Open Access Article
Open Access ArticleTransition metals enhance prebiotic depsipeptide oligomerization reactions involving histidine†
Moran Frenkel-Pinterabc,
Alyssa B. Sargonab,
Jennifer B. Glasscd,
Nicholas V. Hudabc and
Loren Dean Williams *abc
*abc
aNSF/NASA Center for Chemical Evolution, USA. E-mail: loren.williams@chemistry.gatech.edu
bSchool of Chemistry & Biochemistry, Georgia Institute of Technology, Atlanta, GA 30332, USA
cNASA Center for the Origins of Life, Georgia Institute of Technology, Atlanta, GA 30332, USA
dSchool of Earth and Atmospheric Science, Georgia Institute of Technology, Atlanta, GA 30332, USA
First published on 18th January 2021
Abstract
Biochemistry exhibits an intense dependence on metals. Here we show that during dry-down reactions, zinc and a few other transition metals increase the yield of long histidine-containing depsipeptides, which contain both ester and amide linkages. Our results suggest that interactions of proto-peptides with metal ions influenced early chemical evolution.
Around half of all known proteins associate with metal ions or organometallic cofactors.1–4 Metals stabilize protein structure (e.g., zinc fingers) and mediate catalysis (nitrogenases), electron transfer (cytochromes), ligand transport (hemoglobin), and signalling (calmodulin). Metals stabilize nucleic acids; around 500 divalent metal cations associate with a single ribosome.5 The dependence of biochemistry on metals appears ancient and rooted in chemical evolution, preceding emergence of life on Earth.6,7 Long-standing questions about the origins of life might be answered by understanding how metals interact with ancestral biopolymers. For example, metals could have affected oligomerization reactions of early proto-polymers, and could have conferred stability and function, in analogy with their roles in extant biochemistry.
Depsipeptides, containing mixtures of ester and amide linkages, are plausible ancestors of polypeptides.8–14 During drying of hydroxy acids and amino acids at mild temperatures (i.e., 65 °C) ester bonds form first and are converted to peptide bonds by ester–amide exchange. Hydroxy acids and amino acids are thought to have been abundant on the prebiotic earth.15–19
Here we tested the hypothesis that interaction with Zn2+ or other metal ions with the amino acid histidine (His) might affect rates and extents of depsipeptide formation in dry-down reactions. We observed that Zn2+ and other selected transition metals increase yields of long His-containing depsipeptides. The increase in yield is specific for imidazole-containing amino and hydroxy acids and metals that interact directly with them.
We previously showed that His is readily incorporated into depsipeptides during dry-down reactions under mildly acidic conditions (pH ∼3) at 85 °C.12 These conditions are known generally to promote oligomerization in mixtures of hydroxy acids and amino acids into depsipeptides.8–11 Here, mixtures of glycolic acid (glc) and L-His were reacted under these known reaction conditions in the presence or absence of various metals.
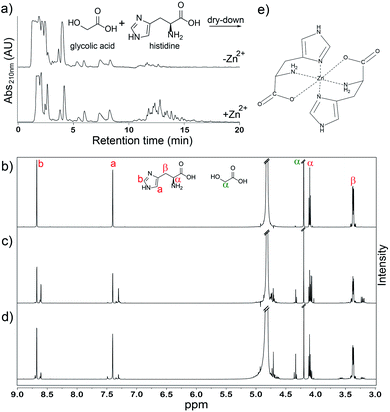
Addition of Zn2+ led to an increase in the average lengths of His-containing depsipeptides. Depsipeptide oligomers are readily detected by high-performance liquid chromatography (HPLC) using a C18 column (Fig. 1a). Longer oligomers exhibit longer retention times20 allowing us to compare various dry-down reactions. The yield of products with a higher retention times on HPLC is increased following addition of Zn2+ to the reaction mixture (Fig. 1a). 1H NMR analysis confirmed that the distribution of oligomeric species changes with addition of Zn2+ to the dry-down reaction (Fig. 1b–d). MS analysis verified the increased abundance of longer His-containing depsipeptides following dry-down with Zn2+ (Fig. S1†). On the other hand, Zn2+ decreases the extent of conversion of His into oligomers. While 42% of His monomer converted into oligomers in the absence of Zn2+, only 22% of His monomer converted into oligomers in its presence (Fig. 1b–d). Oligomerization is indicated by shifts in the β-proton resonance envelope centered at ∼3.37 ppm and in the imidazole proton envelopes at ∼7.40 ppm and ∼8.68 ppm (Fig. 1b–d). Extent of conversion to oligomers was calculated based on the integrals of residual nonreacted imidazole proton resonances (Fig. 1b–d).
The observed increase in depsipeptide length upon addition of Zn2+ might result from direct association between Zn2+ and His monomers (Fig. 1e)21 because the effect is Zn2+ dose-dependent and is maximal at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of Zn2+ to His (Fig. S2†). Specific interaction of Zn2+ with His monomer has been reported previously.22 The increased production of long His-containing depsipeptides with increasing Zn2+ is reversed when the number of Zn2+ equivalents exceeds that of His. Three equivalents of Zn2+ completely inhibited oligomerization reactions (Fig. S3†). The effect of Zn2+ on reactivity of His during the dry-down conditions might arise from either a locked chelate conformation or increased electrophilicity of the carbonyl. Importantly, Zn2+ did not cause polymerization of His in the absence of glycolic acid (Fig. S4†).
1 molar ratio of Zn2+ to His (Fig. S2†). Specific interaction of Zn2+ with His monomer has been reported previously.22 The increased production of long His-containing depsipeptides with increasing Zn2+ is reversed when the number of Zn2+ equivalents exceeds that of His. Three equivalents of Zn2+ completely inhibited oligomerization reactions (Fig. S3†). The effect of Zn2+ on reactivity of His during the dry-down conditions might arise from either a locked chelate conformation or increased electrophilicity of the carbonyl. Importantly, Zn2+ did not cause polymerization of His in the absence of glycolic acid (Fig. S4†).
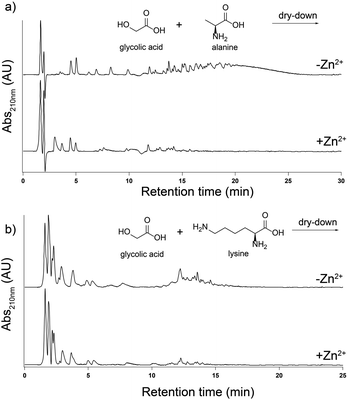
The effect of Zn2+ on depsipeptide formation is not a generic effect and is specific to His. We dried-down mixtures of glc with either alanine (Ala) or lysine (Lys). The addition of Zn2+ to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with these amino acids does not increase the yield of products in any length range. In fact, Zn2+ inhibited depsipeptide formation for both Ala and Lys (Fig. 2).
1 molar ratio with these amino acids does not increase the yield of products in any length range. In fact, Zn2+ inhibited depsipeptide formation for both Ala and Lys (Fig. 2).
In addition to Zn2+, we investigated the effects of Na+, K+, Ca2+, Mg2+, Cu2+, and Co2+ on the oligomerization of His in depsipeptides. We dried-down mixtures of glc and His in the presence or absence of various metals (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of M+ or M2+ to amino acid). Analysis by HPLC showed that Zn2+, Cu2+, and Co2+, but not Na+, K+, or Mg2+, increased the production of longer His-containing depsipeptides (Fig. S5 and S6†). Ca2+ decreased the production of His-containing depsipeptides (Fig. S5†).
1 molar ratio of M+ or M2+ to amino acid). Analysis by HPLC showed that Zn2+, Cu2+, and Co2+, but not Na+, K+, or Mg2+, increased the production of longer His-containing depsipeptides (Fig. S5 and S6†). Ca2+ decreased the production of His-containing depsipeptides (Fig. S5†).
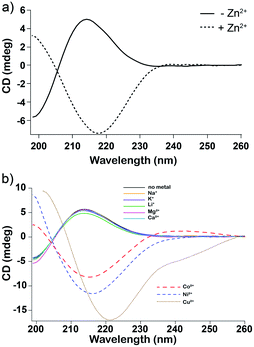
Circular dichroism spectroscopy (CD) supported our hypothesis that enhancement of oligomerization of His in the presence of Zn2+ results from direct association between monomeric His and Zn2+. We added various concentrations of Zn2+ to monomeric glc plus His. The CD spectrum inverts at equal molar ratio of Zn2+ and His (Fig. 3a). We attribute the inversion to formation of His–Zn2+ complex in concert with a change in the conformation of His. An example of a possible complex is shown in Fig. 1e.
CD spectra of glc plus His only report conformational changes of His because glc is achiral. The conformational change upon Zn2+ binding is dose dependent; the change in the CD spectra increases with increasing concentrations of Zn2+ (Fig. S7†). Addition of Zn2+ to the dry-down product mixture of His-containing depsipeptides resulted in far more subtle changes in the CD spectra, which appears to arise from binding of Zn2+ to small amounts of remnant His monomer that was not converted into polymers during the dry-down reaction (Fig. S8†).12 In accordance with the observed species-specific impact of metals on dry-down reactions (Fig. S5†), the inversion of the CD signal of His monomer was also observed for Co2+, Cu2+, and Ni2+, but not for Na+, K+, Li+, Mg2+, or Ca2+. Thus, it appears that low-lying d-orbitals of metals are important for interaction with His. The differences in the electron configuration between the different metals affect their metal coordination properties. Transition metals are more electronegative and have more oxidation states than alkaline and alkaline-Earth metals, and their valence electrons in the d-shell tend to promote stable coordination complexes. By contrast, Zn2+ inhibited oligomerization of Ala or Lys in dry-down reactions. This inhibition is consistent with recent thermodynamic calculations23 that examined effects of metals on the monomer–oligomer equilibria of glycine. Metals shift the equilibria toward the monomer, particularly at neutral and alkaline pH.23
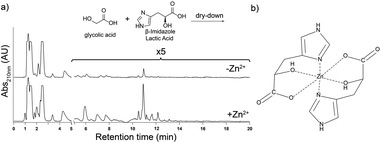
To determine if oligomerization of imidazole-containing monomers other than His is promoted by Zn2+, we dried L-β-imidazole lactic acid (the hydroxy acid analog of His, herein termed his) with glc for one week at 85 °C. The reaction produced polyesters, co-polymers of his and glc (Fig. S9†). Addition of Zn2+ to his and glc dry-down reaction mixtures increased the yield of longer polyester oligomers (Fig. 4a). 1H NMR analysis of these polyesters indicate that Zn2+ did not change the extent of conversion of his monomer into oligomers: 39% of his converted into oligomers in the absence of Zn2+ and 38% of his converted into oligomers in its presence (Fig. S10†). Therefore, Zn2+ does not increase the overall oligomeric yield but rather the distribution of product oligomers, increasing the yield of longer oligomers (Fig. 4a and Fig. S10†). These results imply that a terminal alcohol can support a chelation complex with Zn2+, in analogy with the suggested chelation complex of Zn2+ by His (compare Fig. 4b to Fig. 1e).
Several distinct non-exclusive mechanisms can explain why Zn2+ promotes formation of longer His-containing depsipeptides. Dry-down reactions are conducted under mildly acidic conditions (pH ∼ 3), in which the imidazole side chain (pKa of ∼6) and the α-amino group (pKa of ∼9) of monomeric His are protonated and the carboxylic acid (pKa of ∼2) is deprotonated. Deprotonation of α-amino group would be promoted by His coordination of Zn2+ (Fig. 1), favoring an intermediate in formation of depsipeptides through ester–amide exchange. Zn2+ coordination by His might also lock His in a specific reactive conformation and/or increase the electrophilicity of the His carbonyl group. Zn2+ is expected to pull electron density from His and expose the carbonyl to nucleophilic attack (Fig. 1e). Dehydration would promote His coordination with Zn2+ by depleting competing water molecules.21,24 In principle, it is possible that a complex is formed in which glc and His simultaneously chelate Zn2+, or in which His and glc-based oligomers chelate Zn2+ such that a favored configuration for a nucleophilic attack is reached.
The effects of metals on oligomerization of amino acids by methods other than dry-down reactions has been investigated previously.23,25–30 Concentrated sodium chloride (>3 M) promotes oligomerization in the presence of Cu2+, to increase the yield of glycine (Gly)- and Ala-based peptides.25 Various metals can affect oligomerization of chemically activated amino acids (N-carboxyanhydrides).31,32 Chemical activation studies focused on Gly, the simplest and most reactive amino acid, but resulted in only low yields of very short peptides.23,25–28,33–39 It has been proposed that minerals might catalyze dry-down oligomerization of amino acids.33–39 McKee et al. observed that silica hinders the amidation of Gly in the presence of lactic acid, the hydroxy acid analog of Ala.40 However, silica did lead to an enrichment of amide bonds over ester bonds.40
His may not be a prebiotic amino acid. It has been proposed that the prebiotic chemical ancestor of His might be imidazole-4-acetaldehyde,41–44 which is produced by Strecker synthesis.45,46 The His-containing depsipeptides produced here do not appear to bind to Zn2+ (Fig. S8†). This absence of chelation is consistent with the low number and density of His side chains, and the absence of backbone amines at ester linkages. Longer depsipeptides with greater number and density of His residues may bind Zn2+ and might lead to emergence of small metalloenzymes.
The importance of small proto-metalloproteins on the prebiotic Earth is supported by the cooperative interactions of metals and proteins in extant biology. Mulkidjanian proposed the zinc world theory, according to which the first metabolism was driven by zinc sulfide minerals that catalyzed photochemical reactions.47–49 Primordial cooperation may have existed between metals and proto-peptides prior to the emergence of coded protein synthesis.50–53 For instance, amyloidogenic heptapeptides can function as Zn2+-dependent esterases.51 Zn2+ promotes fibril formation by His-containing peptides, acting as a catalytic cofactor. Short peptides with acidic residues, such as aspartic acid and glutamic acid,52,53 could have protected short RNA molecules against Mg2+-induced degradation.54,55 Coordination of metal ions induces peptide conformational changes and supramolecular assembly.56–60 In addition to effects on peptide self-assembly and function, metal–peptide interactions are utilized for fabrication of nanofiber materials for various applications, including three-dimensional cell culture and tissue engineering.56,61,62
It is generally accepted that Zn2+ concentration has remained fairly constant in seawater through time, whereas the concentrations of Co2+ and Ni2+ were higher in earlier stages of Earth history than in modern seawater.63–72 If accumulation of metals occurred at shallow lakes or similar environments that were subjected to dry-wet cycling, they might have affected distribution of polymers that formed within these environments in a specific manner. Our results suggest that the close relationship between metals and biopolymers has roots in prebiotic chemistry and shaped their co-evolution.
Conclusions
We have partially characterized the interdependence between metals and amino acids that is thought to have roots in prebiotic chemistry and to have shaped the chemical evolution of biopolymers. Specifically, depsipeptides, containing both ester and amide linkages, have been proposed as ancestors of peptides. We examined the effects of metals on depsipeptide formation in dry-down reactions. We postulated that zinc might influence the formation of depsipeptides containing histidine (His). Here we show that through direct association with His, zinc and a few other transition metals dramatically increase the yield of long His-containing depsipeptide oligomers. By contrast, alkali and alkali earth metals do not show the same effect. The results here show that interactions of proto-peptides with metal ions could have influenced early chemical evolution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the NSF and the NASA Astrobiology Program under the NSF Center for Chemical Evolution [CHE-1504217] and by the NASA Astrobiology Program under the NASA Center for the Origins of Life [80NSSC18K1139]. Moran Frenkel-Pinter acknowledges the NASA postdoctoral fellowship program.References
- S. Chakraborty, P. Hosseinzadeh and Y. Lu, Encyclopedia of Inorganic and Bioinorganic Chemistry, 2011, pp. 1–51 Search PubMed.
- T. B. Bartnikas and J. D. Gitlin, Nat. Struct. Mol. Biol., 2001, 8, 733 CrossRef CAS.
- B. L. Vallee and R. Williams, Proc. Natl. Acad. Sci. U. S. A., 1968, 59, 498 CrossRef CAS.
- K. A. McCall, C.-c. Huang and C. A. Fierke, J. Nutr., 2000, 130, 1437S–1446S CrossRef CAS.
- M. S. Bray, T. K. Lenz, J. W. Haynes, J. C. Bowman, A. S. Petrov, A. R. Reddi, N. V. Hud, L. D. Williams and J. B. Glass, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 12164–12169 CrossRef CAS.
- L. Belmonte and S. S. Mansy, Elements, 2016, 12, 413–418 CrossRef CAS.
- M. Frenkel-Pinter, M. Samanta, G. Ashkenasy and L. J. Leman, Chem. Rev., 2020, 120, 4707–4765 CrossRef CAS.
- J. G. Forsythe, S. S. Yu, I. Mamajanov, M. A. Grover, R. Krishnamurthy, F. M. Fernandez and N. V. Hud, Angew. Chem., Int. Ed., 2015, 54, 9871–9875 CrossRef CAS.
- S. S. Yu, R. Krishnamurthy, F. M. Fernandez, N. V. Hud, F. J. Schork and M. A. Grover, Phys. Chem. Chem. Phys., 2016, 18, 28441–28450 RSC.
- S. S. Yu, M. D. Solano, M. K. Blanchard, M. T. Soper-Hopper, R. Krishnamurthy, F. M. Fernández, N. V. Hud, F. J. Schork and M. A. Grover, Macromolecules, 2017, 50, 9286–9294 CrossRef CAS.
- J. G. Forsythe, A. S. Petrov, W. C. Millar, S. S. Yu, R. Krishnamurthy, M. A. Grover, N. V. Hud and F. M. Fernandez, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E7652–E7659 CrossRef CAS.
- M. Frenkel-Pinter, J. W. Haynes, M. C, A. S. Petrov, B. T. Burcar, R. Krishnamurthy, N. V. Hud, L. J. Leman and L. D. Williams, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 16338–16346 CrossRef CAS.
- D. Doran, Y. M. Abul-Haija and L. Cronin, Angew. Chem., Int. Ed., 2019, 58, 11253–11256 CrossRef CAS.
- B. Damer and D. Deamer, Astrobiology, 2020, 20, 429–452 CrossRef.
- S. L. Miller, Science, 1953, 117, 528–529 CrossRef CAS.
- M. P. Bernstein, J. P. Dworkin, S. A. Sandford, G. W. Cooper and L. J. Allamandola, Nature, 2002, 416, 401–403 CrossRef CAS.
- E. T. Peltzer and J. L. Bada, Nature, 1978, 272, 443–444 CrossRef CAS.
- J. Hertzog, H. Naraoka and P. Schmitt-Kopplin, Life, 2019, 9, 48 CrossRef CAS.
- E. Jarosewich, Meteoritics, 1971, 6 Search PubMed.
- C. T. Mant, T. L. Burke, J. A. Black and R. S. Hodges, J. Chromatogr. A, 1988, 458, 193–205 CrossRef CAS.
- L. M. Franklin, S. M. Walker and G. Hill, J. Mol. Model., 2020, 26, 116 CrossRef CAS.
- Kamaluddin, M. Nath, S. W. Deopujari and A. Sharma, Origins Life Evol. Biospheres, 1990, 20, 259–268 CrossRef CAS.
- N. Kitadai, Origins Life Evol. Biospheres, 2017, 47, 13–37 CrossRef CAS.
- J. Burgess, Metal ions in solution, Halsted Press, 1978 Search PubMed.
- B. M. Rode and M. G. Schwendinger, Origins Life Evol. Biospheres, 1990, 20, 401–410 CrossRef CAS.
- S. Saetia, K. R. Liedl, A. H. Eder and B. M. Rode, Origins Life Evol. Biospheres, 1993, 23, 167–176 CrossRef CAS.
- E.-i. Imai, H. Honda, K. Hatori, A. Brack and K. Matsuno, Science, 1999, 283, 831–833 CrossRef CAS.
- J. Napier and J. Yin, Peptides, 2006, 27, 607–610 CrossRef CAS.
- L. Leman, L. Orgel and M. R. Ghadiri, Science, 2004, 306, 283–286 CrossRef CAS.
- A. Kumar, Amino Acids, 2012, 43, 2417–2429 CrossRef CAS.
- D. Ballard, C. H. Bamford and F. Weymouth, Proc. R. Soc. London, Ser. A, 1955, 227, 155–183 CAS.
- T. J. Deming, Macromolecules, 1999, 32, 4500–4502 CrossRef CAS.
- B. M. Rode, Peptides, 1999, 20, 773–786 CrossRef CAS.
- J. D. Bernal, Proc. Phys. Soc., London, 1949, 62, 597 CrossRef.
- N. Lahav and D. White, J. Mol. Evol., 1980, 16, 11–21 CrossRef CAS.
- A. Brack, Dev. Clay Sci., 2006, 1, 379–391 CAS.
- C. Ponnamperuma, A. Shimoyama and E. Friebele, Origins Life, 1982, 12, 9–40 CrossRef CAS.
- B. M. Rode and Y. Suwannachot, Coord. Chem. Rev., 1999, 190, 1085–1099 CrossRef.
- J. G. Lawless and N. Levi, J. Mol. Evol., 1979, 13, 281–286 CrossRef CAS.
- A. D. McKee, M. Solano, A. Saydjari, C. J. Bennett, N. V. Hud and T. M. Orlando, ChemBioChem, 2018, 19, 1913–1917 CrossRef CAS.
- A. Vázquez-Salazar, G. Tan, A. Stockton, R. Fani, A. Becerra and A. Lazcano, Origins Life Evol. Biospheres, 2017, 47, 345–354 CrossRef.
- J. Oró, B. Basile, S. Cortes, C. Shen and T. Yamrom, Origins Life Evol. Biospheres, 1984, 14, 237–242 CrossRef.
- J. Oró and A. Kimball, Arch. Biochem. Biophys., 1962, 96, 293–313 CrossRef.
- J. P. Ferris and L. Orgel, J. Am. Chem. Soc., 1966, 88, 1074 CrossRef CAS.
- C. Shen, L. Yang, S. L. Miller and J. Oro, Origins Life Evol. Biospheres, 1987, 17, 295–305 CrossRef CAS.
- C. Shen, L. Yang, S. Miller and J. Oró, J. Mol. Evol., 1990, 31, 167–174 CrossRef CAS.
- A. Y. Mulkidjanian, Biol. Direct, 2009, 4, 26 CrossRef.
- A. Y. Mulkidjanian and M. Y. Galperin, Biol. Direct, 2009, 4, 27 CrossRef.
- I. Mamajanov, M. Caudan and T. Z. Jia, Protoenzymes: The Case of Hyperbranched Polymer-Scaffolded ZnS Nanocrystals, ChemRxiv, 2020 Search PubMed.
- J. D. Kim, D. H. Pike, A. M. Tyryshkin, G. Swapna, H. Raanan, G. T. Montelione, V. Nanda and P. G. Falkowski, J. Am. Chem. Soc., 2018, 140, 11210–11213 CrossRef CAS.
- C. M. Rufo, Y. S. Moroz, O. V. Moroz, J. Stöhr, T. A. Smith, X. Hu, W. F. DeGrado and I. V. Korendovych, Nat. Chem., 2014, 6, 303 CrossRef CAS.
- D. Ring, Y. Wolman, N. Friedmann and S. L. Miller, Proc. Natl. Acad. Sci. U. S. A., 1972, 69, 765–768 CrossRef CAS.
- S. L. Miller, Biochim. Biophys. Acta, 1957, 23, 480–489 CrossRef CAS.
- J. W. Szostak, J. Syst. Chem., 2012, 3, 2 CrossRef CAS.
- A. Jacobson, Biopolymers, 1965, 3, 249–259 CrossRef CAS.
- R. Zou, Q. Wang, J. Wu, J. Wu, C. Schmuck and H. Tian, Chem. Soc. Rev., 2015, 44, 5200–5219 RSC.
- J. Dong, J. E. Shokes, R. A. Scott and D. G. Lynn, J. Am. Chem. Soc., 2006, 128, 3540–3542 CrossRef CAS.
- J. Dong, J. M. Canfield, A. K. Mehta, J. E. Shokes, B. Tian, W. S. Childers, J. A. Simmons, Z. Mao, R. A. Scott and K. Warncke, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13313–13318 CrossRef CAS.
- W. Ji, C. Yuan, S. Zilberzwige-Tal, R. Xing, P. Chakraborty, K. Tao, S. Gilead, X. Yan and E. Gazit, ACS Nano, 2019, 13, 7300–7309 CrossRef CAS.
- P. W. Mantyh, J. R. Ghilardi, S. Rogers, E. DeMaster, C. J. Allen, E. R. Stimson and J. E. Maggio, J. Neurochem., 1993, 61, 1171–1174 CrossRef CAS.
- M. Reches and E. Gazit, Science, 2003, 300, 625–627 CrossRef CAS.
- D. E. Przybyla, C. M. Rubert Pérez, J. Gleaton, V. Nandwana and J. Chmielewski, J. Am. Chem. Soc., 2013, 135, 3418–3422 CrossRef CAS.
- R. M. Coggon, D. A. Teagle, C. E. Smith-Duque, J. C. Alt and M. J. Cooper, Science, 2010, 327, 1114–1117 CrossRef CAS.
- I. Halevy and A. Bachan, Science, 2017, 355, 1069–1071 CrossRef CAS.
- H. D. Holland, The chemical evolution of the atmosphere and oceans, Princeton University Press, 1984 Search PubMed.
- C. Scott, N. J. Planavsky, C. L. Dupont, B. Kendall, B. C. Gill, L. J. Robbins, K. F. Husband, G. L. Arnold, B. A. Wing and S. W. Poulton, Nat. Geosci., 2013, 6, 125–128 CrossRef CAS.
- E. K. Moore, J. Hao, A. Prabhu, H. Zhong, B. I. Jelen, M. Meyer, R. M. Hazen and P. G. Falkowski, J. Geophys. Res.: Biogeosci., 2018, 123, 743–759 CrossRef CAS.
- J. Glass and C. Dupont, in The Biological Chemistry of Nickel, Royal Society of Chemistry, Cambridge, UK, 2017, pp. 12–26 Search PubMed.
- K. O. Konhauser, L. J. Robbins, E. Pecoits, C. Peacock, A. Kappler and S. V. Lalonde, Astrobiology, 2015, 15, 804–815 CrossRef CAS.
- K. O. Konhauser, E. Pecoits, S. V. Lalonde, D. Papineau, E. G. Nisbet, M. E. Barley, N. T. Arndt, K. Zahnle and B. S. Kamber, Nature, 2009, 458, 750–753 CrossRef CAS.
- S.-J. Wang, R. L. Rudnick, R. M. Gaschnig, H. Wang and L. E. Wasylenki, Nat. Geosci., 2019, 12, 296–300 CrossRef.
- J. Hao, D. A. Sverjensky and R. M. Hazen, Earth Planet. Sci. Lett., 2017, 457, 191–203 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra07965k |
| This journal is © The Royal Society of Chemistry 2021 |