Open Access Article
Open Access ArticleHighly sensitive and selective SO2 MOF sensor: the integration of MFM-300 MOF as a sensitive layer on a capacitive interdigitated electrode†
Valeriya
Chernikova
a,
Omar
Yassine
b,
Osama
Shekhah
 a,
Mohamed
Eddaoudi
a,
Mohamed
Eddaoudi
 *a and
Khaled N.
Salama
*a and
Khaled N.
Salama
 *b
*b
aFunctional Materials Design, Discovery and Development Research Group (FMD3), Advanced Membranes & Porous Materials Center, Division of Physical Sciences and Engineering, King Abdullah University of Science and Technology(KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: mohamed.eddaoudi@kaust.edu.sa
bSensors Lab, Electrical Engineering Program, Computer, Electrical and Mathematical Science and Engineering Division, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: khaled.salama@kaust.edu.sa
First published on 5th March 2018
Abstract
We report on the fabrication of an advanced chemical capacitive sensor for the detection of sulfur dioxide (SO2) at room temperature. The sensing layer based on an indium metal–organic framework (MOF), namely MFM-300, is coated solvothermally on a functionalized capacitive interdigitated electrode. The fabricated sensor exhibits significant detection sensitivity to SO2 at concentrations down to 75 ppb, with the lower detection limit estimated to be around 5 ppb. The MFM-300 MOF sensor demonstrates highly desirable detection selectivity towards SO2vs. CH4, CO2, NO2 and H2, as well as an outstanding SO2 sensing stability.
Sulfur dioxide (SO2) is regarded as one of the most toxic and problematic anthropogenic air pollutants.1 Despite the fact that the world is becoming more receptive to the use of renewable energy alternatives, demand for fossil fuel is ever increasing. Notably, burning of fossil fuels by power plants entails a rise in SO2 emission, posing a serious threat to the environment and human health.2,3 It is to be noted that major health concerns are associated with prolonged exposure to SO2, with a primary one-hour acceptable limit set at 75 parts per billion (ppb).4 Certainly, it is necessary to continuously monitor the concentration of SO2 in ambient air, particularly near emission sources.
Many studies have investigated compact SO2 sensors based on different classes of gas-sensing materials, such as solid electrolytes,5 conducting polymers,6,7 metal oxide semiconductors,8,9 and piezoelectric crystals,10 which are applied on a variety of transduction units. Metal oxide semiconductors operate on a change of resistance when a targeted analyte reacts with the chemisorbed oxygen from air.11 These semiconductors are among the most promising candidates due to their low cost, high sensitivity, and reliability.8,9 Device response and sensitivity are greatly influenced by the exposed surface area and operating temperatures, regulating the amount of oxygen adsorption and the formation of surface ionic species needed for the reaction with the analyte. Nevertheless, these sensors suffer from evident disadvantages such as high-power consumption and complex electrical system design. Manifestly, advanced preparation8 and modification methods12,13 are constantly enhancing the sensing performance of metal oxide sensors. For example, the coating of porous materials, such as zeolites, is used to pre-concentrate targeted gases and to avoid interference from the cross-sensitivity of larger non-targeted gases.13 Despite the aforementioned progress, the quest for room-temperature stable and sensitive gas sensors has inspired researchers to consider alternative materials. For instance, reversible physisorption within porous materials with highly accessible pore systems prompting effective and selective interactions with analytes offers great potential for targeted gas sensing.14
Metal–organic frameworks (MOFs) are crystalline porous materials based on self-assembled metal ions or metal clusters with organic ligands into a periodic networked structure. Uniquely, MOF chemistry offers a myriad of tunable porous structures with unparalleled surface areas and tailor-made pore shapes, sizes and functionalities.15,16 Prominently, various MOFs have shown high capacity and selectivity for harmful gases,17 positioning MOFs as prospective candidates for gas-sensing applications.14,18–23 Nevertheless, our previous work revealed that regulating the MOF pore size and shape is not sufficient to achieve the requisite effective detection of hazardous gases/vapors, and a more specific interaction/affinity between the targeted harmful adsorbates and the host framework is commanded.24,25 Accordingly, we opted to select a MOF with predefined requisites, namely a high SO2 uptake with the ability to congruently discriminate one gas over another and thus maximizing the potential selectivity towards the targeted analyte. A MOF encompassing the aforementioned criteria is prone to offer improved sensor performance in terms of response and selectivity. Although a substantial number of MOFs have been studied for potential SO2 sorption,26–31 to the best of our knowledge, no MOF-based SO2 sensors have been reported so far. MOFs have not been applied for SO2 detection, probably since the majority, and particularly those with open metal sites, are not stable upon exposure to SO2.32 The recently introduced indium based MFM-300 (In) MOF26 was chosen among others such as MFM-300 (Al),24 MFM-202-a,28 M3[Co(CN)6]2 (M = Zn, Co),29 Mg–MOF-74,30 and Ni(bdc)(ted)0.5,31 due to its high SO2 sorption capacity of 8.28 mmol g−1 (298 K and 1 bar), and compatible (mild) synthetic conditions with sensor circuit stability during thin film deposition (Table S1†). MFM-300 (In) MOF is a 3-periodic open framework, isostructural to its aluminum27 and gallium33 analogues, and comprises infinite cis InO4(OH)2 octahedral chains bridged by tetradentate ligands (biphenyl-3,3′,5,5′-tetracarboxylic acid) (Fig. 1). Structural analysis of MFM-300 revealed the decoration of the pore system with OH- groups along the metal chains in the helical direction, thereby creating a periodic array of exposed free OH- groups in the surface of the pores. Markedly, the exposed OH- groups along with four neighbouring C–H groups from benzene rings provide “pocket-like” adsorption sites suitable for SO2 binding, governing its adsorption selectivity toward SO2 (Fig. S1†).26
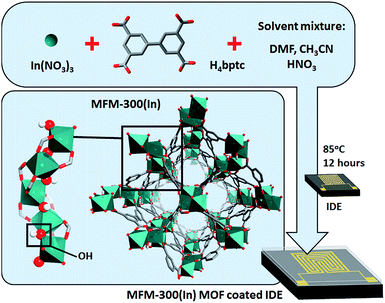 | ||
| Fig. 1 Schematic representation of the optimized solvothermal preparation approach of MFM-300 (In) MOF thin film on the interdigitated electrodes (IDEs). | ||
One of the challenges in integrating MOFs into devices for various related applications is directly related to the ability to fabricate and deploy MOFs as thin films. Delightfully, recent advances have permitted the successful control of MOF thin film growth or deposition on various supports, including surface modification of substrates with self-assembled monolayers (SAMs) which we have used in the present study.34–37 To evaluate the sensing properties of MFM-300 (In), a MOF thin film was coated on interdigitated electrodes (IDEs) and associated changes in capacitance were directly measured as a response to the presence of a given analyte.24,25,38 Particularly, the MFM-300 (In) MOF was grown on a prefunctionalized IDE with an OH-terminated SAM,39–41 using an optimized solvothermal synthetic procedure26 (Fig. 1). The successful fabrication of a highly crystalline, suitably intergrown crystals, and homogeneous MFM-300 (In) MOF thin film was confirmed using X-ray diffraction (XRD) and scanning electron microscopy (SEM) (Fig. 2, S2†).
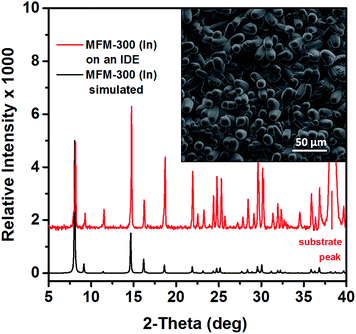 | ||
| Fig. 2 Comparison of calculated X-ray diffraction (XRD) patterns of MFM-300–MOF (In) and thin film grown on the IDE. Top view SEM image of MFM-300–MOF (In) coating. | ||
The gas-sensing tests were performed using the established procedure reported previously,24,25,42 where the samples were first activated under vacuum for one hour, and the chamber was later purged with pure nitrogen. Nitrogen gas was used as a carrier gas to dilute SO2 (and other gases) to the desired concentration (i.e. down the ppb range).
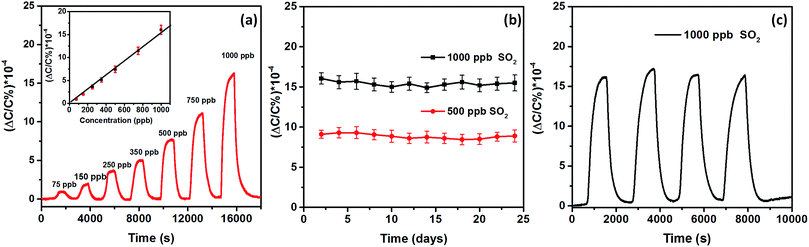
The MFM-300 (In) MOF sensor performance was found to be exceptional as we were able to detect SO2 in the ppb range down to 75 ppb with a linear response from 75 to 1000 ppb (Fig. 3a and b) with a detection limit as low as 5 ppb. The remarkable detection is plausibly governed by the associated changes in film permittivity upon adsorption of SO2 molecules. Reasonably, two types of interactions regulated the adsorption process:24 (i) analyte–framework interaction, in which oxygen centers from SO2 (Oδ−) form hydrogen bonds with the exposed hydrogen (Hδ+) centers from free hydroxyl groups and four aromatic C–H groups from the ligand respectively; and (ii) analyte–analyte interaction, in which adsorbed SO2 interacts with another SO2 through dipoles. These electrostatic changes in the film are reflected in the observed change of capacitance.
These very promising results prompted us to evaluate the stability of the MFM-300 (In) MOF capacitive sensor for SO2 detection at room temperature using reproducibility tests. In these tests, we explored the performance of the sensor in detecting two concentrations of SO2, 500 and 1000 ppb, over a testing period of more than three weeks (Fig. 3c). The results clearly showed that the detection levels were steady/stable with a negligible variation over the range of the tested period of time, attesting to the stability and durability of our SO2 sensor over the range of tested concentrations.42
Subsequently, the effect of relative humidity (RH) on the performance of the MOF sensor was also investigated. The humidity in the chamber was adjusted to the desired level (5–85% RH) and capacitance response was subtracted as a baseline (Fig. S6 and 7†). The capacitance change was recorded at each RH level in the presence of SO2 at 350 and 1000 ppb. Noticeably, distinctive signals for both SO2 concentrations (Fig. 4a), similar to “dry” conditions (Fig. 3a), confirm the strong affinity of the MFM-300 (In) MOF sensor toward SO2 and attest to its practical applicability in the presence of water molecules. The noted sensor performance under humid conditions suggests the possibility of competitive adsorption on hydroxyl groups between water and SO2 at low humidity levels, and therefore, negligible change is observed up to 30% RH.43 At higher humidity levels, adsorbed water in the MOF can increase sorption uptake of the analyte with water compared to the sorption uptake of the same analyte adsorbed on the dry framework, and, thus, increases the effect on capacitance change. With the increase of humidity, the SO2 interacts with adsorbed water by forming additional hydrogen bond interactions.42,43
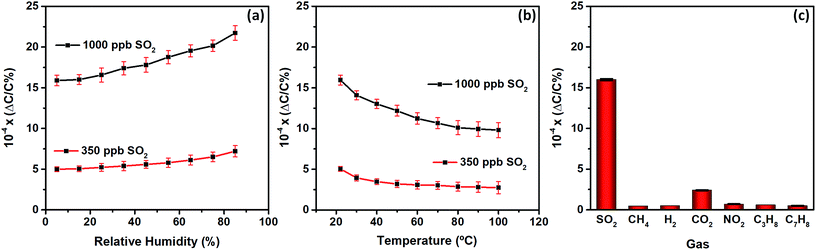 | ||
| Fig. 4 Effects of the (a) relative humidity and (b) temperature on the MFM-300 (In) MOF sensor performance. (c) Selectivity of the MFM-300 (In) MOF sensor to other gases at 1000 ppb. | ||
The temperature (T) dependence of SO2 sensitivity for the MFM-300 (In) MOF sensor was also evaluated in the range of 22–100 °C (Fig. 4b). The sensitivity response logically decreases with the temperature increase, because, in MFM-300 (In) MOF, as in most compounds, equilibrium sorption decreases with increasing temperature. The best sensitivity was obtained at 22 °C. Further, upon increasing the T from 22 °C to 80 °C, we observed a drop by almost 35%, which can be attributed to the lessened interactions toward exposed active sites, resulting in increased molecular diffusion and a decrease in the analyte adsorbed amount.
Finally, we investigated the selectivity of our MFM-300 (In) MOF sensor in the presence of various gases/vapors, including methane (CH4), hydrogen (H2), carbon dioxide (CO2), nitrogen dioxide (NO2), propane (C3H8) and toluene (C7H8) at 1000 ppb level (Fig. 4c). The response of the MFM-300 (In) MOF films to these gases was recorded using the same testing protocol, and the study revealed an excellent selectivity for SO2 compared to the other gases/vapors, with slight cross-sensitivity with CO2. However, the response signal of the MFM-300 (In) MOF to SO2 was almost four times higher than for CO2 and more than 20 times higher than other gases/vapors, which clearly corroborate the exceptional sensing selectivity of the MFM-300 (In) MOF sensor towards SO2.
Table 1 presents a comparison of the performance of our MFM-300 (In) MOF-based capacitor sensor with selected benchmark material-based sensors. Currently, more than 90% of all reported material-based sensors for toxic gases represent a combination of two or more different types of materials, namely composites.50–53 For example, a metal oxide/polymer composite allows SO2 detection at room temperature, while a metal oxide on its own requires heating. In contrast, here we have presented for the first time a pure MOF-based SO2 sensor that shows similar/improved performance compared to the best-reported sensors so far. Prominently for our unveiled SO2 MOF sensor, the high affinity for SO2 of the deposited MOF material, combined with the cheap and easy capacitive measurement, led to the attained superior SO2 sensing performances. Therefore, in the future, the combination of this MOF with other advanced materials could offer great opportunities for the design of better performing sensors based on capacitance or other transduction mechanisms.
| Sensor materials | Sensing mechanism | Conc. ppm | Temp. °C | |
|---|---|---|---|---|
| a Reduced graphene oxide. b Current work. | ||||
| Zeolites | Zeolite A42 | QCM | 50 | 170 |
| Faujasite43 | QCM | 300 | 150 | |
| Metal oxides | Pt-doped TiO2 (ref. 44) | Conductivity | 5 | 200 |
| SnO2/MgO/V2O5 (ref. 45) | Conductivity | 1 | 400 | |
| Pd-doped WO3 (ref. 46) | Conductivity | 1 | 200 | |
| Polymers | Polypyrrole47 | QCM | 10 (vol%) | RT |
| Polyaniline7 | Conductivity | 10 | RT | |
| Composites | Mn–zeolite Y/PEDOT-PSS48 | Conductivity | 1000 | RT |
| SnO2/polyaniline49 | Conductivity | 2 | RT | |
| TiO2/rGOa52 | Conductivity | 5 | RT | |
| MOFsb | MFM-300 | Capacitance | 0.075 | RT |
In conclusion, this study attests to the excellent performance and stability of the first MOF-based SO2 sensor and its superior detection limit, which is considered to be the lowest reported sensitivity by an order of magnitude, in comparison to other sensors at room temperature. Principally, MFM-300 (In) MOF offers a distinctive SO2 detection at concentrations down to 75 ppb with a limit of detection down to 5 ppb. The exceptional stability of the MFM-300 (In) MOF sensor was supported and demonstrated using reproducibility tests. Moreover, the presented results attest to the distinctive and remarkable sensing selectivity of the prepared MOF sensor towards SO2, as shown from the signal intensity associated with the MFM-300 (In) MOF for SO2 detection compared to the associated signal intensities for other evaluated gases/vapors like NO2, CH4, H2 and others. This unique sensing feature of the MFM-300 (In) MOF paves the way for the deployment of MOF-based sensors in various key sensing applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research reported in this publication was supported by funding from King Abdullah University of Science and Technology (KAUST). We also thank Dr Y. Belmabkhout for valuable discussions and Dr P. Bhatt for sorption experiments.Notes and references
- J. N. Galloway, Water, Air, Soil Pollut., 1995, 85, 15–24 CrossRef CAS.
- M. Kampa and E. Castanas, Environ. Pollut., 2008, 151, 362–367 CrossRef CAS PubMed.
- P. L. Ward, Thin Solid Films, 2009, 517, 3188–3203 CrossRef CAS.
- United States Environmental Protection Agency, NAAQS Table, https://www.epa.gov/criteria-air-pollutants/naaqs-table#4, accessed October, 2017.
- J. W. Fergus, Sens. Actuators, B, 2008, 134, 1034–1041 CrossRef CAS.
- A. J. Heeger, Chem. Soc. Rev., 2010, 39, 2354–2371 RSC.
- V. Chaudhary and A. Kaur, Polym. Int., 2015, 64, 1475–1481 CrossRef CAS.
- G. F. Fine, L. M. Cavanagh, A. Afonja and R. Binions, Sensors, 2010, 10, 5469–5502 CrossRef CAS PubMed.
- K. Wetchakun, T. Samerjai, N. Tamaekong, C. Liewhiran, C. Siriwong, V. Kruefu, A. Wisitsoraat, A. Tuantranont and S. Phanichphant, Sens. Actuators, B, 2011, 160, 580–591 CrossRef CAS.
- O. M. Guimaraes, Anal. Lett., 1997, 30, 2159–2174 CrossRef CAS.
- T. Alammar, O. Shekhah, J. Wohlgemuth and A.-V. Mudring, J. Mater. Chem., 2012, 22, 18252–18260 RSC.
- P. Tyagi, S. Sharma, M. Tomar, F. Singh and V. Gupta, Nucl. Instrum. Methods Phys. Res., Sect. B, 2016, 379, 219–223 CrossRef CAS.
- R. Binions, H. Davies, A. Afonja, S. Dungey, D. Lewis, D. E. Williams and I. P. Parkin, J. Electrochem. Soc., 2009, 156, J46 CrossRef CAS.
- D. J. Wales, J. Grand, V. P. Ting, R. D. Burke, K. J. Edler, C. R. Bowen, S. Mintova and A. D. Burrows, Chem. Soc. Rev., 2015, 44, 4290–4321 RSC.
- M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319–330 CrossRef CAS PubMed.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- D. Britt, D. Tranchemontagne and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11623–11627 CrossRef CAS PubMed.
- I. Stassen, N. Burtch, A. Talin, P. Falcaro, M. Allendorf and R. Ameloot, Chem. Soc. Rev., 2017, 46, 3185–3241 RSC.
- M. G. Campbell and M. Dinca, Sensors, 2017, 17, 1108–1118 CrossRef PubMed.
- P. M. Bhatt, Y. Belmabkhout, A. Cadiau, K. Adil, O. Shekhah, A. Shkurenko, L. J. Barbour and M. Eddaoudi, J. Am. Chem. Soc., 2016, 138, 9301–9307 CrossRef CAS PubMed.
- B. Liu, J. Mater. Chem., 2012, 22, 10094–10101 RSC.
- F. Y. Yi, D. Chen, M. K. Wu, L. Han and H. L. Jiang, ChemPlusChem, 2016, 81, 675–690 CrossRef CAS.
- M. G. Campbell and M. Dinca, Sensors, 2017, 17, 1108–1119 CrossRef PubMed.
- C. Sapsanis, H. Omran, V. Chernikova, O. Shekhah, Y. Belmabkhout, U. Buttner, M. Eddaoudi and K. N. Salama, Sensors, 2015, 15, 18153–18166 CrossRef CAS PubMed.
- O. Yassine, O. Shekhah, A. H. Assen, Y. Belmabkhout, K. N. Salama and M. Eddaoudi, Angew. Chem., Int. Ed., 2016, 55, 15879–15883 CrossRef CAS PubMed.
- M. Savage, Y. Cheng, T. L. Easun, J. E. Eyley, S. P. Argent, M. R. Warren, W. Lewis, C. Murray, C. C. Tang, M. D. Frogley, G. Cinque, J. Sun, S. Rudic, R. T. Murden, M. J. Benham, A. N. Fitch, A. J. Blake, A. J. Ramirez-Cuesta, S. Yang and M. Schroder, Adv. Mater., 2016, 28, 8705–8711 CrossRef CAS PubMed.
- S. Yang, J. Sun, A. J. Ramirez-Cuesta, S. K. Callear, W. I. David, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schroder, Nat. Chem., 2012, 4, 887–894 CrossRef CAS PubMed.
- S. Yang, L. Liu, J. Sun, K. M. Thomas, A. J. Davies, M. W. George, A. J. Blake, A. H. Hill, A. N. Fitch, C. C. Tang and M. Schroder, J. Am. Chem. Soc., 2013, 135, 4954–4957 CrossRef CAS PubMed.
- P. K. Thallapally, R. K. Motkuri, C. A. Fernandez, B. P. McGrail and G. S. Behrooz, Inorg. Chem., 2010, 49, 4909–4915 CrossRef CAS PubMed.
- K. Tan, P. Canepa, Q. Gong, J. Liu, D. H. Johnson, A. Dyevoich, P. K. Thallapally, T. Thonhauser, J. Li and Y. J. Chabal, Chem. Mater., 2013, 25, 4653–4662 CrossRef CAS.
- J. B. DeCoste, T. J. Demasky, M. J. Katz, O. K. Farha and J. T. Hupp, New J. Chem., 2015, 39, 2396–2399 RSC.
- S. Han, Y. Huang, T. Watanabe, S. Nair, K. S. Walton, D. S. Sholl and J. Carson Meredith, Microporous Mesoporous Mater., 2013, 173, 86–91 CrossRef CAS.
- C. P. Krap, R. Newby, A. Dhakshinamoorthy, H. Garcia, I. Cebula, T. L. Easun, M. Savage, J. E. Eyley, S. Gao, A. J. Blake, W. Lewis, P. H. Beton, M. R. Warren, D. R. Allan, M. D. Frogley, C. C. Tang, G. Cinque, S. Yang and M. Schroder, Inorg. Chem., 2016, 55, 1076–1088 CrossRef CAS PubMed.
- O. Shekhah, J. Liu, R. A. Fischer and C. Woll, Chem. Soc. Rev., 2011, 40, 1081–1106 RSC.
- O. Shekhah and M. Eddaoudi, Chem. Commun., 2013, 49, 10079–10081 RSC.
- H. C. Streit, M. Adlung, O. Shekhah, X. Stammer, H. K. Arslan, O. Zybaylo, T. Ladnorg, H. Gliemann, M. Franzreb, C. Wöll and C. Wickleder, ChemPhysChem, 2012, 13, 2699–2702 CrossRef CAS PubMed.
- V. Chernikova, O. Shekhah and M. Eddaoudi, ACS Appl. Mater. Interfaces, 2016, 8, 20459–20464 CAS.
- H. Omran and K. N. Salama, IEEE 3rd International Conference on Smart Instrumentation, Measurement and Applications (ICSIMA), 2015, vol. 7559021, pp. 1–4, DOI:10.1109/ICSIMA.
- O. Shekhah, H. Wang, S. Kowarik, F. Schreiber, M. Paulus, M. Tolan, C. Sternemann, F. Evers, D. Zacher, R. A. Fischer and C. Woll, J. Am. Chem. Soc., 2007, 129, 15118–15119 CrossRef CAS PubMed.
- O. Shekhah, C. Busse, A. Bashir, F. Turcu, X. Yin, P. Cyganik, A. Birkner, W. Schuhmann and C. Woll, Phys. Chem. Chem. Phys., 2006, 8, 3375–3378 RSC.
- E. M. S. Azzam, A. Bashir, O. Shekhah, A. R. E. Alawady, A. Birkner, C. Grunwald and C. Wöll, Thin Solid Films, 2009, 518, 387–391 CrossRef CAS.
- A. H. Assen, O. Yassine, O. Shekhah, M. Eddaoudi and K. N Salama, ACS Sens., 2017, 2, 1294–1301 CrossRef CAS PubMed.
- R. Peralta, J. Balmaseda, E. González-Zamora and I. Ibarra, Inorg. Chem. Front., 2015, 2(12), 1080–1084 RSC.
- I. Sasaki, H. Tsuchiya, M. Nishioka, M. Sadakata and T. Okubo, Sens. Actuators, B, 2002, 86, 26–33 CrossRef CAS.
- M. Osada, I. Sasaki, M. Nishioka, M. Sadakata and T. Okubo, Microporous Mesoporous Mater., 1998, 23, 287–294 CrossRef CAS.
- X. Zhang, J. Tie and J. Zhang, Sensors, 2013, 13, 14764–14776 CrossRef PubMed.
- S. C. Lee, B. W. Hwang, S. J. Lee, H. Y. Choi, S. Y. Kim, S. Y. Jung, D. Ragupathy, D. D. Lee and J. C. Kim, Sens. Actuators, B, 2011, 160, 1328–1334 CrossRef CAS.
- M. Stankova, X. Vilanova, J. Calderer, E. Llobet, P. Ivanov, I. Gràcia, C. Cané and X. Correig, Sens. Actuators, B, 2004, 102, 219–225 CrossRef CAS.
- V. Syritski, J. Reut, A. Öpik and K. Idla, Synth. Met., 1999, 102, 1326–1327 CrossRef CAS.
- P. Chanthaanont and A. Sirivat, Adv. Polym. Technol., 2013, 32, 21367 CrossRef.
- C. A. Betty, S. Choudhury and S. Arora, Sens. Actuators, B, 2015, 220, 288–294 CrossRef CAS.
- D. Zhang, J. Liu, C. Jiang, P. Li and Y. e. Sun, Sens. Actuators, B, 2017, 245, 560–567 CrossRef CAS.
- S. Park, C. Park and H. Yoon, Polymers, 2017, 9, 155 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ta10538j |
| This journal is © The Royal Society of Chemistry 2018 |