Nanotherapeutics using all-natural materials. Effective treatment of wound biofilm infections using crosslinked nanoemulsions†
Cheng-Hsuan
Li‡
a,
Ryan F.
Landis‡
a,
Jessa Marie
Makabenta‡
a,
Ahmed
Nabawy
a,
Tiphaine
Tronchet
a,
Danielle
Archambault
b,
Yuanchang
Liu
a,
Rui
Huang
a,
Morgane
Golan
b,
Wei
Cui
b,
Jesse
Mager
b,
Akash
Gupta
 a,
Suzannah
Schmidt-Malan
c,
Robin
Patel
a,
Suzannah
Schmidt-Malan
c,
Robin
Patel
 c and
Vincent M.
Rotello
c and
Vincent M.
Rotello
 *a
*a
aDepartment of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, Massachusetts 01003, USA. E-mail: rotello@chem.umass.edu
bDepartment of Veterinary and Animal Sciences, University of Massachusetts Amherst, 661 North Pleasant Street, Amherst, Massachusetts 01003, USA
cDivision of Clinical Microbiology, Department of Laboratory Medicine and Pathology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA
First published on 18th March 2021
Abstract
Bacterial wound infections are a threat to public health. Although antibiotics currently provide front-line treatments for bacterial infections, the development of drug resistance coupled with the defenses provided through biofilm formation render these infections difficult, if not impossible, to cure. Antimicrobials from natural resources provide unique antimicrobial mechanisms and are generally recognized as safe and sustainable. Herein, an all-natural antimicrobial platform is reported. It is active against bacterial biofilms and accelerates healing of wound biofilm infections in vivo. This antimicrobial platform uses gelatin stabilized by photocrosslinking using riboflavin (vitamin B2) as a photocatalyst, and carvacrol (the primary constituent of oregano oil) as the active antimicrobial. The engineered nanoemulsions demonstrate broad-spectrum antimicrobial activity towards drug-resistant bacterial biofilms and significantly expedite wound healing in an in vivo murine wound biofilm model. The antimicrobial activity, wound healing promotion, and biosafety of these nanoemulsions provide a readily translatable and sustainable strategy for managing wound infections.
New conceptsBacteria-associated infections are emerging threat to public health. Alternative treatment strategies to combat these infections are therefore an urgent priority. In this research, we show that readily available nature-derived components (gelatin, vitamin B2, essential oils) could be used to fabricate effective cross-linked nanoemulsions against refractory bacterial biofilms in vitro and in vivo. In contrast to most previous works, our approach only uses generally recognized as safe (GRAS) materials, further increasing safety and community acceptance. Moreover, all natural-derived therapeutics provide a sustainable path to manage diseases. Our study demonstrates the design of integrating all natural-derived materials for the treatment of bacterial biofilms. We envision that this design and concept contribute to the development of alternative antimicrobial strategies and therapeutics against other life-threatening diseases. |
Antibiotics are the current treatment of choice for bacterial infections.1 Unfortunately, bacteria are rapidly acquiring resistance against these agents through different mechanisms they have developed over billions of years to remove and/or deactivate toxins or evade their activities.2–4 The ability of pathogenic bacteria, including Pseudomonas aeruginosa and Staphylococcus aureus, to form biofilms results in particularly problematic infections in wounds and on implanted devices.5,6 The extracellular polymeric substance (EPS) matrix of biofilms has evolved as a barrier to some antibiotics and host immune responses. The slow growth and presence of persister cells in biofilms further foster resistance against traditional antibiotics.7 Biofilms also impede the wound healing process, resulting in chronic wounds associated with increased morbidity, mortality, and decreased quality of life.8,9 The lack of effective antibiofilm agents has led to an annual multibillion-dollar (US) burden to healthcare systems worldwide.10
Plant-derived essential oils provide a potential resource to combat bacterial biofilm-associated infections.11 Essential oils are produced by plants as protection against infections by bacteria.12 These oils have been extensively used in traditional medicine as antioxidant, anti-inflammatory and antibacterial agents, and their efficacy has been scientifically validated.13,14 The additional benefits of using essential oils, including aroma,15 safety,16 and sustainability, have contributed to their increasing use in therapeutics. The low aqueous solubility of essential oils, however, limits their use in combating planktonic bacterial infections.17 Moreover, these hydrophobic oils are not able to efficiently penetrate into the highly charged EPS of biofilms,18 making them of limited use against biofilm-associated infections.
Synthetic nanoparticles and polymers provide a strategy for enhancing the activity of essential oil-based antimicrobials.19,20 We set out to generate an all-natural platform that delivers active antimicrobials derived from essential oils for treatment of wound biofilms, with the potential for increased safety and community acceptance. In this platform, riboflavin (vitamin B2) is used to cross-link21,22 gelatin that stabilizes nanodroplets of carvacrol, a key antimicrobial component of oregano oil. Our approach combines nature-derived ingredients to generate well-defined nanostructured-materials with size and stability required for potent antimicrobial and antibiofilm applications.23,24 These nanoemulsions eradicated both Gram-positive and -negative bacterial biofilms in vitro. Significantly, the nanoemulsion system was highly active in vivo, reducing bacterial loads in the wound site and enhancing the rate of wound healing in a murine wound biofilm model. Taken together, the described nanoemulsions used only bio-derived GRAS (generally regarded as safe) components to provide a safe, sustainable and effective treatment for wound biofilms.
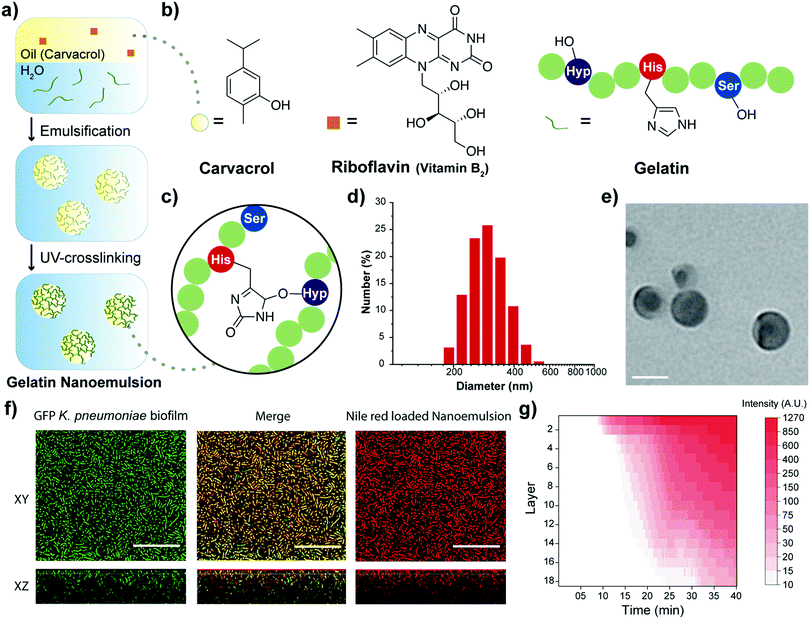
The choice of oil for fabricating the nanoemulsions plays a critical role in therapeutic effects.25 Oregano oil is among the most potent antimicrobial essential oils.19,26 Carvacrol is the primary active component of oregano oil,19 and was chosen to provide the GRAS benefits of oregano oil without the batch-to-batch variability observed with unpurified oils. Moreover, commercial gelatin was chosen as the scaffold for the oil-in-water nanoemulsion engineered to overcome the poor water solubility of carvacrol. Gelatin is naturally-derived, inherently biocompatible, biodegradable, non-cytotoxic, and has low antigenicity.27 Structurally, gelatin is hydrophilic with hydrophobic domains, allowing it to encapsulate and stabilize hydrophobic oils, such as carvacrol, in aqueous condition.28 The gelatin matrix was stabilized through a cross-linking technique used for therapeutic corneal collagen cross-linking.21 This strategy employs riboflavin (vitamin B2) as a photo-initiator for cross-linking gelatin fibers. Nanoemulsions were fabricated by emulsifying a suspension of riboflavin in carvacrol into an aqueous suspension of gelatin (Fig. 1a), generating a transiently stable emulsion. This emulsion was then irradiated (365 nm) to activate the cross-linking process, resulting in stable nanoemulsions.
Dynamic light scattering (DLS), transmission electron microscopy (TEM) and attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) were used to characterize the nanoemulsions. DLS analysis showed that the hydrodynamic diameter of nanoemulsions is ∼310 nm with a narrow size distribution (polydispersity index = 0.017) (Fig. 1d). These nanoemulsions are stable at room temperature for at least 30 days and are degraded in the presence of collagenase (Fig. S1, ESI†). TEM photographs revealed a spherical morphology of the nanoemulsions (Fig. 1e and Fig. S2, ESI†). The size observed in TEM (∼100 nm) is smaller than observed by DLS, presumably due to partial collapse upon removal of carvacrol under vacuum during TEM. Finally, the chemical nature of the cross-linking was demonstrated by the emergence of a band at 1033 cm−1 arising from aliphatic-aromatic ether formation, an additional aromatic ether signature at 1242 cm−1, and appearance of sp3 C–H stretches at 2957 cm−1 (Fig. 1c and Fig. S3, ESI†).
Carvacrol and other hydrophobic materials fail to penetrate biofilms.18 We hypothesized that the cationic charge of gelatin at the low pH29 found in biofilms30 would facilitate transport of nanoemulsions into biofilms. Confocal laser scanning microscopy (CLSM) was used to demonstrate effective and complete penetration of gelatin nanoemulsions into biofilms. Transport was tracked by loading the vehicle with the hydrophobic dye Nile red. Nanoemulsions were incubated with 4 day old biofilms of green fluorescent protein (GFP)-expressing K. pneumoniae IDRL-11999. As shown in Fig. 1f, gelatin nanoemulsions penetrated the biofilm matrix completely, as indicated by visualization of Nile red throughout the thickness of the biofilm, with colocalization of red and green signals. Time-dependent z-stack scanning demonstrated that complete penetration of nanoemulsions occurred within 40 minutes (Fig. 1g).
We next probed the mechanism of action of gelatin nanoemulsions. We hypothesized that gelatin nanoemulsions kill bacteria the same manner as carvacrol, namely through disrupting the cell membrane. We used propidium iodide (PI) staining to monitor the membrane permeability of bacteria.31 Planktonic bacteria (P. aeruginosa, ATCC-27853) were treated with gelatin nanoemulsions or antibiotic ceftazidime (from 0.125× to 4× of their respective minimum inhibitory concentrations (MICs)) in the presence of PI. Gelatin nanoemulsion treatment immediately generated fluorescence inside bacteria, indicating that PI penetrated through the compromised cell membrane and bound to DNA (Fig. S4, ESI†). In contrast, bacteria treated with ceftazidime did not generate fluorescence, as ceftazidime does not directly act on the membrane. Significantly, studies have shown that therapeutics causing membrane disruption are unlikely to induce resistance,19 as demonstrated in a recent effective treatment of burn infection models.32
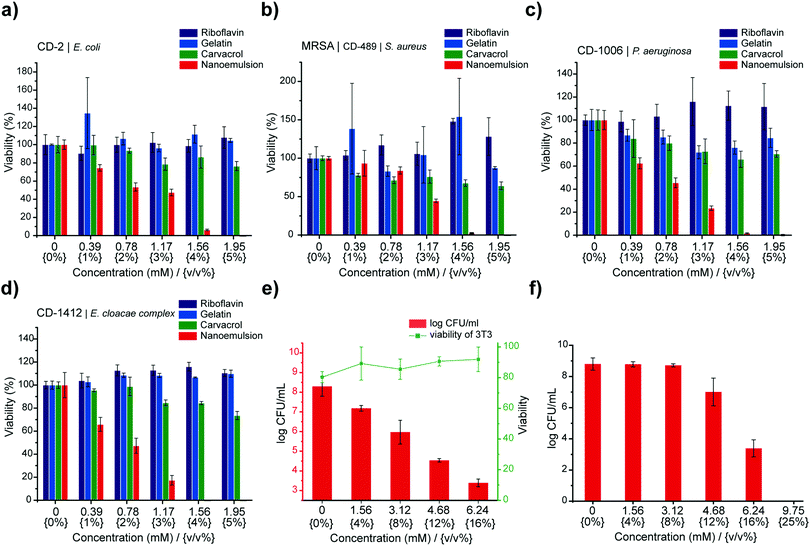
After validating the biofilm penetration profile and the mechanism of action, we used alamarBlue assay to assess antimicrobial activity of nanoemulsions against biofilms of four clinical bacterial isolates (P. aeruginosa CD-1006, methicillin-resistant S. aureus [MRSA] CD-489, Escherichia coli CD-2, and Enterobacter cloacae complex CD-1412). Treatment of these biofilms with nanoemulsions for 3 hours eliminated bacteria within the biofilms at 5% v/v (1.95 mM of carvacrol) (Fig. 2), with individual components of the nanoemulsions having minimal effect. Notably, treatment of bacteria with only gelatin (nutrient33) or riboflavin (nutrient/potential quorum sensing signal34) can enhance biofilm viability. We further quantified the minimum biofilm inhibitory concentrations (MBICs) and minimum biofilm eradication concentrations (MBECs) towards several clinical isolates (Table S1, ESI†), including P. aeruginosa (CD-1006, IDRL-11442, ATCC 27583) and MRSA (IDRL-6169) to represent bacterial species that are common constituents of wound biofilm infections.35 All biofilms were suppressed or eradicated by nanoemulsions at concentrations ranging from 4% v/v (1.56 mM) to 8% v/v (3.12 mM). This activity was mirrored in more challenging dual-species biofilm models (MRSA/P. aeruginosa and MRSA/E. coli) (Table S1, ESI†).36
We next used an in vitro bacterial biofilm-mammalian cell coculture model to evaluate antimicrobial activity and biocompatibility of gelatin nanoemulsions. P. aeruginosa (ATCC-27853) was seeded on the monolayer of NIH 3T3-fibroblast cells for 6 hours to mimic biofilm infections on mammalian cells. Subsequently, the cocultures were treated with gelatin nanoemulsion for three hours. The viabilities of mammalian cells and bacteria were determined using LDH assay and colony counting, respectively. As shown in Fig. 2e, gelatin nanoemulsions effectively reduce bacterial colonies up to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 colony forming units (CFUs), while the toxicity toward 3T3 fibroblast cells remained negligible. Moreover, the activity of the nanoemulsions was determined using a simulated wound fluid (SWF) model, mimicking the interference/deactivation of antimicrobial activity by wound fluids.37 In this experiment, 4 day old MRSA (IDRL-6169) biofilms were grown using tryptic soy broth (TSB)/SWF (1
log10 colony forming units (CFUs), while the toxicity toward 3T3 fibroblast cells remained negligible. Moreover, the activity of the nanoemulsions was determined using a simulated wound fluid (SWF) model, mimicking the interference/deactivation of antimicrobial activity by wound fluids.37 In this experiment, 4 day old MRSA (IDRL-6169) biofilms were grown using tryptic soy broth (TSB)/SWF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. Gelatin nanoemulsions were serially diluted with SWF solution and added to the biofilms; three hours after, antimicrobial efficacy was determined using quantitative colony counting. Nanoemulsions were active against the biofilms in SWF, with 12% v/v (4.68 mM) and 16% v/v (6.24 mM) of nanoemulsions resulting in ∼2 and ∼5
1) solution. Gelatin nanoemulsions were serially diluted with SWF solution and added to the biofilms; three hours after, antimicrobial efficacy was determined using quantitative colony counting. Nanoemulsions were active against the biofilms in SWF, with 12% v/v (4.68 mM) and 16% v/v (6.24 mM) of nanoemulsions resulting in ∼2 and ∼5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 CFU reduction, respectively (Fig. 2f).
log10 CFU reduction, respectively (Fig. 2f).
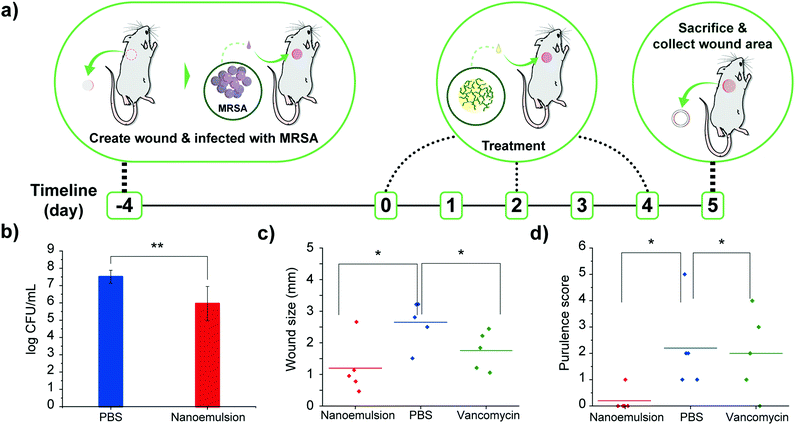
Encouraged by the in vitro efficacy of the nanoemulsions, their in vivo activity was evaluated using a murine wound biofilm model (Fig. 3a) designed to assess efficacy against established biofilms found in chronic wound infections.8,9 In this model, MRSA IDRL-6169 biofilms were established in a wound created using a 5 mm skin puncture, and allowed to mature for 4 days. Mice were then separated into three groups: 100% v/v nanoemulsions (39 mM carvacrol), vancomycin (110 mg kg−1), and saline solution only. Treatments were administered every other day (topical application of nanoemulsions and PBS, and intraperitoneal injection of vancomycin) until the day of sacrifice. Photographs were taken daily, and wound sizes and weights of the mice were monitored every day. The degree of purulence was also evaluated daily using a purulence reaction scoring system described in Fig. S5, ESI.†
Treatment with nanoemulsions significantly reduced bacterial load in the wound as compared with PBS controls, with ∼1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 unit reduction after administration of two treatments (Fig. 3b), a reduced degree of killing relative to in vitro studies owing to the much greater complexity of the in vivo environment. Significantly, vancomycin only had ∼0.5
log10 unit reduction after administration of two treatments (Fig. 3b), a reduced degree of killing relative to in vitro studies owing to the much greater complexity of the in vivo environment. Significantly, vancomycin only had ∼0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 bacterial reduction (Fig. S6, ESI†). The antimicrobial effect of the nanoemulsions was mirrored by their enhanced wound healing. The group treated with nanoemulsions showed a greater reduction in wound size after treatment as compared to those treated with vancomycin or PBS (Fig. 3c), with the two control group wound beds still containing pus (Fig. S7–S9, ESI†). The wounds were likewise better healed: mice in the nanoemulsion group had a purulence score 0, with normal-appearing healed wound beds. In contrast, vancomycin and PBS groups had purulence scores ∼2, indicating the presence of pus (Fig. 3d and Fig. S10, ESI†).
log10 bacterial reduction (Fig. S6, ESI†). The antimicrobial effect of the nanoemulsions was mirrored by their enhanced wound healing. The group treated with nanoemulsions showed a greater reduction in wound size after treatment as compared to those treated with vancomycin or PBS (Fig. 3c), with the two control group wound beds still containing pus (Fig. S7–S9, ESI†). The wounds were likewise better healed: mice in the nanoemulsion group had a purulence score 0, with normal-appearing healed wound beds. In contrast, vancomycin and PBS groups had purulence scores ∼2, indicating the presence of pus (Fig. 3d and Fig. S10, ESI†).
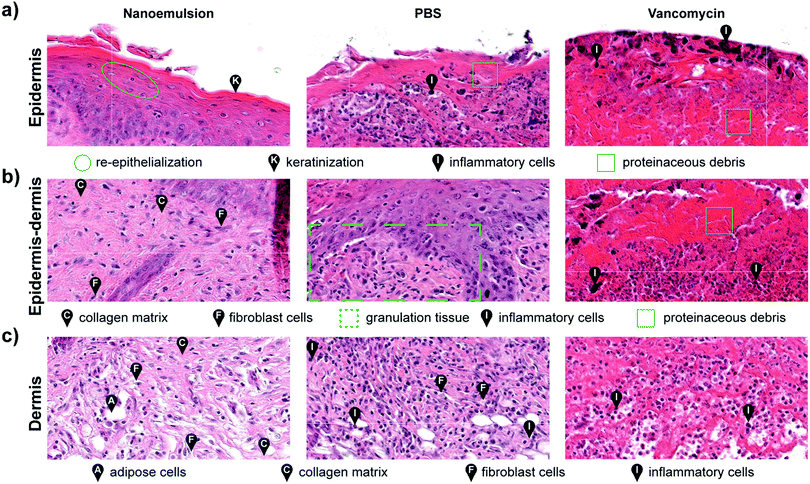
Histological analysis of the wound beds similarly indicated enhanced healing with the nanoemulsions. Hematoxylin and eosin staining (H&E staining) revealed regeneration of keratin and epithelial layers, and collagen matrix for the nanoemulsion group. In contrast, for the vancomycin and PBS groups, inflammatory cells were still abundantly found in the area, indicating that the wounds were in the early stages of the wound healing cascade (Fig. 4 and Fig. S11, ESI†). Notably, the healed skin had a normal appearing epidermis and dermis, suggesting that nanoemulsions do not alter morphology of the skin undergoing repair. The relative lack of inflammatory cells could also suggest an anti-inflammatory role for the nanoemulsions in wound healing.
Conclusions
In summary, integration of nanostructured biomaterials with essential oil payloads provides effective treatment of challenging wound biofilms. Emulsification of oregano oil and gelatin followed by vitamin B2 cross-linking provided stable nanoemulsions comprised solely of naturally-occurring components. These described nanoemulsions effectively penetrate into biofilms, and kill embedded Gram-positive and -negative bacteria effectively in vitro. This antibacterial effect is observed in an in vivo murine model, and translates into enhancement in wound healing both in terms of wound size and degree of purulence. Crucially, this platform is highly modular, providing a general platform for the delivery of a wide variety of oils and other payloads. Overall, integration of the inherent bactericidal activity of essential oils with materials properties provided by biomaterials presents a new path of treatment for wound biofilms, with potential for treating other life-threatening bacterial infections.Author contributions
R. F. L. and V. M. R. conceived, designed and fabricated gelatin nanoemulsions. R. F. L., C.-H. L., A. N. and R. H. characterized gelatin nanoemulsions. T. T., C.-H. L., J. M. M. and A. N. performed antimicrobial experiments in vitro. S. S. M. and R. P. helped design in vivo experiments and edited the manuscript. J. M. M., A. N., Y. L. and A. G. performed antimicrobial experiments in vivo. W. C. assisted in in vivo study. D. A. and J. M. carried out histological experiments and analysed data. C.-H. L., J. M. M. and V. M. R. wrote and revised the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no competing final interest.Acknowledgements
This research was supported by the NIH (AI134770). Clinical samples obtained from the Cooley Dickinson Hospital Microbiology Laboratory (Northampton, MA) were kindly provided by Dr Margaret Riley. The microscopy data was gathered in the Light Microscopy Facility and Nikon Center of Excellence at the Institute for Applied Life Sciences, UMass Amherst with support from the Massachusetts Life Sciences Center.Notes and references
- N. F. Col and R. W. O’Connor, Rev. Infect. Dis., 1987, 9, S232–S243 CrossRef PubMed.
- J. M. A. Blair, M. A. Webber, A. J. Baylay, D. O. Ogbolu and L. J. V. Piddock, Nat. Rev. Microbiol., 2015, 13, 42–51 CrossRef CAS PubMed.
- R. Aminov, Front. Microbiol., 2010, 1, 134 CrossRef PubMed.
- X. Liu, Z. Wang, X. Feng, E. Bai, Y. Xiong, X. Zhu, B. Shen, Y. Duan and Y. Huang, Bioconjugate Chem., 2020, 31, 1425–1437 CrossRef CAS PubMed.
- G. A. James, E. Swogger, R. Wolcott, E. deLancey Pulcini, P. Secor, J. Sestrich, J. W. Costerton and P. S. Stewart, Wound Repair Regen., 2008, 16, 37–44 CrossRef PubMed.
- C. R. Arciola, D. Campoccia, P. Speziale, L. Montanaro and J. W. Costerton, Biomaterials, 2012, 33, 5967–5982 CrossRef CAS PubMed.
- K. Lewis, Curr. Top. Microbiol. Immunol., 2008, 322, 107–131 CAS.
- S. L. Percival, S. M. McCarty and B. Lipsky, Adv. Wound Care, 2014, 4, 373–381 CrossRef PubMed.
- C. Xu, O. U. Akakuru, X. Ma, J. Zheng, J. Zheng and A. Wu, Bioconjugate Chem., 2020, 31, 1708–1723 CrossRef CAS PubMed.
- K. Järbrink, G. Ni, H. Sönnergren, A. Schmidtchen, C. Pang, R. Bajpai and J. Car, Syst. Rev., 2017, 6, 1–7 CrossRef PubMed.
- M. Simões, R. N. Bennett and E. A. S. Rosa, Nat. Prod. Rep., 2009, 26, 746–757 RSC.
- B. K. Tiwari, V. P. Valdramidis, C. P. O’Donnell, K. Muthukumarappan, P. Bourke and P. J. Cullen, J. Agric. Food Chem., 2009, 57, 5987–6000 CrossRef CAS PubMed.
- M. T. Baratta, H. J. D. Dorman, S. G. Deans, D. M. Biondi and G. Ruberto, J. Essent. Oil Res., 1998, 10, 618–627 CrossRef CAS.
- M. H. Farzaei, R. Bahramsoltani, Z. Abbasabadi and R. Rahimi, J. Pharm. Pharmacol., 2015, 67, 1467–1480 CrossRef CAS PubMed.
- M. Etman, M. Amin, A. H. Nada, M. Shams-Eldin and O. Salama, Drug Discoveries Ther., 2011, 5, 150–156 CrossRef CAS PubMed.
- G. Freire Rocha Caldas, A. V. Araújo, G. S. Albuquerque, J. D. C. Silva-Neto, J. H. Costa-Silva, I. R. A. De Menezes, A. C. L. Leite, J. G. M. Da Costa, A. G. Wanderley, G. F. R. Caldas, A. V. Araújo, G. S. Albuquerque, J. D. C. Silva-Neto, J. H. Costa-Silva, I. R. A. de Menezes, A. C. L. Leite, J. G. M. da Costa and A. Gonçalves-Wanderley, Evidence-Based Complement. Altern. Med., 2013, 2013, 856168 Search PubMed.
- C. Samperio, R. Boyer, W. N. Eigel, K. W. Holland, J. S. McKinney, S. F. O’Keefe, R. Smith and J. E. Marcy, J. Agric. Food Chem., 2010, 58, 12950–12956 CrossRef CAS PubMed.
- A. Iannitelli, R. Grande, A. Di Stefano, M. Di Giulio, P. Sozio, L. J. Bessa, S. Laserra, C. Paolini, F. Protasi and L. Cellini, Int. J. Mol. Sci., 2011, 12, 5039–5051 CrossRef CAS PubMed.
- R. F. Landis, C.-H. Li, A. Gupta, Y.-W. Lee, M. Yazdani, N. Ngernyuang, I. Altinbasak, S. Mansoor, M. A. S. Khichi, A. Sanyal and V. M. Rotello, J. Am. Chem. Soc., 2018, 140, 6176–6182 CrossRef CAS PubMed.
- A. Guarda, J. F. Rubilar, J. Miltz and M. J. Galotto, Int. J. Food Microbiol., 2011, 146, 144–150 CrossRef CAS PubMed.
- G. Wollensak, E. Spoerl and T. Seiler, Am. J. Ophthalmol., 2003, 135, 620–627 CrossRef CAS PubMed.
- J. Ribes, N. Beztsinna, R. Bailly, S. Castano, E. Rascol, N. Taib-Maamar, E. Badarau and I. Bestel, Bioconjugate Chem., 2021, 32, 553–562 CrossRef CAS PubMed.
- J. M. V. Makabenta, A. Nabawy, C.-H. Li, S. Schmidt-Malan, R. Patel and V. M. Rotello, Nat. Rev. Microbiol., 2021, 19, 23–36 CrossRef CAS PubMed.
- Y. Liu, L. Shi, L. Su, H. C. Van der Mei, P. C. Jutte, Y. Ren and H. J. Busscher, Chem. Soc. Rev., 2019, 48, 428–446 RSC.
- N. Terjung, M. Löffler, M. Gibis, J. Hinrichs and J. Weiss, Food Funct., 2012, 3, 290–301 RSC.
- A. Nostro, A. R. Blanco, M. A. Cannatelli, V. Enea, G. Flamini, I. Morelli, A. S. Roccaro and V. Alonzo, FEMS Microbiol. Lett., 2004, 230, 191–195 CrossRef CAS PubMed.
- A. O. Elzoghby, J. Controlled Release, 2013, 172, 1075–1091 CrossRef CAS PubMed.
- E. Dickinson, Food Hydrocolloids, 2009, 23, 1473–1482 CrossRef CAS.
- B. Gaihre, M. S. Khil, D. R. Lee and H. Y. Kim, Int. J. Pharm., 2009, 365, 180–189 CrossRef CAS PubMed.
- J. M. Vroom, K. J. De Grauw, H. C. Gerritsen, D. J. Bradshaw, P. D. Marsh, G. K. Watson, J. J. Birmingham and C. Allison, Appl. Environ. Microbiol., 1999, 65, 3502–3511 CrossRef CAS PubMed.
- D. J. Arndt-Jovin and T. M. Jovin, in Fluorescence Microscopy of Living Cells in Culture Part B. Quantitative Fluorescence Microscopy—Imaging and Spectroscopy, ed. D. L. Taylor and Y.-L. Wang, Academic Press, 1989, vol. 30, pp. 417–448 Search PubMed.
- M. Lu, S. Wang, T. Wang, S. Hu, B. Bhayana, M. Ishii, Y. Kong, Y. Cai, T. Dai, W. Cui and M. X. Wu, Sci. Transl. Med., 2021, 13, eaba3571 CrossRef CAS PubMed.
- S. A. Koser, B. D. Chinn and F. Saunders, J. Bacteriol., 1938, 36, 57–65 CrossRef CAS PubMed.
- S. Rajamani, W. D. Bauer, J. B. Robinson, J. M. Farrow, E. C. Pesci, M. Teplitski, M. Gao, R. T. Sayre and D. A. Phillips, Mol. Plant-Microbe Interact., 2008, 21, 1184–1192 CrossRef CAS PubMed.
- R. Serra, R. Grande, L. Butrico, A. Rossi, U. F. Settimio, B. Caroleo, B. Amato, L. Gallelli and S. de Franciscis, Expert Rev. Anti-Infect. Ther., 2015, 13, 605–613 CrossRef CAS PubMed.
- S. DeLeon, A. Clinton, H. Fowler, J. Everett, A. R. Horswill and K. P. Rumbaugh, Infect. Immun., 2014, 82, 4718–4728 CrossRef PubMed.
- W. A. Craig and P. G. Welling, Clin. Pharmacokinet., 1977, 2, 252–268 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental methods, MBICs and MBBCs of gelatin nanoemulsions against bacterial biofilms, information of bacterial strains, stability and biodegradation of gelatin nanoemulsions determined by DLS, IR spectra of nanoemulsions, photographs of infected wounds, purulence scoring system, purulence scores of wounds, images of histological samples. See DOI: 10.1039/d0mh01826k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |