Understanding cation effects in electrochemical CO2 reduction†
Abstract
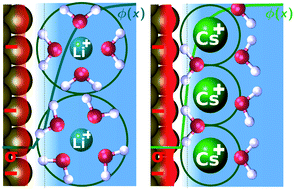
Solid–liquid interface engineering has recently emerged as a promising technique to optimize the activity and product selectivity of the electrochemical reduction of CO2. In particular, the cation identity and the interfacial electric field have been shown to have a particularly significant impact on the activity of desired products. Using a combination of theoretical and experimental investigations, we show the cation size and its resultant impact on the interfacial electric field to be the critical factor behind the ion specificity of electrochemical CO2 reduction. We present a multi-scale modeling approach that combines size-modified Poisson–Boltzmann theory with ab initio simulations of field effects on critical reaction intermediates. The model shows an unprecedented quantitative agreement with experimental trends in cation effects on CO production on Ag, C2 production on Cu, CO vibrational signatures on Pt and Cu as well as Au(111) single crystal experimental double layer capacitances. The insights obtained represent quantitative evidence for the impact of cations on the interfacial electric field. Finally, we present design principles to increase the activity and selectivity of any field-sensitive electrochemical process based on the surface charging properties: the potential of zero charge, the ion size, and the double layer capacitance.
- This article is part of the themed collection: 2019 Energy and Environmental Science HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
