Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular nanomotors with “pH taxis” for active drug delivery in the tumor microenvironment†
Motilal
Mathesh
,
Jiawei
Sun
,
Frans
van der Sandt
and
Daniela A.
Wilson
 *
*
Institute of Molecules and Materials, Radboud University, Heyendaalseweg 135, 6525 AJ, Nijmegen, The Netherlands. E-mail: d.wilson@science.ru.nl
First published on 22nd October 2020
Abstract
Self-propelled nanomotors demonstrating autonomous motion in biologically relevant fuel are currently being studied to overcome the use of external physical or chemical stimuli as precise delivery agents. In this context, the tumor microenvironment (TME) with slightly acidic pH is used for developing cargo-releasing artificial systems triggered by such conditions. However, there is still a need for fabrication of smart nanomotors that can sense the acidic pH prevalent in the TME rather than using an external fuel source for selective activation and thereafter migrating towards tumors for active drug delivery. Herein, supramolecular assembly-based nanomotors are fabricated by in-situ grown CaCO3 nanoparticles and studied for their motility behaviour in endogenously generated acidic pH by HeLa cells and further exploited as an active delivery vehicle for DOX molecules to the cells for their anticancer efficacy. The nanomotors are activated in slightly acidic pH showcasing “pH taxis” towards tumor cells without the need for any sophisticated/complicated technologies or an external fuel source for active and targeted delivery of drugs.
Introduction
Advancements in the field of artificial micro/nanomotors have shifted the niche of drug delivery systems (DDS) from passive to active autonomous systems. These active systems overcome the drawbacks of a passive delivery system that relies on passive accumulation in the target site, thus reducing the efficacy and bioavailability and increasing the associated high systemic toxicity. Active synthetic micro/nanomotors are also sought after due to their attractive capabilities in the field of biomedicine1 such as biosensing,2 cargo delivery3 and nanosurgery4 and can be actuated and controlled by an external source such as magnetic,5–7 electric,8,9 light10–13 and ultrasound (US) acoustic wave sources.14–17 With these advancements, researchers have fabricated artificial systems utilizing more than one chemical or physical stimuli for actuation for in-vivo studies and biomedical applications. For instance, our group has recently fabricated bowl-shaped stomatocyte nanomotors loaded with platinum/nickel (Pt/Ni) nanoparticles for propelled motion and enhanced directionality. Pt nanoparticles decomposed H2O2 to produce O2 for propulsion, and the directionality was achieved by an external magnet in the presence of magnetic Ni nanoparticles.6 In terms of biomedical applications, Pt-loaded stomatocytes were observed to show chemotactic behavior towards H2O2 produced by neutrophil cells.18 Furthermore, once the Pt-loaded stomatocytes were fabricated by mixing PEG-b-PS and PEG-b-PCL, they were observed to degrade at acidic pH owing to the biodegradability of PCL and deliver doxorubicin (DOX) to cancer cells.19 Other groups fabricated US-powered nanowire motors comprising nanoporous gold structures incorporated with DOX, where the drug release was triggered by near-infrared light due to photothermal effects.20 Even though such systems have been studied extensively for biomedical applications, the use of a non-biocompatible fuel/external power source for guidance is not appropriate in a biological environment and has drawbacks in terms of delicate manipulation and requires specialists and sophisticated equipment.21 In order to circumvent this problem, designing artificial systems powered by fuels inherently present in the body fluids or their constituents would be beneficial.22Recently, fabrication of nanoconstructs for active delivery of drugs by using the in-vivo conditions of the tumor microenvironment (TME) is being studied. The most commonly used active DDS for tumor cells are based on the difference in redox states, pH and biomolecules between tumor cells and healthy cells.23 Herein, we focus on slightly acidic conditions observed in tumor cells that have been utilized for powering mico/nanomotors. The pH in the TME is slightly acidic (pH 6.5–6.8) that decreases to 4.5–5.5 in the endo/lysosomes of tumor cells,24 due to the Warburg effect, i.e., metabolism through glycolysis that produces lactate.25 Gao et al. fabricated pH-responsive biocatalytic micromotors by the super-assembly of catalase and succinylated β-lactoglobulin that propel in the presence of H2O2 and release DOX upon endocytosis and particle degradation in cellular acidic compartments. The mechanism is based on reversible gelation of succinylated β-lactoglobulin depending on pH conditions. Even though the system was successful in showcasing the proof-of-concept study for exploiting physiological conditions, H2O2 was supplemented externally to cell culture.26 In another example, Janus nanoparticles comprising hydroxyapatite were fabricated with urease and hyaluronic acid that facilitated tumor penetration due to active motion, thus enhancing uptake by tumor cells. Upon delivery into tumor cells, the increasing solubility of hydroxyapatite helped in the controlled release of drugs to tumor cells.24 Also, tadpole-like structures with size less than 100 nm with functionalized catalase were fabricated to propel in the presence of H2O2 in the TME.27 Even though motion was achieved in both systems, the fuel required for propulsion was supplied externally and was not endogenously produced by the tumor microenvironment. Another major drawback was functionalization of enzymes on the outside of the nanomotors that can be deactivated in the TME. Inorganic nanoparticles such as calcium carbonate (CaCO3), known to dissolve under acidic conditions,28 are biocompatible and can be used for pH-responsive cancer therapy. Until now, they have been widely used for passive delivery of drugs that can be anchored onto CaCO3 particles by co-precipitation, and once they are in the vicinity of tumor cells, they release the drugs due to low pH conditions and cause cell death.29 Sen et al. showed the release of Ca2+, HCO3−, OH+ and H+ ions upon dissolution of CaCO3 to induce particle movement. The mechanism is based on the diffusioosmosis process created by the difference in diffusion coefficients of the generated ions,30 which could be utilized to power much smaller motors in the nanometer range under weak acidic conditions.31 We envisioned that CaCO3 nanoparticles could be used to achieve active motion of our stomatocyte nanomotors in the slightly acidic environment present in the TME as potential DDS for biomedical applications. Our group pioneered the fabrication of supramolecular assemblies by a “bottom-up approach” called stomatocytes,32 which are made up of amphiphilic di-block copolymers providing a soft interface to the cells and thus can be utilized for delivery of drug molecules.19 Herein, for the first time, we fabricated nanomotors by growing in-situ CaCO3 nanoparticles inside supramolecular architectures which play the role of a nanoreactor and studied their motion behavior in the presence of HeLa cells to simulate the TME. The smart nanomotors were observed to be activated under slightly acidic pH conditions and were able to propel autonomously towards HeLa cells, as a truly “pH tactic” system. Furthermore, DOX molecules were loaded into CaCO3 stomatocytes (CaCO3-sto) structures by co-precipitation and the hybrid motile system was used for actively delivering into HeLa cells. This approach avoids the use of complicated functionalization for active delivery of cargoes in the biological environment and also the use of an external fuel source to power nanomotors, which opens up opportunities for new applications under in-vivo conditions.
Results and discussion
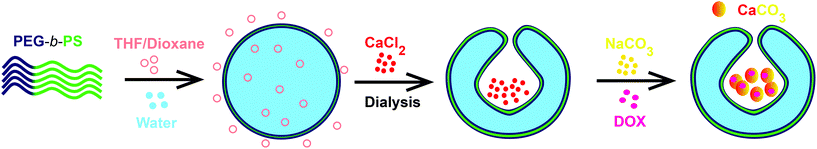
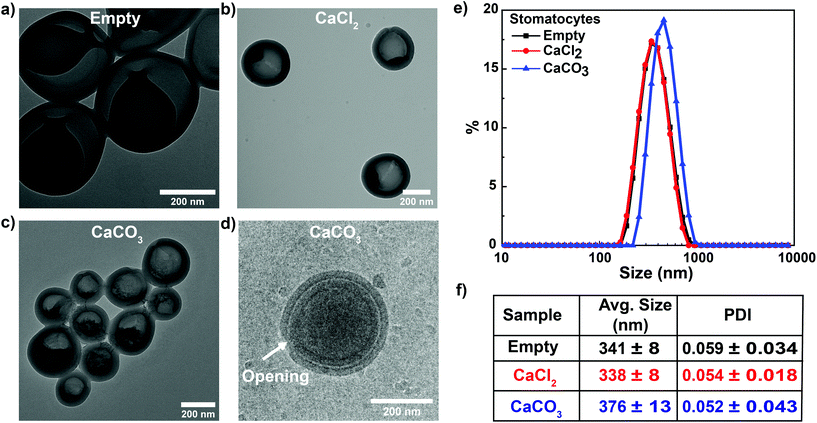
The schematic for the facile approach used to grow “in-situ” CaCO3 nanoparticles in the stomach of stomatocytes for having autonomous motion under weak acidic conditions is shown in Fig. 1. In order to avoid any complicated methods for CaCO3 nanoparticle formation, we focused on a well-known, simple and easy precipitation reaction between CaCO3 and Na2CO3.33Briefly, the CaCl2 salt was initially encapsulated in the stomach during the shape transformation of polymersomes into stomatocytes by dialyzing the polymersomes with an aqueous solution of CaCl2 followed by dropwise addition of Na2CO3 solution and stirring overnight at 500 rpm. During the procedure, Na2CO3 solution can pass through the opening of stomatocytes to react with CaCl2 and form CaCO3 nanoparticles by a precipitation reaction. The as-formed stomatocytes were studied by TEM that indicated the presence of the CaCl2 salt as a dark region (Fig. 2b) before Na2CO3 addition and a porous structure formation in the stomach (Fig. 2c) after Na2CO3 addition due to the formation of CaCO3 nanoparticles. A well-defined opening was also observed by cryo-TEM (Fig. 2d), which is important for the decomposition of CaCO3 nanoparticles under acidic pH conditions, which further leads to powering the nanomotors. From DLS measurements, the sizes of empty and CaCl2 stomatocytes were observed to be around 340 nm and 376 nm for in-situ formed CaCO3-sto (Fig. 2e). It is noteworthy that CaCO3 was in the nanosize regime due to the templating effect from stomatocytes during its formation. Previously, such a technique was reported for metal nanoparticle entrapment in stomatocytes where the stomach had a similar effect on the formation of Pt nanoparticles by controlling and confining the nucleation sites.34
In tumor cells, lactic acid is produced as a by-product due to anaerobic glucose metabolism inducing acidosis,35 with pH values around 6.0–6.5 in the tumor intracellular environment36 and 4.5–5.5 in the endo/lysosomes37 of tumor cells. In order to simulate the above acidic conditions induced naturally by HeLa cells, CaCO3-sto were studied for the autonomous motion in buffers of pH 4.6, 6 and 7 using nanoparticle tracking analysis (NTA). The mean-square displacement (MSD) curves were obtained by following trajectories in NTA and fitted by linear fit or parabolic fit for Brownian and propelled motion, respectively. According to the self-diffusiophoretic model proposed by Golestanian and coworkers, the speed of the nanomotors was deduced by the following equation:38
| r2 = 4DΔt + (vΔt)2 |
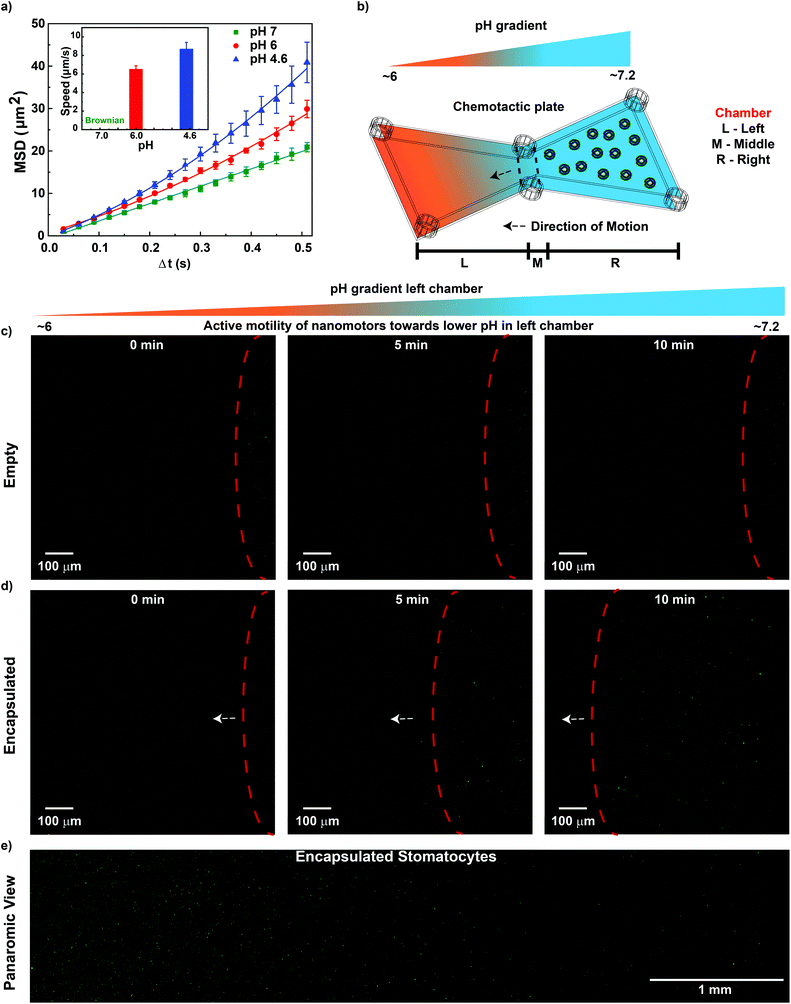
Under neutral pH conditions, CaCO3-sto showed Brownian motion, and with a decrease in the pH from 6 to 4.6, an increase in the speed was observed from 6.5 to 9 μm s−1 (Fig. 3a). In comparison, only Brownian motion was observed for the control CaCl2-sto samples (ESI Fig. S2†) at all pH values, instigating CaCO3 dissolution in slightly acidic pH as the driving force. The motion mechanism behind CaCO3-sto is due to the self-dissolution of CaCO3 nanoparticles inside stomatocytes under acidic conditions to ions such as Ca2+, HCO3− and OH−. These self-generated ions introduce a chemical gradient around the stomatocytes due to the difference in their diffusion coefficient and cause diffusioosmotic flows31 powering the stomatocytes. A similar self-diffusiophoresis mechanism has been studied to actuate nanomotors in the presence of glucose using cascade enzymatic reactions involving glucose oxidase and catalase.39 Previously, pH-mediated propulsion of artificial systems has been studied by fabrication of tubular polyaniline/Zn microrockets powered by acidic conditions with speeds reaching up to 100 bodylengths per s.40 In another example, silica particles coated with polymer multilayers disintegrating by a change in the pH were observed to have active motion and the self-propulsion was reported to result from a broken symmetry due to the polymer concentration gradient along the surface of swimmers.41 However, the former system requires highly acidic conditions which are not present in the TME and the transition metal Zn is also known to corrode slowly.42 The latter system used the pH gradient created by NaOH solution in μ-slides that is not present in the TME. Herein, we overcome these issues by using biocompatible and low-cost CaCO3 that can disintegrate in an acidic environment to self-generate a chemical gradient by release of ions in the pH value regime found in the TME and propel the nanomotors. To the best of our knowledge, this is the first study demonstrating the use of CaCO3 to drive supramolecular assembly-based nanomotors.
In order to push the system towards biomedical applications, it is important to study the motion in the physiologically present fuel. Hence, once the autonomous motion of CaCO3-sto was observed, the next step was to extrapolate the system and study its motion behavior in the presence of conditioned tumor cell media that represent the physiologically present fuel. For this purpose, a μ-slide chemotaxis plate from Ibidi was used that contains two chambers on either side separated by a middle chamber that can be used for long-term chemotaxis studies.43 A schematic representation is shown in Fig. 3b, illustrating the different chambers and the establishment of a pH gradient. The left and middle chambers were filled with conditioned HeLa cell media (pH 6.0); the right chamber was filled with a dilute solution of CaCO3-sto (encapsulated) as the test sample and empty stomatocytes in the case of control experiments. For reliability, all measurements were carried out at the same position in the left chamber of the chemotactic plate. From the chemotaxis experiment, it was observed that CaCO3-sto has directed movement towards lower pH conditions in comparison with Brownian movement for empty stomatocytes. This was witnessed with the increase in the number of particles in the left chamber with increasing time resulting from the migration of CaCO3-sto particles towards lower pH conditions (Fig. 3c and d). The panoramic view of the left chamber of the chemotactic plate (after 1 h) showed an increase in the number of particles with increasing pH gradient that was not observed for empty stomatocytes (ESI Fig. S3†). The control experiments rule out the possibility of the Marangoni effect, drift effects and convection gradients for the movement of CaCO3-sto in the chemotactic plate. Further proof for the migration of particles in the pH gradient was obtained by monitoring the middle and left chambers at 0 and after 30 min (ESI Fig. S4†). In the case of empty stomatocytes, there was no migration observed in both the chambers at the two time points, but for CaCO3-sto, a decrease in the number of particles was observed in the middle chamber and also significant particles were observed to be migrated to the end of the left chamber after 30 min. This showcases the directed motion of CaCO3-sto in the pH gradient generated by HeLa cell cultured media, making it the first truly “pH tactic” supramolecular assembly-based system without the use of any external fuel source/stimuli.
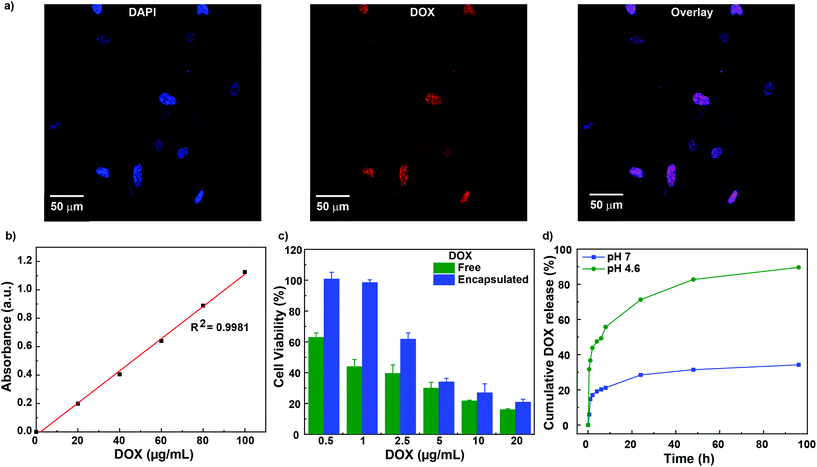
Once the “pH taxis” of the fabricated stomatocytes was observed in the physiologically relevant fuel generated by conditioned HeLa cells, their application for drug delivery was further studied. For this purpose, DOX-loaded CaCO3-sto (DOX/CaCO3-sto) were fabricated and used as DDS towards HeLa cells. Initial studies were conducted for studying the cellular uptake of free DOX or DOX/CaCO3-sto by incubating them with HeLa cells and visualized using confocal laser scanning microscopy (CLSM). After 4 h of incubation, the DOX molecules were observed to be taken up by HeLa cells for both systems and were present in the cell nucleus (Fig. 4a). In the case of free DOX, the cellular uptake was observed to be due to passive diffusion to the nucleus, whereas for CaCO3-sto-loaded DOX, it was due to the release of the entrapped DOX in the acidic environment of the endosomes.44 Previously, pH/redox-sensitive DOX-loaded nanoparticles were prepared from poly(ethylene glycol)-block-poly(L-lysine) (PEG-b-PLL) for improving cancer therapy and similar results were observed under acidic conditions.45 However, the system was based on passive delivery. Herein, we expect the use of CaCO3 to both power and actuate stomatocytes to actively target tumor tissues and cells due to the slightly acidic pH, and also for active delivery of drugs due to the ease of entrapment, thereby increasing the bioavailability and efficacy.
Furthermore, cytotoxicity assay was carried out by incubating HeLa cells with free DOX and DOX-entrapped CaCO3-sto to study the in-vitro efficacy. First, a calibration curve was obtained for free DOX to calculate the entrapment in CaCO3-sto (Fig. 4b, ESI methods†). Once the entrapped amount of DOX was calculated, equivalent amounts of free DOX and DOX/CaCO3-sto were used for cytotoxicity assays. Free DOX showed higher toxicity in lower concentrations in killing cancer cells, since it can easily diffuse into cells as a molecule in comparison with the entrapped DOX, which must enter cells by endocytosis and be released through endosomes in in-vitro experiments (Fig. 4c). However, with the increase of the DOX concentration, DOX/CaCO3-sto had a similar toxicity trend to the free DOX, since the problem encountered for entering the membrane at lower concentrations is overcome, and higher DOX/CaCO3-sto accumulation in cells leads to a high release of DOX. Previously, we have successfully shown cellular uptake of our stomatocyte system to act as delivery agents for tumor cells.46 Moreover, DOX/CaCO3-sto also has a big advantage as a targeted delivery tool in the biological system, as the motors can be actuated at low pH in the TME by dissolution of CaCO3 invoking pH taxis behavior towards tumor cells and hence the DOX release would take place. This behavior gives rise to active targeted therapy that is expected to be more effective than passive therapy. Also, the dissolution of CaCO3 can result in a pH increase in the TME and inhibit tumor growth,28 thereby providing an added advantage. In addition, our DDS can escape the mononuclear phagocytic system (MPS) at the blood barrier47 and enhance the cellular availability of DOX by releasing them without disrupting the membrane of polymersomes since they are entrapped in the stomach of the stomatocyte instead of the lumen of the polymersome membrane as reported in our previous studies.19 It is also possible that some DOX molecules interact with PEG moieties of the stomatocyte structure and bind to them, which can cause cell death, even after repeated washing steps. However, the main focus of this study was to power nanomotors by encapsulating with CaCO3 and to demonstrate them as a truly “pH tactic” system that can propel by an endogenously produced pH gradient in the TME and as a model example to showcase their capabilities in drug delivery to tumor cells.
In order to prove our methodology that DOX release takes place only at acidic pH due to dissolution of CaCO3, we carried out DOX release experiments at basic (pH 7.0) and acidic pH (pH 4.6) for 96 hours. As anticipated, only at acidic pH, an increase in DOX release was observed up to 90% and was very low under basic pH conditions (Fig. 4d). In the initial period, some DOX release was observed even at basic pH that may be due to leaching of drug molecules from the CaCO3 surface. However, at later stages, only under acidic conditions, the release phenomenon was observed as anticipated, thus showcasing our system to be applicable for controlled drug release in the tumor microenvironment where the pH is close to 4.6.
Conclusions
We have fabricated active DDS with biocompatible CaCO3-loaded stomatocytes that power motion under slightly acidic conditions provided by the TME. CaCO3-sto were not only observed to show “pH tactic” directional control and movement in the presence of conditioned HeLa cell culture media but could also be used for delivering drug molecules to HeLa cells under in-vitro conditions. We believe, under in-vivo conditions, our system will have higher efficacy in comparison with free DOX with increased bioavailability at the target site due to its active motion. This fabricated system showcases a facile method to power micro/nanomotors in the endogenously produced physiologically relevant fuel and can substantiate further as active DDS. We envision such a system to be highly beneficial for biomedical applications and can be further extrapolated for the development of fully biodegradable systems with the use of polymers that can degrade under physiological conditions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M.M. would like to acknowledge funding from the European Union's Horizon 2020 framework programme under the Marie Skłodowska-Curie Individual Fellowships Grant Agreement No. 794657. D.A.W. and J.S. acknowledge support from the Ministry of Education, Culture and Science (Gravity Program 024.001.035). The authors would like to thank I. Alexopoulos from General Instruments at Radboud University for assistance with confocal images.References
- J. Li, B. E.-F. de Ávila, W. Gao, L. Zhang and J. Wang, Sci. Rob., 2017, 2, eaam6431 CrossRef.
- B. E.-F. de Ávila, A. Martín, F. Soto, M. A. Lopez-Ramirez, S. Campuzano, G. M. Vásquez-Machado, W. Gao, L. Zhang and J. Wang, ACS Nano, 2015, 9, 6756–6764 CrossRef.
- O. Felfoul, M. Mohammadi, S. Taherkhani, D. de Lanauze, Y. Zhong Xu, D. Loghin, S. Essa, S. Jancik, D. Houle, M. Lafleur, L. Gaboury, M. Tabrizian, N. Kaou, M. Atkin, T. Vuong, G. Batist, N. Beauchemin, D. Radzioch and S. Martel, Nat. Nanotechnol., 2016, 11, 941–947 CrossRef CAS.
- G. Chatzipirpiridis, O. Ergeneman, J. Pokki, F. Ullrich, S. Fusco, J. A. Ortega, K. M. Sivaraman, B. J. Nelson and S. Pané, Adv. Healthcare Mater., 2015, 4, 209–214 CrossRef CAS.
- A. Ghosh and P. Fischer, Nano Lett., 2009, 9, 2243–2245 CrossRef CAS.
- F. Peng, Y. Tu, Y. Men, J. C. van Hest and D. A. Wilson, Adv. Mater., 2017, 29, 1604996 CrossRef.
- Y. Liu, D. Ge, J. Cong, H. G. Piao, X. Huang, Y. Xu, G. Lu, L. Pan and M. Liu, Small, 2018, 14, 1704546 CrossRef.
- K. Kim, X. Xu, J. Guo and D. Fan, Nat. Commun., 2014, 5, 1–9 Search PubMed.
- A. M. Boymelgreen, T. Balli, T. Miloh and G. Yossifon, Nat. Commun., 2018, 9, 1–8 CrossRef CAS.
- J. Wang, Z. Xiong, X. Zhan, B. Dai, J. Zheng, J. Liu and J. Tang, Adv. Mater., 2017, 29, 1701451 CrossRef.
- M. Xuan, Z. Wu, J. Shao, L. Dai, T. Si and Q. He, J. Am. Chem. Soc., 2016, 138, 6492–6497 CrossRef CAS.
- C. Chen, F. Mou, L. Xu, S. Wang, J. Guan, Z. Feng, Q. Wang, L. Kong, W. Li, J. Wang and Q. Zhang, Adv. Mater., 2017, 29, 1603374 CrossRef.
- B. Dai, J. Wang, Z. Xiong, X. Zhan, W. Dai, C.-C. Li, S.-P. Feng and J. Tang, Nat. Nanotechnol., 2016, 11, 1087–1092 CrossRef CAS.
- W.-P. Li, C.-H. Su, Y.-C. Chang, Y.-J. Lin and C.-S. Yeh, ACS Nano, 2016, 10, 2017–2027 CrossRef CAS.
- F. Soto, G. L. Wagner, V. Garcia-Gradilla, K. T. Gillespie, D. R. Lakshmipathy, E. Karshalev, C. Angell, Y. Chen and J. Wang, Nanoscale, 2016, 8, 17788–17793 RSC.
- Z. Wu, T. Li, J. Li, W. Gao, T. Xu, C. Christianson, W. Gao, M. Galarnyk, Q. He and L. Zhang, ACS Nano, 2014, 8, 12041–12048 CrossRef CAS.
- D. Wang, C. Gao, W. Wang, M. Sun, B. Guo, H. Xie and Q. He, ACS Nano, 2018, 12, 10212–10220 CrossRef CAS.
- F. Peng, Y. Tu, J. C. van Hest and D. A. Wilson, Angew. Chem., Int. Ed., 2015, 54, 11662–11665 CrossRef CAS.
- Y. Tu, F. Peng, A. A. M. André, Y. Men, M. Srinivas and D. A. Wilson, ACS Nano, 2017, 11, 1957–1963 CrossRef CAS.
- V. Garcia-Gradilla, S. Sattayasamitsathit, F. Soto, F. Kuralay, C. Yardımcı, D. Wiitala, M. Galarnyk and J. Wang, Small, 2014, 10, 4154–4159 CAS.
- M. Xuan, J. Shao, X. Lin, L. Dai and Q. He, Chemphyschem, 2014, 15, 2255–2260 CrossRef CAS.
- X. Ma, A. Jannasch, U.-R. Albrecht, K. Hahn, A. Miguel-López, E. Schäffer and S. Sánchez, Nano Lett., 2015, 15, 7043–7050 CrossRef.
- C. Ding, L. Tong, J. Feng and J. Fu, Molecules, 2016, 21, 1715 CrossRef.
- Z. Chen, T. Xia, Z. Zhang, S. Xie, T. Wang and X. Li, Chem. Eng. J., 2019, 375, 122109 CrossRef CAS.
- H. Wu, Z. Ding, D. Hu, F. Sun, C. Dai, J. Xie and X. Hu, J. Pathol., 2012, 227, 189–199 CrossRef CAS.
- S. Gao, J. Hou, J. Zeng, J. J. Richardson, Z. Gu, X. Gao, D. Li, M. Gao, D. W. Wang and P. Chen, Adv. Funct. Mater., 2019, 29, 1808900 CrossRef.
- H. Li, Z. Sun, S. Jiang, X. Lai, A. Böckler, H. Huang, F. Peng, L. Liu and Y. Chen, Nano Lett., 2019, 19, 8749–8757 CrossRef CAS.
- A. Som, R. Raliya, L. Tian, W. Akers, J. E. Ippolito, S. Singamaneni, P. Biswas and S. Achilefu, Nanoscale, 2016, 8, 12639–12647 RSC.
- Z. Dong, L. Feng, W. Zhu, X. Sun, M. Gao, H. Zhao, Y. Chao and Z. Liu, Biomaterials, 2016, 110, 60–70 CrossRef CAS.
- J. J. McDermott, A. Kar, M. Daher, S. Klara, G. Wang, A. Sen and D. Velegol, Langmuir, 2012, 28, 15491–15497 CrossRef CAS.
- M. Guix, A. K. Meyer, B. Koch and O. G. Schmidt, Sci. Rep., 2016, 6, 1–7 CrossRef.
- I. Ortiz-Rivera, M. Mathesh and D. A. Wilson, Acc. Chem. Res., 2018, 51, 1891–1900 CrossRef CAS.
- Y. Ueno, H. Futagawa, Y. Takagi, A. Ueno and Y. Mizushima, J. Controlled Release, 2005, 103, 93–98 CrossRef CAS.
- D. A. Wilson, R. J. M. Nolte and J. C. M. van Hest, J. Am. Chem. Soc., 2012, 134, 9894–9897 CrossRef CAS.
- L. E. Gerweck and K. Seetharaman, Cancer Res., 1996, 56, 1194–1198 CAS.
- F. A. Gallagher, M. I. Kettunen, S. E. Day, D.-E. Hu, J. H. Ardenkjær-Larsen, R. i. t. Zandt, P. R. Jensen, M. Karlsson, K. Golman, M. H. Lerche and K. M. Brindle, Nature, 2008, 453, 940–943 CrossRef CAS.
- C.-Y. Sun, Y. Liu, J.-Z. Du, Z.-T. Cao, C.-F. Xu and J. Wang, Angew. Chem., Int. Ed., 2016, 55, 1010–1014 CrossRef CAS.
- R. Golestanian, T. B. Liverpool and A. Ajdari, Phys. Rev. Lett., 2005, 94, 220801 CrossRef.
- P. Schattling, B. Thingholm and B. Städler, Chem. Mater., 2015, 27, 7412–7418 CrossRef CAS.
- W. Gao, A. Uygun and J. Wang, J. Am. Chem. Soc., 2012, 134, 897–900 CrossRef CAS.
- M. Fernández-Medina, X. Qian, O. Hovorka and B. Städler, Nanoscale, 2019, 11, 733–741 RSC.
- L. Yin, H. Cheng, S. Mao, R. Haasch, Y. Liu, X. Xie, S.-W. Hwang, H. Jain, S.-K. Kang, Y. Su, R. Li, Y. Huang and J. A. Rogers, Adv. Funct. Mater., 2014, 24, 645–658 CrossRef CAS.
- P. Zengel, A. Nguyen-Hoang, C. Schildhammer, R. Zantl, V. Kahl and E. Horn, BMC Cell Biol., 2011, 12, 21 CrossRef.
- A. Helenius, I. Mellman, D. Wall and A. Hubbard, Trends Biochem. Sci., 1983, 8, 245–250 CrossRef CAS.
- G. Zhang, Y. Zhu, Y. Wang, D. Wei, Y. Wu, L. Zheng, H. Bai, H. Xiao and Z. Zhang, RSC Adv., 2019, 9, 20513–20517 RSC.
- J. Sun, M. Mathesh, W. Li and D. A. Wilson, ACS Nano, 2019, 13, 10191–10200 CrossRef CAS.
- M. A. Dobrovolskaia and S. E. McNeil, Nat. Nanotechnol., 2007, 2, 469 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, Experimental section, additional figures for MSD curves and fluorescence imaging. See DOI: 10.1039/d0nr04415f |
| This journal is © The Royal Society of Chemistry 2020 |