Open Access Article
Open Access ArticleHosta plantaginea (Lam.) Aschers (Yuzan): an overview on its botany, traditional use, phytochemistry, quality control and pharmacology
Li Yanga and
Jun-Wei He *b
*b
aKey Laboratory of Modern Preparation of TCM, Ministry of Education, Jiangxi University of Traditional Chinese Medicine, Nanchang 330004, China
bResearch Center of Natural Resources of Chinese Medicinal Materials and Ethnic Medicine, Jiangxi University of Traditional Chinese Medicine, Nanchang 330004, China. E-mail: hjwjn2008@163.com
First published on 30th October 2019
Abstract
Hosta plantaginea (Lam.) Aschers, as a traditional folk medicine, has been widely used both as a single herb and in prescriptions in Asia mainly due to its anti-inflammatory and analgesic effects. A total of 101 compounds including steroids, flavonoids, alkaloids and others have been isolated from H. plantaginea. Modern pharmacology has revealed that H. plantaginea possesses various therapeutic effects such as anti-inflammatory, analgesic and antibacterial effects both in vitro and in vivo. Although a number of reports on the chemical constituents and pharmacological activities of this plant are available, there is limited research on the bioactive constituents and the mechanism of the biological activities of H. plantaginea. Thus, it is essential to strengthen the research on bioactive constituents and their mechanisms as well as their structure–function relationships in H. plantaginea. Up to now, only three compounds have been established for the quality control of H. plantaginea. However, a comprehensive review on the botany, traditional use, phytochemistry, quality control and pharmacology information about this plant has not been reported so far; thus, a systematic and comprehensive review is very necessary. Therefore, this paper provided a comprehensive overview on the botany, traditional use, phytochemistry, quality control and pharmacology of H. plantaginea and also provided evidence for its further research and clinical applications.
1. Introduction
Hosta Tratt. is a genus in the family Liliaceae, which comprises about 43 species mainly distributed in the temperate and sub-tropical zones of Asia, particularly in Japan and China.1–6 Hosta plants are commonly used ornamentally or for medicine and are thus widely cultivated in parks and botanical gardens throughout the region.2,7 Only four native species have been found in China, namely, H. plantaginea, H. ventricosa (Salisb.) Stearn, H. ensata F. Maekawa and H. albofarinosa D. Q. Wang, which are widely cultivated in parks and/or commonly used as folk medicine in China.2,8,9 Among them, H. plantaginea is a perennial herb widely cultivated in China; it is used as a traditional Chinese medicine (TCM) and is known as Yu zan (Chinese name ). The dried whole plants, leaves, roots and flowers of this plant have been used as local and traditional medicine in China, Japan and South Korea.10–12 Chinese people call the flowers of H. plantaginea yu-zan-hua, and they are also named bai-e-hua, bai-he-hua and nei-xiao-hua; they have long been commonly used in traditional Mongolian medicine for the treatment of inflammatory and painful diseases, such as sore throat, muteness, lung heat and toxic heat.10,13–16 Moreover, yu-zan-hua was the main herb in the prescriptions of the Yuzan qingyan shiwuwei pill, Yuzan qingyan shiwuwei powder and Qinyan liuwei powder (Fig. 1).
). The dried whole plants, leaves, roots and flowers of this plant have been used as local and traditional medicine in China, Japan and South Korea.10–12 Chinese people call the flowers of H. plantaginea yu-zan-hua, and they are also named bai-e-hua, bai-he-hua and nei-xiao-hua; they have long been commonly used in traditional Mongolian medicine for the treatment of inflammatory and painful diseases, such as sore throat, muteness, lung heat and toxic heat.10,13–16 Moreover, yu-zan-hua was the main herb in the prescriptions of the Yuzan qingyan shiwuwei pill, Yuzan qingyan shiwuwei powder and Qinyan liuwei powder (Fig. 1).
 | ||
| Fig. 1 (a) Whole plants; (b) fresh flowers; (c) dried flowers; (d–f) three prescriptions containing the flowers of H. plantaginea. | ||
H. plantaginea is enriched in multiple structurally diverse and biologically important steroids and flavonoids. Modern pharmacology has revealed that H. plantaginea has anti-inflammatory, analgesic, antibacterial, antifungal, anti-cancer, antioxidant and other biological activities.17–19 Up to now, a large number of studies focused on the phytochemistry and pharmacology of H. plantaginea have been published. However, a comprehensive review on the botany, traditional use, phytochemistry, quality control and pharmacology information about this plant has not been reported; thus, a systematic and comprehensive review is very necessary. In this review, we systematically summarized the progress in the botany, traditional use, phytochemistry and quality control studies of H. plantaginea over the past decades, with all the elucidated compounds listed. The biological characterization of the extracts and constituents isolated from this plant were also discussed. It is hoped that the information presented in this paper will be useful for the full utilization of H. plantaginea for new drug development and pharmaceutical applications.
2. Botany
2.1 Morphology
As a perennial herbaceous plant, H. plantaginea grows to 40–80 centimeter. The calyx has several to a dozen flowers: the flowers are sessile, ovate or lanceolate, 2.5–7 cm long, and 1–1.5 cm wide; the inner sepals are very small. Also, the flowers are solitary or sometimes in clusters of 2 or 3 and fragrant; the pedicel is about 1 cm. The perianth is white, funnel form, and 10–13 cm in size. The stamens are slightly shorter than or subequaling perianth; the filaments are adnate to the perianth tube near base. The capsule is cylindrical, about 6 × 1 cm, and 3-angled. The flowering stages range from July to August. The rhizome is 1.5–2 cm thick and stout. The petiole grows from 20 to 40 cm in length; the leaf blade is ovate-cordate, -orbicular, or ovate, 14–25 × 8–16 cm, glabrous, veins in 6–10 pairs, base cordate, margin slightly undulate, and apex abruptly acute. Scape 40–80 cm. Raceme: several to more than 10-flowered, bracts 2 subtending each flower, outer one ovate or lanceolate, 2.5–7 × 1–1.5 cm; the inner one is very small.2,92.2 Geographical distribution of H. plantaginea
H. plantaginea is chiefly distributed in underwood, grassy slope, or rocky regions at lower altitudes below 2000 meters from Sichuan, Hubei, Hunan, Jiangsu, Anhui, Zhejiang and other provinces of China. The requirements for growing the plants are not stringent, and they need little sunshine and appropriate temperatures. Moreover, this plant is mostly cultivated in parks as an ornamental plant.2,93. Traditional use
3.1 Medicinal use
H. plantaginea was listed for medicinal use first in “Ben Cao Pin Hui Jing Yao” during the Ming Dynasty more than five hundred years ago. Because of their versatile biological and pharmacological activities, the H. plantaginea plants have been traditionally used in China, Japan and South Korea. In traditional Chinese medicine, the flowers were used as an oral medicine for sore throat, muteness, lung heat, and toxic heat. The whole plants or leaves were applied to inflammatory mass, hemorrhoids and snake bite, and the roots were taken orally for inflammatory mass, vomiting blood and osteophytes.10,13,14 In the TCM culture, H. plantaginea is described as acrid in taste, a little cold in nature and attributive to the stomach, lung and liver meridians. According to Bencao Gangmu and Zhong's previous reports,20,21 the roots of H. plantaginea were used as Sinopodophyllum emodi (Wall) Ying to treat cancer in Japan because of their very similar morphology and efficacy. Meanwhile, the roots were applied to dermatitis in South Korea.22The prescriptions related to the flowers of H. plantaginea recorded in TCM were the Yuzan qingyan shiwuwei pill, Yuzan qingyan shiwuwei powder and Qinyan liuwei powder, which are used for the treatment of sore throat, muteness, lung heat and toxic heat. Studies on the side effects and safety evaluations of this plant are very limited although it is widely used in TCM.
3.2 Non-medicinal use
In addition to its use in medicine, the flowers, young leaves and buds of H. plantaginea are used as a daily food material to eat or drink in some cities of China.23,24 The flowers of H. plantaginea are very beautiful with concentrated fragrance; thus, the plant is often placed at home or office.25,26 Moreover, H. plantaginea is commonly used ornamentally and thus widely cultivated in the parks and botanical gardens in China.3,4,6,27,28 Additionally, H. plantaginea is an ombrophyte and has the advantages of rapid reproduction, easy growth and strong resistance; thus, it is commonly used for vegetation protection.27,284. Phytochemistry
Up to now, 101 chemical constituents have been isolated and identified from H. plantaginea, including steroids, flavonoids, alkaloids, phenylethanols, acetophenones, and others (Table 1). Among them, steroids and flavonoids are considered to be the primary bioactive constituents of this herbal medicine.| No. | Secondary metabolites | Part | Ref. |
|---|---|---|---|
| Steroids | |||
| 1 | Gitogenin | Flowers | 16, 29 and 30 |
| 2 | (25R)-2α,3β,17β,24β-Tetrahydroxy-5α-spirostanane | Flowers | 31 |
| 3 | Gitogenin-3-O-β-D-galactopyranoside | Flowers | 30 |
| 4 | Gitogenin-3-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Flowers, leaves | 24, 30 and 32 |
| 5 | Gitogenin-3-O-α-L-rhamanopyranosyl(1→2)-β-D-galactopyranoside | Flowers, leaves | 24 and 30 |
| 6 | 12-Hydroxy-gitogenin-3-O-α-L-rhamnopyranosyl-(1→2)-β-D-galactopyranoside | Leaves | 24 and 32 |
| 7 | Gitogenin-3-O-β-D-glucopyranosyl-(1→4)-O-[α-L-rhamnopyranosyl(1→2)]-β-D-galactopyranoside | Flowers | 30 |
| 8 | Gitogenin-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Flowers, underground | 11, 16, 29, 30 and 33 |
| 9 | Gitogenin-3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Flowers, underground | 11, 16, 29, 30 and 33 |
| 10 | Gitogenin-3-O-β-D-glucopyranosyl-(1→2)-O-[O-α-L-rhamnopyranosyl-(1→4)-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galsctopyranoside | Flowers, underground | 11, 16, 29, 30 and 33 |
| 11 | Gitogenin-3-O-β-D-xylopyranosyl-(1→4)-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Flowers | 30 |
| 12 | Tigogenin-3-O-β-D-glucopyranosyl(1→4)-O-[α-L-rhamnopyranosyl-(1→2)]-β-D-galactopyranoside | Flowers, leaves | 24 and 30 |
| 13 | Hostaside IV | Leaves | 24 |
| 14 | Manogenin | Flowers | 16 |
| 15 | 9-Dehydromanogenin | Flowers, leaves | 31 and 32 |
| 16 | (25R)-2α,3β,24β-Trihydroxy-5α-spirost-9(11)-en-12-one | Flowers | 31 |
| 17 | Hostasaponin A | Rhizomes | 34 |
| 18 | Hostasaponin B | Rhizomes | 34 |
| 19 | 9,11-Dehydromanogenie-3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | Leaves | 24 and 32 |
| 20 | β-Sitosterol | Flowers, leaves | 32 and 33 |
| 21 | β-Stigmasterol | Leaves | 32 |
| 22 | β-Sitosterol-3-O-β-D-glucopyranoside | Flowers, leaves | 16, 29, 32, 33 and 35 |
| 23 | Stigmasterol-3-O-β-d-glucoside | Flowers | 35 |
| 24 | (2α,3β,5α,25R)-2,3-Dihydroxy-22-methoxyfurostan-26-yl-β-D-glucopyranoside | Underground | 11 |
| 25 | 16,22-Oxido-26-hydroxycholest-4-en-3-one | Flowers | 31 |
| 26 | (2α,3β,5α,16β)-Pregn-20-ene-20-carboxylic acid-2,16-dihydroxy-γ-lactone-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl | Underground | 11 |
| 27 | 2α,3β-Dihydroxy-2α-pregn-16-en-20-one | Rhizomes | 34 |
| 28 | Hostaside III | Leaves | 24 |
| 29 | Hostaside I | Leaves | 24 |
| 30 | Hostaside II | Leaves | 24 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Flavonoids | |||
| 31 | Kaempferol | Flowers | 29, 36 and 37 |
| 32 | Astragalin | Flowers | 37 |
| 33 | Kaempferol-7-O-β-D-glucoside | Flowers | 29 and 35–37 |
| 34 | Kaempferol-3,7-di-O-β-D-glucoside | Flowers | 37 |
| 35 | Kaempferol-3-O-β-D-sophoroside | Flowers | 37 |
| 36 | Plantanone A | Flowers | 37 |
| 37 | Kaempferol-3-O-rutinoside | Flowers | 29, 36 and 37 |
| 38 | Kaempferol-3-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside-7-O-β-D-glucopyranoside | Flowers | 39 |
| 39 | Hostaflavone A | Flowers | 39 |
| 40 | Kaempferol-3-O-rutinoside-7-O-glucoside | Flowers, leaves | 32 and 37 |
| 41 | Kaempferol-3-O-(2′′-O-β-D-glucopyranosyl)-β-D-rutinoside | Leaves | 32 |
| 42 | Kaempferol-3-O-β-D-glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside | Flowers | 37 |
| 43 | Plantanone B | Flowers | 37 |
| 44 | Kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside | Flowers | 37 |
| 45 | Kaempferol-3-O-{β-D-glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranosyl}-7-O-β-D-glucopyranoside | Flowers | 37 |
| 46 | Plantanone C | Flowers | 41 |
| 47 | Quercetin | Flowers | 36 |
| 48 | Hostaflavanone A | Flowers | 38 |
| 49 | 5,7-Dimethoxy-4′-hydroxyflavan | Flowers | 40 |
| 50 | 5,7-Dimethoxy-8-methyl-4′-hydroxyflavan | Flowers | 40 |
| 51 | Epicatechin | Flowers | 40 |
| 52 | Catechin | Flowers | 40 |
| 53 | Epigallocatechin | Flowers | 40 |
| 54 | Gallocatechin | Flowers | 40 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Alkaloids | |||
| 55 | Hostasinine A | Whole plants | 42 |
| 56 | Hostasine | Whole plants | 43 |
| 57 | 8-Demethoxyhostasine | Whole plants | 43 |
| 58 | 8-Demethoxy-10-O-methylhostasine | Whole plants | 43 |
| 59 | 10-O-Methylhostasine | Whole plants | 43 |
| 60 | (+)-Haemanthamine | Whole plants | 43 |
| 61 | O-Demethylhaemanthamine | Whole plants | 43 |
| 62 | Haemanthidine | Whole plants | 43 |
| 63 | Yemenine C | Whole plants | 43 |
| 64 | Lycorine | Whole plants | 43 |
| 65 | Pseudolycorine | Whole plants | 43 |
| 66 | O-Methylcorenine | Whole plants | 43 |
| 67 | 9-O-Demethyl-7-O-methyllycorenine | Whole plants | 43 |
| 68 | Albomaculine | Whole plants | 43 |
| 69 | 7-Deoxy-trans-dihydronarciclasine | Whole plants | 43 |
| 70 | 8-O-Demethylmaritidine | Whole plants | 43 |
| 71 | Ungermine | Whole plants | 43 |
| 72 | Norsanguinine | Whole plants | 43 |
| 73 | (1S,3S)-1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid | Flowers | 38 |
| 74 | (1R,3S)-1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid | Flowers | 38 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Phenylethanols and acetophenones | |||
| 75 | Phenethyl-O-β-D-glucopyranoside | Flowers | 38 and 40 |
| 76 | Phenethanol-β-D-gentiobioside | Flowers | 34 |
| 77 | Phenethyl-O-rutinoside | Flowers | 34 |
| 78 | 4-Hydroxylacetophenone | Flowers | 40 |
| 79 | Acetophenone-4-O-β-D-glucoside | Flowers | 40 |
| 80 | 2-Hydroxyl-6-methoxyacetophenone-4-O-β-D-glucoside | Flowers | 40 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Phenylpropanoids | |||
| 81 | anti-1-Phenylpropane-1,2-diol | Flowers | 38 |
| 82 | anti-1-Phenylpropane-1,2-diol-2-O-β-D-glucopyranoside | Flowers | 38 |
| 83 | Coumaric acid | Flowers | 40 |
| 84 | 3-(4-Hydroxy-3-methoxyphenyl) acrylic acid methyl ester | Flowers | 40 |
| 85 | 3,4-Dihydroxycinnamyl alcohol-3-O-glucoside | Flowers | 40 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Terpenoids | |||
| 86 | Hoplanoside A | Flowers | 44 |
| 87 | Lomacarinoside A | Flowers | 44 |
| 88 | (4S)-4-Hydroxy-3,5,5-trimethyl-2-cyclohexen-1-one,4-[3-(β-D-glucopyranosyloxy)butyl] | Flowers | 45 |
| 89 | Roseoside | Flowers | 45 |
| 90 | Plantaginoside | Flowers | 45 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Aliphatics | |||
| 91 | Docosanol | Leaves | 32 |
| 92 | Arachic acid | Flowers | 29 and 36 |
| 93 | Hexadecanoic acid | Flowers | 29 and 36 |
| 94 | Hostacerebroside A | Flowers | 46 |
| 91 | Docosanol | Leaves | 32 |
| 92 | Arachic acid | Flowers | 29 and 36 |
| 93 | Hexadecanoic acid | Flowers | 29 and 36 |
| 94 | Hostacerebroside A | Flowers | 46 |
| 95 | 1-O-β-D-Glucopyranosyl-(2S,3R,4E,8Z)-2-[(2-hydroxytetradecanoyl)amido]-4,8-octadecadienyl-1,3-diol | Flowers | 31 |
| 96 | (2S)-1-O-Linenoyl-3-O-β-D-galactopyranosylglycerol | Flowers | 31 |
| 97 | (2S)-1-O-(10,13)-Octadecenoyl-3-O-β-D-galactopyranosyl glycerol | Flowers | 31 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Others | |||
| 98 | 4-Hydroxybenzaldehyde | Flowers | 40 |
| 99 | 4-Hydroxy-3-methoxybenzene | Flowers | 31 |
| 100 | (2-Methyl) heptyl phthalate | Flowers | 31 |
| 101 | (2-Methylphenyl) (4-hydroxy-3-methoxyphenyl)-1,4-diene-3-pentanone | Flowers | 31 |
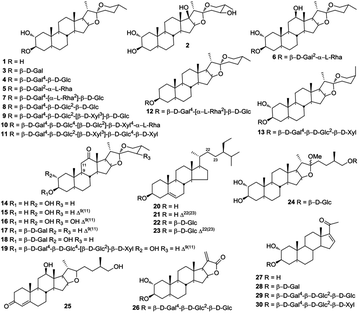
4.1 Steroids
Steroids were regarded as the major bioactive principle of H. plantaginea, and phytochemical researchers were focused on these species since 1997.11 Until now, 30 steroid compounds have been isolated and identified from H. plantaginea (Fig. 2). The sugar moieties in the carbohydrate part of steroidal saponins are β-D-glucose, β-D-galactose, α-L-rhamnose and β-D-xylose; in contrast, in terms of aglycones as the core of these compounds, there are five different types, namely, spirostanes (1–19), stigmastanes (20–23), furostanes (24 and 25), C22 steroids (26), and C21 steriods (27–30), as shown in Fig. 2, where the sugar groups are usually attached to the C-3 position of aglycones. By comparison, it can be clearly seen that spirostanes are mainly found as glycosides in this plant, including four different subtypes: gitogenin (1–11), tigogenin (12), neogitogenin (13), manogenin (14–19).4.2 Flavonoids
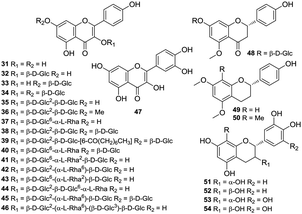
Flavonoids consist of a large group of polyphenolic compounds with benzo-γ-pyrone structures, which are ubiquitously present in plants; there is no exception for the H. plantaginea plants. Flavonoids are another major bioactive constituents in H. plantaginea and they are divided into three categories, namely, 17 flavonols (31–47), one flavanone (48), and six flavans (49–54) (Fig. 3). Among them, kaempferol (31) and its derivatives (32–46) as well as quercetin (47) belong to flavonols, which are the main active ingredients of H. plantaginea and most of them are flavonoid glycosides with 3- and/or 7-linked glycans. In fact, 22 flavonoids were isolated and identified from the flowers of H. plantaginea by us and in Yu's previous work in 2017–2019.37–41 This is the first report on the isolation of flavonones and flavans from the Liliaceae family.384.3 Alkaloids
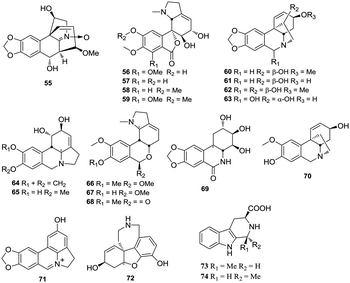
The H. plantaginea plants are also rich in alkaloids, and some of them show notable inhibitory activities against acetylcholinesterase (AChE) and tobacco mosaic virus (TMV).43 To date, 20 alkaloids have been isolated and identified from H. plantaginea. These alkaloids include 18 benzylphenethylamine alkaloids (55–72) and two β-carboline alkaloids (73 and 74) (Fig. 4). Hostasinine A (55) is a benzylphenethylamine alkaloid with a novel skeleton featuring a C-4–C-6 linkage and a nitrone moiety. Moreover, other 17 benzylphenethylamine alkaloids (56–72) represent five skeletal types of alkaloids, namely, lycoreniene-type (56–59 and 66–68), isocarbostyril-type (69), crinine-type (60–63 and 70), lycorine-type (64, 65 and 71), and galanthamine-type (72).4.4 Phenylethanols and acetophenones
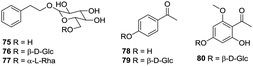
Phenylethanols and acetophenones represent a relatively small class of compounds in H. plantaginea. To date, only three phenylethanols, namely, phenethyl-O-β-D-glucopyranoside (75), phenethanol-β-D-gentiobioside (76) and phenethyl-O-rutinoside (77) have been obtained from the ethanolic extract of H. plantaginea.38,40 Moreover, three acetophenones, namely, 4-hydroxylacetophenone (78), acetophenone-4-O-β-D-glucoside (79) and 2-hydroxyl-6-methoxyacetophenone-4-O-β-D-glucoside (80) have also been isolated from H. plantaginea (Fig. 5).404.5 Phenylpropanoids
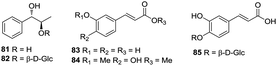
Phenylpropanoids also represent a relatively small class of compounds in H. plantaginea. To date, only two phenylpropanols (81 and 82) and three phenylpropionic acids (83–85) have been obtained from the ethanolic extract of H. plantaginea (Fig. 6).38,404.6 Terpenoids
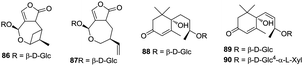
To date, only two monoterpenes (86 and 87) and three megastigmanes (88–90) have been obtained from the ethanolic extract of H. plantaginea (Fig. 7).44,454.7 Aliphatics
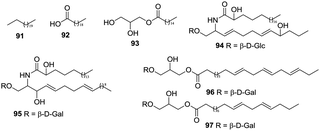
Some studies have been carried out to investigate the aliphatic compounds in H. plantaginea. Seven aliphatics (91–97) have been isolated from the flowers and leaves of H. plantaginea (Fig. 8).4.8 Others
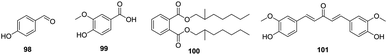
In addition to the above-mentioned main components, other components have also been found in the flowers of H. plantaginea, such as 4-hydroxybenzaldehyde (98),40 4-hydroxy-3-methoxybenzene (99),31 (2-methyl)heptyl phthalate (100),31 and (2-methylphenyl) (4-hydroxy-3-methoxyphenyl)-1,4-diene-3-pentanone (101)31 (Fig. 9).5. Quality Control
Quality control is very important for the use of TCMs. Many rapid, sensitive and stable technologies such as UPLC-MS, HPLC and UV have been applied for the qualitative and quantitative analyses of H. plantaginea.47–52 To date, only three compounds, namely, kaempferol (31), kaempferol-7-O-β-D-glucoside (33) and kaempferol-3-O-rutinoside (37) have been used as quantitative markers by HPLC or UPLC-MS. Interestingly, the contents of compound 31 were found to be 0.0025%, 0.33–0.56%, 0.29–0.44% and 0.020% in the flowers of H. plantaginea by different research groups.47,49–51 Moreover, the contents of compounds 33 and 37 were found to be 0.0058% and 0.090% by UPLC-MS, respectively.46 In addition, the content of total saponins was found to be 1.61–4.70% in the flowers of H. plantaginea by ultraviolet-visible (UV) spectrophotometry.52 Furthermore, Li and co-authors established the fingerprint of the flowers of H. plantaginea by HPLC.48 In fact, the harvest times, geographical locations, and other factors can affect the contents of the active compounds in the flowers of H. plantaginea, which should be considered when assessing their clinical efficacies. Therefore, there is an urgent need to determine other bioactive markers using various chromatographic and spectroscopic means to establish the comprehensive quality standards for this medicinal plant.6. Pharmacology
H. plantaginea has long been used in China, Japan, Korea and other countries because of its various pharmacological effects. In recent years, research reports on the chemical constituents and pharmacological activities of H. plantaginea have shown an increasing trend. Modern pharmacology has revealed that H. plantaginea has anti-inflammatory, analgesic, antibacterial, antifungal, anti-cancer, antioxidant and other biological activities.6.1 Anti-inflammatory and analgesic activities
In TCM, the whole plants, leaves, roots and flowers of H. plantaginea have been used as local and traditional ethnic medicine for the treatment of sore throat, inflammatory mass, dysuria, lung heat, snake bite and others. In agreement with the traditional usage of H. plantaginea, several studies have illustrated that this plant possesses anti-inflammatory and analgesic effects both in vitro and in vivo. The anti-inflammatory abilities against cyclooxygenase (COX)-1 and -2 enzymes were evaluated. Flavonoid compounds 31–40, 42–45 and 48 showed significant inhibitory activities against COX-1 and COX-2 at a concentration of 50 mM, with the inhibition ratios ranging from 53.00% to 80.55% for COX-1 and from 52.19% to 66.29% for COX-2. Further detailed testing showed that these compounds inhibited the COX-1 and COX-2 enzymes with the IC50 values of 12.90–46.16 μM in comparison with the positive control celecoxib with the IC50 values of 9.00 μM for COX-1 and 1.04 μM for COX-2.37–39 Thus, flavonoids are supposed to contribute to the anti-inflammatory effect of H. plantaginea.In vivo, at the doses of 1.0, 2.0 and 4.0 g kg−1 (raw herb), different concentrations of ethanol (50%, 65%, 80% and 95%) crude extracts obtained from the flowers of H. plantaginea showed moderate anti-inflammatory effects in a xylene-induced ear oedema mouse model.53 Moreover, the crude extract, ethyl acetate fraction and n-BuOH fraction showed a significant anti-inflammatory effect in xylene-induced mouse ear oedema, acetic acid-induced writhing and carrageenan-induced oedema from different research groups.32,54,55 In addition, the leaves of H. plantaginea, Yu-Zan-Qing-Yan-Shi-Wu-Wei powder and Yu-Zan-Qing-Yan-Shi-Wu-Wei pill have good therapeutic effects on patients with acute and chronic pharyngitis.56–58
In 2010, the analgesic activity of H. plantaginea was studied in vivo.59 The oral administration of a 50% ethanol extract of the H. plantaginea flowers (0.5 and 2.0 g kg−1, raw herb) caused a significant analgesic effect in an acetic acid-induced writhing mouse model with the inhibition of 39.28% and 53.41%, respectively; the positive control drug xiao-yan-tong pill displayed inhibition of 67.07% at a concentration of 0.1 g kg−1. Moreover, 50% ethanol extract of the flowers of H. plantaginea also had a significant antinociceptive effect in the hot plate mouse model at a dose of 2.0 g kg−1 compared with the control group.
6.2 Anti-cancer activity
The crude extracts and steroids from H. plantaginea have significant activities against tumor cells such as the L615, MDA-MB-231, MCF-7, SMMC-7721, HL-60, Jurkat, K562, HepG2, MDCK, and YAC-A cell lines in vitro. However, no study has been conducted on the anti-cancer activity of H. plantaginea in vivo.The ethanol extract from the flowers of H. plantaginea exhibited significant anti-cancer activity against the L615 tumor cells.14 The steroid compounds 2, 15, and 25 exhibited significant anti-cancer activities against MDA-MB-231, MCF-7 and SMMC-7721 tumor cells.31 Moreover, compounds 8–10 exhibited significant anti-cancer activities against the leukaemia HL-60 cells in a dose-dependent manner with the IC50 values of 2.9, 1.0 and 1.7 μg mL−1, respectively.11 In addition, compounds 1, 4, 5, and 7–11 exhibited significant or moderate anti-cancer activities against leukaemia HL-60, Jurkat, and K562 cells and others (Table 2).30,60 Thus, steroids are supposed to contribute to the anti-cancer effect of H. plantaginea.
| Compounds | HL-60 (μM) | Jurkat (μM) | K562 (μM) | HepG2 (μM) | MCF-7 (μM) | SGC-7901 (μM) | MDCK (μg mL−1) | YAC-1 (μg mL−1) | SMMC-7721 (μg mL−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Not determined. | |||||||||
| 1 | >40 | >40 | >40 | 9.95 | >40 | 39.5 | 287.38 | 5.38 | 2.84 |
| 4 | 20.58 | 16.96 | 17.65 | 8.17 | >40 | 9.92 | —a | —a | —a |
| 5 | 18.28 | 13.46 | 15.25 | 7.14 | >40 | 7.32 | —a | —a | —a |
| 7 | 3.11 | 4.32 | 5.25 | 1.14 | 1.51 | 1.72 | 5.12 | —a | —a |
| 8 | 3.50 | 3.95 | 4.35 | 1.13 | 1.23 | 1.85 | —a | —a | —a |
| 9 | 2.45 | 2.84 | 2.76 | 0.16 | 0.56 | 0.44 | 51.16 | 24.23 | —a |
| 10 | 2.92 | 4.21 | 3.14 | 1.13 | 0.89 | 0.34 | 22.57 | 12.83 | 16.17 |
| 11 | 2.41 | 4.54 | 3.24 | 1.16 | 1.02 | 0.59 | —a | —a | —a |
| 10-Hydroxycamptothecin | 0.04 | 0.03 | 1.60 | 11.90 | 16.81 | 19.80 | —a | —a | —a |
| Cisplatin | 1.92 | 3.66 | 17.63 | 4.58 | 57.25 | 5.79 | —a | —a | —a |
6.3 Antioxidant activity
Recently, some studies have revealed the anti-oxidative effect of the compounds in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay in vitro.37–39,45 The flavonoid compounds 31, 33 and 48 exhibited strong antioxidant effects with the IC50 values of 36.3, 77.6 and 83.2 μM, respectively, compared to the positive control L-ascorbic acid (Vc) (33.9 μM). Moreover, three megastigmane glycosides 88–90 showed very strong antioxidant effects with the IC50 values of 1.34, 1.66 and 1.28 μM, respectively, compared to the positive control Vc (1.01 μM).6.4 Anti-acetylcholinesterase and anti-viral activities
Three benzylphenethylamine alkaloids from the whole plants of H. plantaginea, namely, 8-demethoxy-10-O-methylhostasine (58), ungermine (71) and norsanguinine (72) demonstrated significant anti-acetylcholinesterase (AChE) activity, with the IC50 values of 2.32, 3.85 and 1.43 μM, respectively, compared to the positive control tacrine (0.20 μM).42,43 Moreover, 7-deoxy-trans-dihydronarciclasine (69) showed strong inhibitory activity against tobacco mosaic virus (TMV) than the positive control ribavirin, with the IC50 values of 1.80 and 2989.60 μM, respectively.436.5 Other biological activities
In modern research, the H. plantaginea plant has been reported to have a variety of activities besides the above-mentioned fields. The roots of H. plantaginea are considered to be effective on recalcitrant contact dermatitis in patients.22 Additionally, the whole plants of H. plantaginea can improve bone hyperplasia in patients.617. Conclusion and future perspectives
This review summarized information on the botany, traditional use, phytochemistry, quality control and pharmacology of H. plantaginea. The amount of data gathered from different studies revealed that this medicinal plant is rich in many secondary metabolites and vast biological active constituents. However, the bioactive constituent action mechanisms and their structure–function relationships need to be investigated for further development into therapeutics.Systematic efficacy studies are necessary to examine the standardized extracts of H. plantaginea and to identify the bioactive constituents responsible for the pharmacological effects. Furthermore, the anti-inflammatory activity and its mechanisms are limited, which consequently limit the use of this medicinal plant to treat inflammation-related diseases. The secondary metabolites of steroids and flavonoids from H. plantaginea may be drug candidates for treating inflammation-related diseases because of their potent anti-inflammatory activities. To this end, no study has been performed to evaluate the toxic effects of H. plantaginea. However, further clinical evaluation must be performed to perceive the detailed effect of this plant on humans.
We believe that this review can be of particular value by providing fundamental insights into the medicinal value of this plant. Moreover, this review can provide a reference for clinical medication, sustainable development and utilization of this plant.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by grants from the National Natural Science Foundation of China (No. 81503357), the Natural Science Foundation of Jiangxi Province (No. 20192BAB215059, 20171BBH80026 and 20161BAB215209), and the Jiangxi University of Traditional Chinese Medicine (JXSYLXK-ZHYAO031).Notes and references
- L. G. Fu and T. Q. Chen, Higher plants of China, 2002, vol. 13, pp. 91–92 Search PubMed.
- J. Tang and F. Z. Wang, Flora of China, 1980, vol. 14, pp. 49–52 Search PubMed.
- Q. J. Li, Y. F. He, C. Wang, Y. R. Chang, L. P. Wang, Q. K. Wang and Y. Q. Liu, J. Anhui Agric. Sci., 2010, 38, 11826–11829 CAS.
- J. Z. Zhang, A. P. Si, G. F. Sun, L. Shi and Q. X. Zhang, Acta Hortic. Sin., 2004, 31, 549–554 Search PubMed.
- W. G. Sehmid, The genus Hosta, Timber Press, London, UK, 1991, pp. 428–428 Search PubMed.
- Q. Y. Li and Y. P. Xia, Zhongguo Yuanlin, 2004, 20, 77–79 Search PubMed.
- J. X. Liu, C. H. Zhao, X. R. Liu, Y. Z. Xi and Y. L. Zhang, Plant Syst. Evol., 2011, 294, 99–107 CrossRef.
- D. Q. Wang, Guihaia, 1989, 9, 297–298 Search PubMed.
- X. Q. Chen and E. B. David, Flora of China, 2000, vol. 24, pp. 204–205 Search PubMed.
- State Administration of Traditional Chinese Medicine, Zhonghua Bencao, 1999, 8, 107–109 Search PubMed.
- Y. Mimaki, A. Kameyama, M. Kuroda, Y. Sashida, T. Hirano, K. Oka, K. Koike and T. Nikaido, Phytochemistry, 1997, 44, 305–310 CrossRef CAS.
- D. J. Park, H. J. Im, H. G. Kim, W. H. Yang, S. H. Yong and M. S. Choi, J. Environ. Biol., 2018, 39, 507–516 CrossRef CAS.
- M. R. Jia and Y. Zhang, Dictionary of Chinese ethnic medicine, 2016, pp. 468–468 Search PubMed.
- State Administration of Traditional Chinese Medicine, Zhonghua Bencao, 2004, 32, 142 Search PubMed.
- Y. Xin, Chin. Tradit. Pat. Med., 2015, 37, 653–656 Search PubMed.
- J. H. Zhang, H. X. Xie, P. F. Xue, H. G. Zhang, X. Y. Liu and M. S. Baiji, Chin. Pharm. J., 2010, 45, 335–337 CAS.
- J. W. He, L. Yang and G. Y. Zhong, Chin. Tradit. Herb. Drugs, 2016, 47, 4295–4300 Search PubMed.
- L. Yang, Y. Q. Wang, J. W. He, X. M. Wang and J. X. Zhu, Chin. Med. Mat., 2016, 39, 216–222 CAS.
- L. Yang, J. J. Zhao, Y. Q. Fang, J. X. Zhu, X. M. Wang, J. W. He and G. Y. Zhong, Chin. J. Exp. Tradit. Med. Formulae, 2016, 22, 230–234 Search PubMed.
- G. Y. Zhong, L. S. Xu, G. J. Xu and T. Namba, China J. Chin. Mater. Med., 2002, 27, 89–94 Search PubMed.
- S. Z. Li, Compendium of Materia Medica, 1977, vol. 2, pp. 1208–1208 Search PubMed.
- S. J. Yun, J. Y. Lee, G. H. Kim, T. H. Kim, A. Y. Lee, S. H. Lee and J. S. Hong, J. Eur. Acad. Dermatol. Venereol., 2018, 32, e28–e29 CrossRef CAS.
- Y. Xin and W. L. J. Ao, Zhong guo yao xue da hui ji di shi jie zhong guo yao shi zhou da hui, 2010, p. 6 Search PubMed.
- M. Y. Wang, Y. Peng, C. S. Peng, J. Q. Qu and X. B. Li, J. Asian Nat. Prod. Res., 2018, 20, 501–509 CrossRef CAS.
- Q. Liu, G. F. Sun, J. Z. Zhang and X. D. Li, Sci. Agric. Sin., 2015, 48, 4323–4334 CAS.
- M. Yu, Y. Yu, L. P. Xu, H. Z. Liu and S. Y. Liu, North. Hortic., 2015, 27, 198–201 Search PubMed.
- H. Dai, Y. M. Fang, L. B. Huang and M. Zhang, Journal of Jiangsu Forestry Science & Technology, 2014, 41, 37–41 Search PubMed.
- M. X. Guan and R. Dong, North. Hortic., 2013, 25, 182–185 Search PubMed.
- J. H. Zhang, Inner mongolia medical college, 2009 Search PubMed.
- J. Q. Liu, C. F. Wang, M. H. Qiu and W. X. Hu, Chin. Tradit. Herb. Drugs, 2010, 41, 520–526 CAS.
- X. Liu, Studies on the bioactive constituents from Zingiber officinale Rosc and Hosta plantaginea (Lam.) Aschers, Third Military Medical University, 2017 Search PubMed.
- J. Y. Qu, M. Y. Wang, C. M. Wang, G. Y. Zhong and X. B. Li, Chin. Tradit. Herb. Drugs, 2011, 42, 217–221 CAS.
- W. Y. Li, Preliminary study on the chemical compositions and bioactivities of Mongolian medicine Hosta plantaginea (Lam.) Ascherson, Huazhong University of Science and Technology, 2009 Search PubMed.
- M. Y. Wang, Z. H. Xu, Y. Peng, G. Y. Zhong and X. B. Li, Chem. Nat. Compd., 2016, 52, 1047–1051 CrossRef CAS.
- J. He, Study on quality standard and chemical constituents of Hosta ventricosa stearn, Guangzhou University of Chinese Medicine, 2010 Search PubMed.
- H. X. Xie, J. H. Zhang, H. G. Zhang and P. F. Xue, China J. Chin. Mater. Med., 2009, 44, 733–735 CAS.
- J. W. He, L. Yang, Z. Q. Mu, Y. Y. Zhu, G. Y. Zhong, Z. Y. Liu, Q. G. Zhou and F. Cheng, RSC Adv., 2018, 8, 18175–18179 RSC.
- L. Yang, S. T. Jiang, Q. G. Zhou, G. Y. Zhong and J. W. He, Molecules, 2017, 22, 1825 CrossRef.
- J. W. He, X. Y. Huang, Y. Q. Wang, J. Liang, R. H. Liu, G. Y. Zhong and L. Yang, Nat. Prod. Res., 2019, 33, 1599–1604 CrossRef CAS.
- H. Yu, Q. H. Wang, J. J. Han, B. Y. Q. E. Bao and W. L. J. Ao, Chin. Tradit. Pat. Med., 2017, 39, 107–111 Search PubMed.
- Q. G. Zhou, L. Yang, J. W. He and G. Y. Zhong, China J. Chin. Mater. Med., 2019, 44, 3312–3315 Search PubMed.
- Y. H. Wang, S. Gao, F. M. Yang, Q. Y. Sun, J. S. Wang, H. Y. Liu, C. S. Li, Y. T. Di, S. L. Li, H. P. He and X. J. Hao, Org. Lett., 2007, 9, 5279–5281 CrossRef CAS.
- Y. H. Wang, Z. K. Zhang, F. M. Yang, Q. Y. Sun, H. P. He, Y. T. Di, S. Z. Mu, Y. Lu, Y. Chang, Q. H. Zheng, M. Ding, J. H. Dong and X. J. Hao, J. Nat. Prod., 2007, 70, 1458–1461 CrossRef CAS.
- Q. H. Wang, J. J. Han and B. Y. Q. E. Bao, J. Food Biochem., 2017, 41, e12320 CrossRef.
- X. H. Bao, Q. F. Wang, B. Y. Q. E. Bao, J. J. Han and W. L. J. Ao, Chem. Nat. Compd., 2017, 53, 614–617 CrossRef CAS.
- H. X. Xie and P. F. Xue, China Pharm, 2014, 23, 12–13 CAS.
- J. N. Ma, C. M. Mao, Z. J. Tian and W. D. Liu, Chin. Tradit. Pat. Med., 2017, 39, 639–641 Search PubMed.
- H. F. Li, M. J. Wang and H. N. Zhu, China Med. Her., 2017, 14, 34–37 Search PubMed.
- W. L. J. Ao, M. G. Bai, H. G. J. L. T. Wang and S. Weijimis, Drug Stand. China, 2012, 13, 347–350 CAS.
- X. J. Li, P. F. Xue, A. H. Ju, J. P. Gao and Y. L. Ren, Neimenggu Yixueyuan Xuebao, 2011, 33, 307–310 Search PubMed.
- J. He, Y. Gao and W. M. Li, Tradit. Chin. Drug Res. Clin. Pharmacol., 2010, 21, 192–194 CAS.
- H. X. Xie, P. F. Xue, H. G. Zhang, L. Q. Fan and H. M. Bian, J. Beijing Univ. Tradit. Chin. Med., 2009, 32, 624–625 CAS.
- J. W. He, L. Yang, J. X. Zhu, X. M. Wang, Z. R. Zou, W. W. He and G. Y. Zhong, J. Jiangxi Norm. Univ., 2016, 40, 183–185 Search PubMed.
- Y. Xin and Dalahu, Asia-Pacific Traditional Medicine, 2015, 11, 5–7 Search PubMed.
- C. Y. Li, P. F. Xue, M. N. Liu, P. P. Wang and L. Wang, Lishizhen Med. Mater. Med. Res., 2015, 26, 1559–1560 Search PubMed.
- A. L. Guan and M. D. L. Naren, J. Med. Pharm. Chin. Minor., 2015, 21, 21–22 Search PubMed.
- J. L. Bao, Shijie Zuixin Yixue Xinxi Wenzhai, 2016, 16, 172 Search PubMed.
- Z. M. He, Journal of Sichuan Traditional Chinese Medicine, 1994, 13, 53 Search PubMed.
- H. X. Xie, P. F. Xue, J. Zhou, L. Fan and F. H. Yuan, Acta. Acad. Med. Nei. Mongol., 2010, 32, 36–38 Search PubMed.
- M. M. Wu, X. J. Li, P. F. Xue, S. L. Shi, C. Y. Li, H. X. Xie and J. H. Zhang, Chin. Med. J. Res. Pract., 2018, 32, 16–18 Search PubMed.
- X. Tang, Zhongguo Xiangcun Yisheng Zazhi, 1992, 4, 32 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |