Additionally, the characteristic peaks of Mn3+ species are also observed at 642.35 and 653.86 eV with the corresponding satellite peaks located at 643.46 eV and 661.46 eV. The deconvoluted Ni 2p spectrum of Ni-BDC reveals the presence of peaks at 856.09 and 873.85 eV with two strong satellite peaks at 860.68 and 876.77 eV (Fig. 6d(iii)), which are indicative of Ni2+. Additionally, the characteristic peaks of Ni3+ species are observed at binding energies of 857.12 and 874.93 eV with two satellite peaks at 863.95 eV and 880.81 eV. The deconvoluted Cu 2p spectrum of Cu-BDC reveals the existence of peaks at 934.68 and 954.60 eV with two strong satellite peaks at 940.15 and 960.75 eV (Fig. 6d(iv)), corresponding to Cu2+. Additionally, the characteristic peaks of Cu3+ species are observed at binding energies of 935.15 and 955.72 eV with two satellite peaks located at 944.26 eV and 963.55 eV.
After glucose adsorption, the chemical composition and chemical state of the M-BDC MOFs were also analyzed by XPS, as presented in Fig. 6e–h. The XPS survey spectrum of the M-BDC MOFs (M = Zr, Mn, Ni, Cu) presented in Fig. 6e(i–iv) displays peaks at ∼285, 531.5, 333.19, 856.36, and 935.18 eV, belonging to C 1s, O 1s, Zr 2p, Mn 2p, Ni 2p, and Cu 2p peaks, respectively.74,75 After glucose adsorption, the Mn 2p peaks in the Mn-BDC sample disappear, indicating the unstable nature of this MOF in water, unlike Zr-BDC, Ni-BDC, and Cu-BDC.
As seen from Fig. S7 (ESI†), the overall atomic percentage of carbon increases after glucose adsorption for all M-BDC MOFs. This may be due the additional absorption of carbon from glucose by the M-BDC MOFs. Hence, the glucose adsorption mechanism involves the removal of carbon from glucose molecules. Compared to Mn-BDC, Ni-BDC, and Cu-BDC, the increase in the atomic percentage of carbon (C–Cis more pronounced in Zr-BDC, which explains for the superior glucose adsorption by Zr-BDC (Fig. S7, ESI†). In contrast, the atomic percentages of carbonyl carbon (C–O) and carboxylate carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) are decreased for all M-BDC MOFs. The possible glucose adsorption mechanism by M-BDC MOFs is illustrated in Fig. 7.
O) are decreased for all M-BDC MOFs. The possible glucose adsorption mechanism by M-BDC MOFs is illustrated in Fig. 7.
The refractive index of the standard or bare SPR sensor chip is 1.61, while for the Zr-BDC thin film prepared by spin coating it is 1.208 and the refractive index of Cu-BDC is 1.34.77,78 Unfortunately, the refractive index values for Mn-BDC and Ni-BDC have not been reported so far. However, the magnitude of the refractive index is strongly influenced by the pore width of the material; the larger the pore width, the smaller the refractive index.78 When viewed using Zr-BDC and Cu-BDC data, the sensitivity of Zr-BDC to glucose is higher than that of Cu-BDC despite the smaller refractive index. Therefore, it can be concluded that the refractive index value of M-BDC does not really affect the sensitivity. The main characteristic that affects the changes in the refractive index and the angular change is the porosity of M-BDC MOFs, which can adsorb as much glucose as possible so that its relative permittivity increases.79,80 However, this still needs to be studied further.
To further quantify the thermodynamic stability of each M-BDC MOF, we also calculated the decomposition reaction enthalpy of M-BDC (M = Zr, Ni, Cu) in the presence of water molecules using first-principles density functional theory (DFT) calculations (Fig. S9, ESI†). We considered two decomposition reaction models following eqn of (4) and (5). Here, reaction (4) represents a model where the H2O dissociates into OH− and H+ which subsequently attach to the metal ligand and to the oxygen of the dissociated part of the ligand. Meanwhile, reaction (5) represents a model where H2O is molecularly attached to the metal ligand (without any dissociation). For the two considered reaction models, we found that all dissociation reactions of M-BDC are endothermic, indicating that the degradation of M-BDC is not a spontaneous process. However, further examination of the calculated enthalpies shows that Cu-BDC and Zr-BDC indeed have better thermodynamic stability as compared to Ni-BDC for both reaction models (as shown by the overall higher dissociation enthalpy). These data support the slight degradation of Mn-BDC and Ni-BDC by water molecules, as described earlier in the mechanism section. For future development, it is necessary to modify the M-BDC MOF to increase its water stability.
Following glucose detection, the M-BDC MOF samples were examined using XRD to assess the compositional stability. As shown in Fig. S10 (ESI†), the peak positions of Zr-BDC remain the same after the glucose sensing process; however the intensity of the peaks slightly decreases. In comparison, the peaks of Cu-BDC are shifted after glucose sensing, which may be attributed to a change in lattice parameters (Fig. S10a, ESI†). On Mn-BDC, there is only one peak remaining after glucose sensing while other peaks have either shifted or disappeared (Fig. S10b, ESI†). Meanwhile, Ni-BDC becomes amorphous after the glucose sensing test (Fig. S10c, ESI†). The M-BDC MOF samples that experience changes in lattice parameters or become amorphous after the glucose sensing process are likely to have undergone a phase transformation.81 These results clearly demonstrate the superior stability of Zr-BDC compared to Cu-BDC, Mn-BDC, and Ni-BDC.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
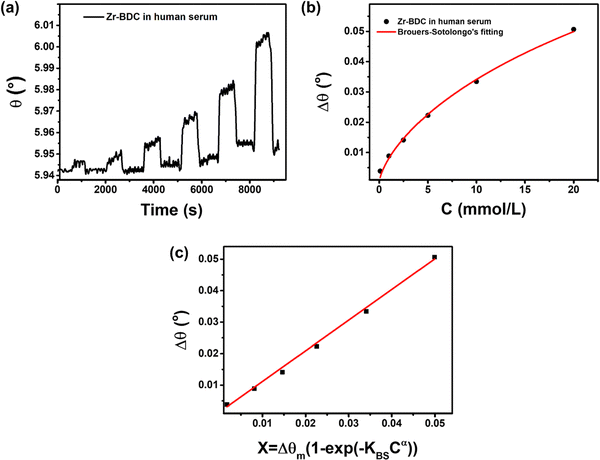
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The dynamic response of the Zr-BDC-functionalized SPR sensor toward different concentrations of glucose (0.1–20 mmol L−1) is shown in Fig. 8a with changes in the response values summarized in Table S6 (ESI†). Isotherm analysis of the C vs. Δθ curve shows good agreement with the Brouers–Sotolongo model with R2 = 0.99576 (Fig. 8b). This suggests that the mechanism of glucose detection in human serum follows the same model as in PBS only. However, there are differences in the parameters obtained, such as Δθm, α, and KBS, with values of 0.1267 mmol g−1, 0.066, and 0.676, respectively. Furthermore, the exponent α value is less than 1, indicating that the initial adsorption kinetics process occurs quickly so that the active site on the hierarchical Zr-BDC MOF can enable the adsorption of many glucose molecules.57 A slight reduction in the Δθm value is also observed for SPR measurement in a human serum/PBS mixture compared to that in PBS only because the initial concentration adsorbed can affect the adsorption capacity.82
1. The dynamic response of the Zr-BDC-functionalized SPR sensor toward different concentrations of glucose (0.1–20 mmol L−1) is shown in Fig. 8a with changes in the response values summarized in Table S6 (ESI†). Isotherm analysis of the C vs. Δθ curve shows good agreement with the Brouers–Sotolongo model with R2 = 0.99576 (Fig. 8b). This suggests that the mechanism of glucose detection in human serum follows the same model as in PBS only. However, there are differences in the parameters obtained, such as Δθm, α, and KBS, with values of 0.1267 mmol g−1, 0.066, and 0.676, respectively. Furthermore, the exponent α value is less than 1, indicating that the initial adsorption kinetics process occurs quickly so that the active site on the hierarchical Zr-BDC MOF can enable the adsorption of many glucose molecules.57 A slight reduction in the Δθm value is also observed for SPR measurement in a human serum/PBS mixture compared to that in PBS only because the initial concentration adsorbed can affect the adsorption capacity.82
The glucose detection performance of the Zr-BDC-functionalized SPR sensor in a human serum/PBS mixture is better than that in PBS alone. The calibration curve in Fig. 8c produces a linear equation Δθ = 0.97396X + 0.00142 with an R2 value of 0.99806. The slope of the line is not much different than that obtained in PBS solution, but the LOD is much lower in human serum/PBS mixture (0.482 mmol L−1 (S/N = 3)), indicating the enhanced sensing performance. This improvement is attributed to the presence of human serum in solution, which can increase the mass of the captured molecules, hence increasing its stability and refractive index. Next, from the dynamic response-recovery curves, the sensitivities obtained from calibration measurements in the human serum/PBS mixture and pure PBS are 0.97702 and 0.97396, respectively, were compared. The high sensitivity of the Zr-BDC-functionalized SPR sensor can be developed further and used in practical applications.
As shown in Table 3, although some previous SPR sensors exhibit a lower LOD than our Zr-BDC-functionalized SPR sensor, and they require the use of GOx or phenyl boronic acid (PBA) as a glucose receptor. Meanwhile, other SPR sensors only work within a small concentration range of glucose (0–5 mmol L−1), whereas the glucose concentration in the blood of diabetics is usually more than 11.1 mmol L−1 under normal conditions and higher than 7 mmol L−1 in a fasting state.83–86 With a LOD of 0.482 mmol L−1, the developed Zr-BDC-functionalized SPR sensor can detect glucose concentrations in people with diabetes (0 to 20 mmol L−1). Furthermore, the LOD value of our SPR sensor is below that required by the Food and Drug Administration (FDA, United States) for point-of-care diabetes detection tool, which is 10 mg dL−1 or 0.555 mmol L−1.87 Therefore, the Zr-BDC-functionalized SPR sensor has the potential to be used in a POC device and compete with other detection devices that use other natural glucose receptors.
| SPR type | Recognition layer | Detection step | Medium solution | Concentration range | LOD (S/N = 3) | Ref. |
|---|---|---|---|---|---|---|
| Fiber optic | SiO2/GOx | Separated | PBS | 0–80 mg dL−1 (0–4.44 mmol L−1) | 0.142 mg mL−1 (0.788 mmol L−1) | 83 |
| Fiber optic | AuNps/P-mercaptophenylboronic acid (PMBA) | Separated | PBS | 0–1.7 mmol L−1 | 0.00078 mmol L−1 | 84 |
| Fiber optic | Ag/Au film/4-mercaptophenylboronic acid (4-MPBA) | Separated | PBS | 0–0.1 mmol L−1 | 0.00112 mmol L−1 | 85 |
| Prism coupler | Cr-Au/Ta2O5 | Separated | DI water and 2% lipofundin | 0–500 mg dL−1 (0–27.75 mmol L−1) | 3.72 mg dL−1 (0.21 mmol L−1) | 87 |
| Prism coupler | Glucose oxidase@silica mesocellular foams/SiO2 nanoparticles (GOx@SiMCFs/SiNPs) | Separated | PBS | 0–200 mg dL−1 (0–11.21 mmol L−1) | 2.22 mmol L−1 | 14 |
| Prism coupler | Au/Zr-BDC film | Continued | Human serum – PBS | 0.1–20 mmol L−1 | 0.482 mmol L−1 | This work |
4. Conclusions
This study has demonstrated that the modification of a standard SPR sensor chip with hierarchical M-BDC MOFs to enhance the sensing response towards small molecules, such as glucose. The adsorption mechanism of glucose by M-BDC MOFs varies depending on the metal center. The adsorption isotherms of Cu-BDC, Mn-BDC, and Ni-BDC follow the Langmuir–Freundlich adsorption model. In contrast, the adsorption of glucose on Zr-BDC follows the Brouers–Sotolongo adsorption model. Among the M-BDC MOFs, the glucose adsorption is fastest in Zr-BDC as it occurs by diffusion with KAV = 0.66681 s−1. The FTIR spectra of the M-BDC MOFs after glucose adsorption show the strengthening of the C–O and O–H stretching vibrations, except for Mn-BDC. Therefore, the binding of glucose to M-BDC MOFs occurs through interactions with the O–H and C–O groups. The calibration curves obtained from the dynamic response data of M-BDC MOFs reveal that Zr-BDC exhibits a higher slope value (i.e., higher sensitivity) than other M-BDC MOFs. Furthermore, it has a better LOD for glucose sensing in concentration range of 0.1–20 mmol L−1 than Cu-BDC, Ni-BDC, Mn-BDC and bare Au substrate in the concentration range of 5–20 mmol L−1. The Zr-BDC-functionalized SPR sensor also displays good selectivity towards glucose in the presence of other interfering compounds, such as ascorbic acid, uric acid, urea, and maltose. In addition, it shows a stronger binding towards glucose than fructose and sucrose. The reusability test of the Zr-BDC-functionalized SPR sensor shows that its dynamic response decreases from 100% to 91.5%, 52.72%, 49.46%, and 37.04% after the 2nd, 3rd, 4th, and 5th measurement, respectively. In the future, the reusability aspect of this sensor will be improved further by hybridization with other materials. The glucose sensing test in a human serum/PBS mixture shows an increase in the glucose sensing performance of the Zr-BDC-functionalized SPR sensor than in PBS alone with a ten times smaller LOD. In our future work, this sensor will be further improved to enable its application in point-of-care devices for diabetes detection.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial grants provided by Lembaga Pengelola Dana Pendidikan (LPDP), Ministry of Finance of Indonesia. This work is also partially supported by Indonesia Ministry of Education, Culture, Research, and Technology, and the research grant of Institut Teknologi Bandung (ITB). J. H. acknowledges the funding from Japan Society for the Promotion of Science (JSPS) KAKENHI Program (Grant no. 20K05453). Y. V. K. acknowledges the funding from Advance Queensland (AQIRF043-2020-CV).References
- World Health Organization, Global report on diabetes, World Health Organization, Geneva, 2016 Search PubMed.
- V. Scognamiglio, Biosens. Bioelectron., 2013, 47, 12–25 CrossRef CAS PubMed.
- H.-C. Wang and A.-R. Lee, J. Food Drug Anal., 2015, 23, 191–200 CrossRef CAS PubMed.
- International Diabetes Federation, IDF Diabetes Atlas Ninth Edition, 2019 Search PubMed.
- E. Wijaya, C. Lenaerts, S. Maricot, J. Hastanin, S. Habraken, J.-P. Vilcot, R. Boukherroub and S. Szunerits, Curr. Opin. Solid State Mater. Sci., 2011, 15, 208–224 CrossRef CAS.
- X. Guo, J. Biophoton., 2012, 5, 483–501 CrossRef CAS PubMed.
- J. Homola, S. S. Yee and G. Gauglitz, Sens. Actuators, B, 1999, 54, 3–15 CrossRef CAS.
- W. W. Lam, L. H. Chu, C. L. Wong and Y. T. Zhang, Sens. Actuators, B, 2005, 105, 138–143 CrossRef CAS.
- N. K. Singh, B. Jain and S. Annapoorni, Sens. Actuators, B, 2011, 156, 383–387 CrossRef CAS.
- J. Li, D.-f Lu, Z. Zhang, Q. Liu and Z.-m Qi, Sens. Actuators, B, 2014, 203, 690–696 CrossRef CAS.
- H. V. Hsieh, Z. A. Pfeiffer, T. J. Amiss, D. B. Sherman and J. B. Pitner, Biosens. Bioelectron., 2004, 19, 653–660 CrossRef CAS PubMed.
- W. Wu, J. Shen, Y. Li, H. Zhu, P. Banerjee and S. Zhou, Biomaterials, 2012, 33, 7115–7125 CrossRef CAS PubMed.
- L. Kumar, R. Gupta, D. Thakar, V. Vibhu and S. Annapoorni, Plasmonics, 2013, 8, 487–494 CrossRef CAS.
- Y. Yuan, N. Yuan, D. Gong and M. Yang, Photon. Sens., 2019, 9, 309–316 CrossRef CAS.
- M.-S. Steiner, A. Duerkop and O. S. Wolfbeis, Chem. Soc. Rev., 2011, 40, 4805–4839 RSC.
- M. Lobry, D. Lahem, M. Loyez, M. Debliquy, K. Chah, M. David and C. Caucheteur, Biosens. Bioelectron., 2019, 142, 111506 CrossRef CAS PubMed.
- A. Stephenson-Brown, H.-C. Wang, P. Iqbal, J. A. Preece, Y. Long, J. S. Fossey, T. D. James and P. M. Mendes, Analyst, 2013, 138, 7140–7145 RSC.
- X. Sun and T. D. James, Chem. Rev., 2015, 115, 8001–8037 CrossRef CAS PubMed.
- N. Pal, B. Saha, S. K. Kundu, A. Bhaumik and S. Banerjee, New J. Chem., 2015, 39, 8035–8043 RSC.
- N. Pal, S. Banerjee and A. Bhaumik, J. Colloid Interface Sci., 2018, 516, 121–127 CrossRef CAS PubMed.
- P. J. Lynch, A. Amorim Graf, S. P. Ogilvie, M. J. Large, J. P. Salvage and A. B. Dalton, J. Mater. Chem. B, 2020, 8, 7733–7739 RSC.
- X. Xuan, M. Qian, L. Pan, T. Lu, L. Han, H. Yu, L. Wan, Y. Niu and S. Gong, J. Mater. Chem. B, 2020, 8, 9094–9109 RSC.
- H. Zou, D. Tian, C. Lv, S. Wu, G. Lu, Y. Guo, Y. Liu, Y. Yu and K. Ding, J. Mater. Chem. B, 2020, 8, 1008–1016 RSC.
- D. Chu, Y. Wang, D. Li, X.-Q. Chu, D. Ge and X. Chen, Dalton Trans., 2022, 51, 15354–15360 RSC.
- X. Zha, W. Yang, L. Shi, Q. Zeng, J. Xu and Y. Yang, Dalton Trans., 2023, 52, 2631–2640 RSC.
- W. Liu and X.-B. Yin, TrAC, Trends Anal. Chem., 2016, 75, 86–96 CrossRef CAS.
- C.-H. Chuang and C.-W. Kung, Electroanalysis, 2020, 32, 1885–1895 CrossRef CAS.
- Y. Xu, Q. Li, H. Xue and H. Pang, Coord. Chem. Rev., 2018, 376, 292–318 CrossRef CAS.
- H. Li, L. Shi, C. Li, X. Fu, Q. Huang and B. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 34095–34104 CrossRef CAS PubMed.
- L. Hang, F. Zhou, D. Men, H. Li, X. Li, H. Zhang, G. Liu, W. Cai, C. Li and Y. Li, Nano Res., 2017, 10, 2257–2270 CrossRef CAS.
- G. Zhu, M. Zhang, L. Lu, X. Lou, M. Dong and L. Zhu, Sens. Actuators, B, 2019, 288, 12–19 CrossRef CAS.
- G. Gumilar, Y. V. Kaneti, J. Henzie, S. Chatterjee, J. Na, B. Yuliarto, N. Nugraha, A. Patah, A. Bhaumik and Y. Yamauchi, Chem. Sci., 2020, 11, 3644–3655 RSC.
- Z. Zeng, J. Lyu, P. Bai and X. Guo, Ind. Eng. Chem. Res., 2018, 57, 9200–9209 CrossRef CAS.
- A. Göçenoğlu Sarıkaya, B. Osman, T. Çam and A. Denizli, Sens. Actuators, B, 2017, 251, 763–772 CrossRef.
- A. M. M. Vargas, A. L. Cazetta, M. H. Kunita, T. L. Silva and V. C. Almeida, Chem. Eng. J., 2011, 168, 722–730 CrossRef CAS.
- M. A. Ahmad, N. Ahmad and O. S. Bello, Appl. Water Sci., 2015, 5, 407–423 CrossRef CAS.
- M. A. H. D. Allah, S. N. Taqui, U. T. Syed and A. A. Syed, SN Appl. Sci., 2019, 1, 330 CrossRef CAS.
- M. Brdar, M. Šćiban, A. Takači and T. Došenović, Chem. Eng. J., 2012, 183, 108–111 CrossRef CAS.
- N. Ayawei, A. N. Ebelegi and D. Wankasi, J. Chem., 2017, 2017, 3039817 Search PubMed.
- R. Seetharaj, P. V. Vandana, P. Arya and S. Mathew, Arabian J. Chem., 2019, 12, 295–315 CrossRef CAS.
- U. S. Kestur, H. Lee, D. Santiago, C. Rinaldi, Y.-Y. Won and L. S. Taylor, Cryst. Growth Des., 2010, 10, 3585–3595 CrossRef CAS.
- J. Henzie, V. Etacheri, M. Jahan, H. Rong, C. N. Hong and V. G. Pol, J. Mater. Chem. A, 2017, 5, 6079–6089 RSC.
- Y. Borodko, S. M. Humphrey, T. D. Tilley, H. Frei and G. A. Somorjai, J. Phys. Chem. C, 2007, 111, 6288–6295 CrossRef CAS.
- S. Chowdhury, N. L. Torad, A. Ashok, G. Gumilar, W. Chaikittisilp, R. Xin, P. Cheng, M. I. Ul Hoque, M. A. Wahab, M. R. Karim, B. Yuliarto, M. S. Hossain, Y. Yamauchi and Y. V. Kaneti, Chem. Eng. J., 2022, 450, 138065 CrossRef CAS.
- W. Qian, Y. Tan, Y. Yu, L. Zhang, X. Wu and B. Xue, J. Alloys Compd., 2022, 918, 165741 CrossRef CAS.
- M. Shete, P. Kumar, J. E. Bachman, X. Ma, Z. P. Smith, W. Xu, K. A. Mkhoyan, J. R. Long and M. Tsapatsis, J. Membrane Sci., 2018, 549, 312–320 CrossRef CAS.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS PubMed.
- A. Carton, A. Mesbah, T. Mazet, F. Porcher and M. François, Solid State Sci., 2007, 9, 465–471 CrossRef CAS.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- S. Gao, Y. Sui, F. Wei, J. Qi, Q. Meng and Y. He, J. Mater. Sci., 2018, 53, 6807–6818 CrossRef CAS.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H.-C. Zhou, Adv. Mater., 2018, 30, 1704303 CrossRef PubMed.
- K. Tan, N. Nijem, P. Canepa, Q. Gong, J. Li, T. Thonhauser and Y. J. Chabal, Chem. Mater., 2012, 24, 3153–3167 CrossRef CAS.
- K. Tan, N. Nijem, Y. Gao, S. Zuluaga, J. Li, T. Thonhauser and Y. J. Chabal, CrystEngComm, 2015, 17, 247–260 RSC.
- Ü. Kökçam-Demir, A. Goldman, L. Esrafili, M. Gharib, A. Morsali, O. Weingart and C. Janiak, Chem. Soc. Rev., 2020, 49, 2751–2798 RSC.
- Y. Keren, M. Borisover and N. Bukhanovsky, Chemosphere, 2015, 138, 462–468 CrossRef CAS PubMed.
- M. A. Al-Ghouti and D. A. Da'ana, J. Hazard. Mater., 2020, 393, 122383 CrossRef CAS PubMed.
- S. Karoui, R. Ben Arfi, K. Mougin, A. Ghorbal, A. A. Assadi and A. Amrane, J. Hazard. Mater., 2020, 387, 121675 CrossRef CAS PubMed.
- F. Brouers and T. J. Al-Musawi, J. Mol. Liq., 2015, 212, 46–51 CrossRef CAS.
- F. Brouers, J. Modern Phys., 2014, 5, 1594–1601 CrossRef.
- R. Abazari, A. R. Mahjoub and J. Shariati, J. Hazard. Mater., 2019, 366, 439–451 CrossRef CAS PubMed.
- N. A. Oladoja, Desalination Water Treat., 2016, 57, 15813–15825 CrossRef CAS.
- J. C. Soares, A. C. Soares, P. A. R. Pereira, V. D. C. Rodrigues, F. M. Shimizu, M. E. Melendez, C. Scapulatempo Neto, A. L. Carvalho, F. L. Leite, S. A. S. Machado and O. N. Oliveira, Phys. Chem. Chem. Phys., 2016, 18, 8412–8418 RSC.
- N. K. Gupta, A. Sengupta, V. G. Rane and R. M. Kadam, Sep. Sci. Technol., 2017, 52, 2049–2061 CrossRef CAS.
- C. Nanthamathee and P. Dechatiwongse, Mater. Chem. Phys., 2021, 258, 123924 CrossRef CAS.
- C. E. Housecroft and A. G. Sharpe, Inorganic chemistry, Pearson Prentice Hall, Harlow, England, 2nd edn, 2005 Search PubMed.
- M. Zhang, K. Gu, X. Huang and Y. Chen, Phys. Chem. Chem. Phys., 2019, 21, 19226–19233 RSC.
- H.-A. Tajmir-Riahi, Carbohyd. Res., 1988, 183, 35–46 CrossRef CAS PubMed.
- S. Zuluaga, E. M. A. Fuentes-Fernandez, K. Tan, F. Xu, J. Li, Y. J. Chabal and T. Thonhauser, J. Mater. Chem. A, 2016, 4, 5176–5183 RSC.
- D. Lv, J. Chen, Y. Chen, Z. Liu, Y. Xu, C. Duan, H. Wu, Y. Wu, J. Xiao, H. Xi, Z. Li and Q. Xia, AIChE J., 2019, 65, e16616 CrossRef.
- J. J. Low, A. I. Benin, P. Jakubczak, J. F. Abrahamian, S. A. Faheem and R. R. Willis, J. Am. Chem. Soc., 2009, 131, 15834–15842 CrossRef CAS PubMed.
- J. Canivet, A. Fateeva, Y. Guo, B. Coasne and D. Farrusseng, Chem. Soc. Rev., 2014, 43, 5594–5617 RSC.
- T. Sathvika, S. Balaji, M. Chandra, A. Soni, V. Rajesh and N. Rajesh, Chem. Eng. J., 2019, 360, 879–889 CrossRef CAS.
- J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-G. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642–5650 RSC.
- Y. Wu, X. Song, S. Li, J. Zhang, X. Yang, P. Shen, L. Gao, R. Wei, J. Zhang and G. Xiao, J. Ind. Eng. Chem., 2018, 58, 296–303 CrossRef CAS.
- W. Zheng, W. Bi, X. Gao, Z. Zhang, W. Yuan and L. Li, Sustain. Energy Fuels, 2020, 4, 5757–5764 RSC.
- L. G. Beka, X. Bu, X. Li, X. Wang, C. Han and W. Liu, RSC Adv., 2019, 9, 36123–36135 RSC.
- Y. Huang, C.-A. Tao, R. Chen, L. Sheng and J. Wang, Nanomaterials, 2018, 8, 676 CrossRef PubMed.
- E. Redel, Z. Wang, S. Walheim, J. Liu, H. Gliemann and C. Wöll, Appl. Phys. Lett., 2013, 103, 091903 CrossRef.
- R. Dorey, in Ceramic Thick Films for MEMS and Microdevices, ed. R. Dorey, William Andrew Publishing, Oxford, 2012, pp. 85–112 DOI:10.1016/B978-1-4377-7817-5.00004-3.
- G. Gumilar, J. Henzie, B. Yuliarto, A. Patah, N. Nugraha, M. Iqbal, M. A. Amin, M. S. A. Hossain, Y. Yamauchi and Y. V. Kaneti, J. Mater. Chem. A, 2022, 10, 6662–6678 RSC.
- A. Ali, Y. W. Chiang and R. M. Santos, Journal, 2022, 12, 205 CAS.
- N. H. Othman, N. H. Alias, M. Z. Shahruddin, N. F. Abu Bakar, N. R. Nik Him and W. J. Lau, J. Environ. Chem. Eng., 2018, 6, 2803–2811 CrossRef CAS.
- Y. Yuan, X. Yang, D. Gong, F. Liu, W. Hu, W. Cai, J. Huang and M. Yang, Opt. Express, 2017, 25, 3884–3898 CrossRef CAS PubMed.
- W.-L. Zheng, Y.-N. Zhang, L.-K. Li, X.-G. Li and Y. Zhao, Biosens. Bioelectron., 2022, 198, 113798 CrossRef CAS PubMed.
- X. Wang, X. Sun, Y. Hu, L. Zhang, L. Zeng, Q. Liu and J. Duan, IEEE Sens. J., 2022, 22, 20413–20420 CAS.
- B. L. Furman, Reference Module in Biomedical Sciences, Elsevier, 2017 DOI:10.1016/B978-0-12-801238-3.98060-7.
- Q.-H. Phan, Y.-R. Lai, W.-Z. Xiao, T.-T.-H. Pham and C.-H. Lien, Opt. Express, 2020, 28, 24889–24899 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tb00138e |
| This journal is © The Royal Society of Chemistry 2023 |