Open Access Article
Open Access ArticleOccupational exposure to graphene based nanomaterials: risk assessment
Marco
Pelin
 a,
Silvio
Sosa
a,
Silvio
Sosa
 a,
Maurizio
Prato
a,
Maurizio
Prato
 *bcd and
Aurelia
Tubaro
*bcd and
Aurelia
Tubaro
 *a
*a
aDepartment of Life Sciences, University of Trieste, 34127 Trieste, Italy. E-mail: tubaro@units.it; ssosa@units.it; mpelin@units.it; Tel: +39 040 5588835
bDepartment of Chemical and Pharmaceutical Sciences, University of Trieste, 34127 Trieste, Italy. E-mail: prato@units.it; Tel: +39 040 5583598
cCIC BiomaGUNE, Parque Tecnológico de San Sebastián, Paseo Miramón, 182, 20009 San Sebastián, Guipúzcoa, Spain
dBasque Foundation for Science, Ikerbasque, Bilbao 48013, Spain
First published on 22nd August 2018
Abstract
Graphene-based materials (GBMs) are a family of novel materials including graphene, few layer graphene (FLG), graphene oxide (GO), reduced graphene oxide (rGO) and graphene nanoplatelets (GNP). Currently, the risk posed by them to human health is associated mainly with the occupational exposure during their industrial and small-scale production or waste discharge. The most significant occupational exposure routes are inhalation, oral, cutaneous and ocular, inhalation being the majorly involved and most studied one. This manuscript presents a critical up-to-date review of the available in vivo toxicity data of the most significant GBMs, after using these exposure routes. The few in vivo inhalation toxicity studies (limited to 5-days of repeated exposure and only one to 5 days per week for 4 weeks) indicate inflammatory/fibrotic effects at the pulmonary level, not always reversible after 14/90 days. More limited in vivo data are available for the oral and ocular exposure routes, whereas the studies on cutaneous toxicity are at the initial stage. A long persistence of GBMs in rodents is recorded, while contradictory genotoxic data are reported. Data gap identification is also provided. Based on the available data, the occupational exposure limit cannot be determined. More experimental toxicity studies according to specific guidelines (tentatively validated for nanomaterials) and more information on the actual occupational exposure level to GBMs are needed. Furthermore, ADME (Absorption, Distribution, Metabolism, Excretion), genotoxicity, developmental and reproductive toxicity data related to the occupational exposure to GBMs have to be implemented. In addition, sub-chronic and/or chronic studies are still needed to completely exclude other toxic effects and/or carcinogenicity.
1. Introduction
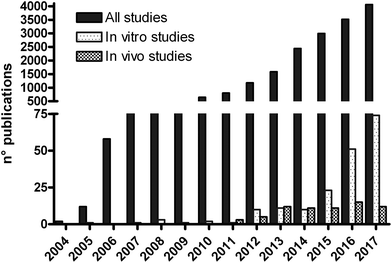
In recent years, the development of carbon-based nanomaterials (CBNs) and nanotechnology has constantly increased, offering a wide range of novel opportunities and solutions in different areas of research and application, which involve the environment, manufacturing technology and health care. As a consequence, an emerging area of concern in toxicology is represented by the manufactured nanomaterials.1One of the last discovered CBNs is graphene, consisting of two-dimensional, single atom thick sheets of planar sp2 bound carbons arranged in a honeycomb-like structure, with a high surface area on both sides of the planar axis.2 Furthermore, different graphene-based materials (GBMs) have been obtained by oxidation and/or functionalization of graphene, and characterized by a variable lateral size, thickness, surface area, shape, carbon-to-oxygen ratio and possible surface functionalization.3–6 GBMs are promising tools for a broad range of possible applications in electronics, energy technology, sensors and biomedicine.6,7 However, GBMs are surrounded by a plethora of unanswered questions regarding their safety. Although their potential toxicity has already been highlighted, limited toxicity studies on GBMs are available and the risk posed by them to human health remains largely unexplored. In fact, despite more than 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 scientific publications on GBMs are available since their discovery by Novoselov et al.,8 only about 250 of them reported toxicity data and about 70 (0.4% of total publications) included in vivo toxicity findings on laboratory animals (Fig. 1), the key component of the hazard identification process.
000 scientific publications on GBMs are available since their discovery by Novoselov et al.,8 only about 250 of them reported toxicity data and about 70 (0.4% of total publications) included in vivo toxicity findings on laboratory animals (Fig. 1), the key component of the hazard identification process.
The main risk to human health posed by GBMs appears to be associated with the occupational exposure to these materials, their applications being still at the experimental stage.5 During their industrial or small-scale production and waste discharge, humans can be exposed to GBMs mainly by inhalation, cutaneous and ocular routes, the respiratory tract, the skin and the eyes being in direct contact with the work environment. Ingestion can also occur by accidental oral intake and/or by secondary swallowing of inhaled GBMs.
In the occupational hazard assessment, various types of data are used, including human data, data from laboratory animal studies, data from in vitro studies and non-testing data that can be derived from the physicochemical properties of a substance. For GBMs, human data, case reports and medical surveys of workers are not available so far. Thus, on the basis of the occupational exposure routes, we carried out a critical review of the literature on GBM toxicity provided by laboratory animal studies, together with monitoring data in the work environment. In vivo toxicity studies were focused on few layer graphene (FLG), graphene oxide (GO), reduced graphene oxide (rGO) and graphene nanoplatelets (GNP), which are considered as starting materials for further functionalization and of interest for industrial production.
2. Methods of literature review
A systematic review of the literature on GBM toxicity in laboratory mammals and monitoring data in occupational settings was performed with no time restriction, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.9 The electronic databases (PubMed, Scopus and ToxLine) were used as data sources, using the term “graphene”.Inclusion criteria were: (1) in vivo studies on FLG, GO, rGO and GNP in laboratory mammals after exposure routes mimicking possible occupational exposure in humans; (2) monitoring data in the work environment; (3) full text articles; (4) English language. Exclusion criteria included editorials, not related abstracts and studies carried out on functionalized and/or composite forms of graphene (i.e. polymer- or polysaccharide-conjugated graphene). For each study, information including the physicochemical properties of the administered GBMs, dosage and routes of exposure as well as outcomes was extracted independently.
3 In vivo toxicity studies related to occupational exposure
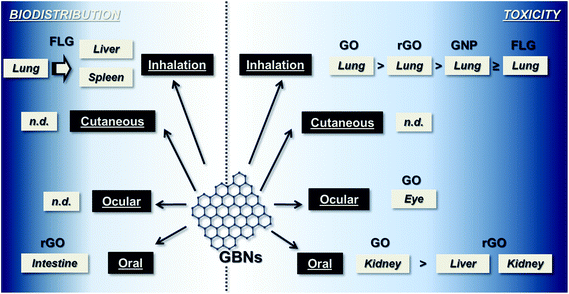
As shown in Fig. 2, very few and incomplete data related to GBM toxic effects after the main occupational exposure routes are available, so far.Respiratory exposure
The majority of in vivo toxicity studies were carried out to assess the effects at the respiratory level after exposure to GBMs by inhalation, intratracheal instillation or pharyngeal aspiration. Studies in rodents after acute exposure to GBMs by intratracheal instillation or pharyngeal aspiration revealed relatively severe lung inflammation, as reported in a recent review.10 Among the investigated GBMs, on evaluating inflammatory cells and/or inflammatory markers in the broncho-alveolar lavage fluid as indices of lung inflammation, GO appeared to be the most toxic one compared to rGO, GNP or FLG, which appeared to be the least toxic GBM at the pulmonary level.10–17 In contrast, in a very recent comparative study in male C57BL/6 mice (8 weeks-old), pharyngeal aspiration of GO induced lower toxic effects than rGO.18 However, it should be noted that the physicochemical properties of the tested materials are not always completely reported in these studies (Table 1), making a direct comparison of the effects of GBMs difficult. For instance, chemical composition is not always reported for GO and/or rGO, giving no information on the oxidation state of these materials. Similarly, thickness, surface area and chemical composition data are missing in the majority of the studies.| Lateral size (nm) | Thickness | Surface area | Chemical composition | Impurity | Density | Aggregation | In vivo exposure route | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| nm | Layers | |||||||||
| it = intratracheal exposure; pa = pharyngeal aspiration. n/a = data not available. | ||||||||||
| FLG | 60–590 | 0.97–3.94 | 4–6 | n/a | C 89% | n/a | n/a | Aggregated | it | 14 |
| O 6% | ||||||||||
| N 3.6% | ||||||||||
| H 1.4% | ||||||||||
| n/a | 1.2–5.0 | n/a | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm2 000 nm2 |
n/a | n/a | n/a | Aggregated | it | 11 | |
5000–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
n/a | 10 | 100 m2 g−1 | n/a | n/a | 2 g cm−3 | Aggregated | pa | 12 | |
| GO | 2000–3000 | 2 | 2–3 | 338–441 m2 g−1 | n/a | n/a | n/a | n/a | it | 19 |
| n/a | 0.5–2.0 | n/a | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm2 000 nm2 |
n/a | n/a | n/a | n/a | it | 11 | |
| 100–150 | n/a | n/a | n/a | n/a | n/a | n/a | Aggregated | pa | 18 | |
| 2000–3000 | n/a | 2–3 | n/a | n/a | Inorganic impurities (<1.5%) | n/a | n/a | it | 16 | |
| rGO | 100–150 | n/a | n/a | n/a | n/a | n/a | n/a | Aggregated | pa | 18 |
| 1000–2000 | n/a | 2–3 | 411 m2 g−1 | n/a | Inorganic impurities (<1.5%) | n/a | n/a | it | 16 | |
| GNP | 2000 | 3–4 | n/a | 735 m2 g−1 | n/a | n/a | n/a | n/a | it | 13 |
2000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8–25 | 28–84 | 106–747 m2 g−1 | n/a | n/a | n/a | Aggregated | pa | 15 | |
| <2000 | <5 nm | <4 | >700 m2 g−1 | n/a | n/a | n/a | n/a | it | 17 | |
The studies after intratracheal instillation also suggest that the level of graphene dispersion seems to affect its lung toxicity: highly dispersed graphene induced modest acute lung inflammation without fibrosis in male C57BL/6 mice (8–12 week-old) and its toxicity appeared to be lower than that of aggregated graphene, which lodged in the airways and induced local fibrosis.11 Furthermore, graphene appears to be accumulated mainly in the lungs, as recorded for 14C-FLG (5 μg per mouse) in male ICR mice (4 weeks-old): 47% of the dose was still detected in the lungs after 4 weeks and the remaining was distributed in the liver and spleen.14 Similarly, after intratracheal instillation, GNP (2.5–5.0 mg kg−1) was retained in the lungs of male ICR mice (6 weeks-old) for up to 28 days.13 Very recently, a reproductive toxicity study was carried out in male NMRI mice (age/weight not specified) after the intratracheal instillation of commercial GO once a week for 7 consecutive weeks (18 μg per mouse per instillation; cumulative dose: 126 μg per mouse). The increased neutrophil number in the broncho-alveolar lavage fluid suggested pulmonary inflammation.19
In conclusion, these few studies after intratracheal instillation suggest the lung as the target organ and the storage depot of GBMs, with the following toxicity rank: FLG < GNP < rGO < GO. Different pieces of evidence of lung inflammation and fibrosis were observed both after acute exposure and after one exposure per week for 7 consecutive weeks. Moreover, a long persistence of 14C-FLG and GNP in the lungs was observed.
However, intratracheal instillation or pharyngeal aspiration involves a non-physiological delivery of GBMs, which may lead to a less homogeneous distribution of materials as well as higher local concentrations and toxic effects than those occurring by occupational inhalation exposure.10 For these reasons, inhalation exposure by head–nose or only-nose delivery systems, mimicking the usual human exposure scenario, is more suitable for the hazard identification and characterization.
Currently, only five in vivo studies after inhalation exposure to FLG, GO or GNP are reported. Each study was carried out in rats exposed to an atmosphere containing particles with an aerodynamic diameter small enough to reach the broncho-alveolar region (Table 2). The first study was carried out in male Wistar rats (7 weeks-old) head–nose exposed for 5 days (6 h per day) to FLG containing 3D-graphite impurities. The atmosphere contained a mean concentration ranging from 0.54 to 10.1 mg m−3 with calculated particle depositions using apparent and agglomerate densities of 0.26 and 0.29 mg per lung. Three and 24 days after the last treatment, the broncho-alveolar lavage fluid of rats exposed to 3.05 or 10.1 mg FLG per cm2 showed cytological, cytokine and enzyme activity changes related to acute/sub-acute inflammation. In parallel, a dose-dependent accumulation of single macrophages or small aggregates of alveolar macrophages loaded with black particles (recognized as graphene) were found in the lungs of all FLG-treated rats (mainly in the lumen of alveoli; only a few in the alveolar wall, alveolar ducts and terminal bronchioles). In addition, lung microgranulomas were also observed after the recovery period of 24 days, without any alteration of the lung parenchyma. For this study, the authors declared to have followed the Organization for Economic Co-operation and Development (OECD) test guideline (TG) 412, which indicates a 4-week exposure period (5 days per week; 6 h day−1). However, the rats were exposed to FLG only for 1 week (5 days; 6 h day−1).20
| Lateral size (nm) | Thickness | Surface area | Chemical composition | Impurity | Density | Aggregation | Test atmosphere (μm) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| nm | Layers | |||||||||
| n/a = data not available. | ||||||||||
| FLG | <10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
9 | n/a | 131 m2 g−1 | C 84.1% | 3D-graphite; sulfur impurity | 0.02 g mL−1 | ∼40 μm, crumpled napkin | MMAD≤0.4; particle size = 0.473–0977 | 20 |
| O 8.8% | ||||||||||
| S 5.4% | ||||||||||
| Na 0.6% | ||||||||||
| Si 0.4% | ||||||||||
| 550 | 8 | n/a | 100 m2 g−1 | C 76.8% | n/a | n/a | n/a | MMAD = 0.567; particle size = 0.010–0.130 | 21 | |
| O 10.4% | ||||||||||
| Na 10.5% | ||||||||||
| P 2.4% | ||||||||||
| GO | 10–120 | n/a | n/a | n/a | C 56.8% | n/a | 0.46–3.76 mg m−3 | n/a | Equivalent hydrodynamic diameter = 0.15–0.25 | 23 |
| O 20.2% | ||||||||||
| K 11.3% | ||||||||||
| Na 8.3% | ||||||||||
| Cl 3.4% | ||||||||||
| 500–5000 | 1 | 1–2 | 8.46 m2 g−1 | C 42–45% | n/a | 1.7 g mL−1 | Stacked platelet structure | MMAD = 0.203; particle size = 0.265–34 | 24 | |
| O 35–40% | ||||||||||
| GNP | <2000 | 0.35–0.38 | 20–30 | 750 m2 g−1 | C 96% | n/a | 0.2 g mL−1 | n/a | MMAD = 0.123; particle size = 0.265–34 | 22 |
| O 4% | ||||||||||
In another study, male Sprague-Dawley rats (6 weeks-old) were nose-only exposed for 5 days (6 h day−1) to commercial FLG. The mean atmosphere FLG concentrations were 0.68 or 3.86 mg m−3, corresponding to deposited doses of 3.6 or 20.3 μg per rat per day, respectively. The exposure to FLG did not change the body weight or organ weight of the rats, also during the recovery period of 28 days. No significant difference was observed in the blood levels of lactate dehydrogenase, protein and albumin between the FLG-treated rats and controls. Histopathological analysis showed FLG ingestion by alveolar macrophages.21
In a study on GNP, male Sprague-Dawley rats (6 weeks-old) were nose-only exposed for 4 weeks (6 h day−1, 5 days per week) to commercial GNP and monitored up to 90 days after exposure, according to the OECD TG 412. In the test atmosphere, the mass concentration of GNP particles ranged from 0.12 to 1.88 mg m−3, corresponding to daily deposited doses of 0.6–9.9 μg per rat. Particles of the inhaled GNP were observed in alveolar macrophages up to 90 days post-exposure, with translocation also to lung lymph nodes. However, they did not induce any lung pathology, inflammation, change in blood biochemical parameters or genotoxic effects at the pulmonary level, evaluated using the comet assay. The authors reported a No-Observed-Adverse-Effect-Level (NOAEL) >1.88 mg m−3.22
Minor signs of toxicity were recorded for GO: male Sprague-Dawley rats (6 weeks-old) nose-only exposed for 6 h to GO showed alveolar macrophages with ingested GO, also during the recovery period of 14 days.23 These results are in line with a very recent inhalation study in which male Sprague-Dawley rats (6 weeks-old) were nose-only exposed for 5 days (6 h day−1) to a GO having different physicochemical properties (Table 2) and monitored up to 21 days after exposure. The delivered mass concentrations of GO ranged from 0.76 to 9.78 mg m−3, corresponding to 3.25 × 103–9.97 × 103 particles per cm3. No significant effects were observed in the hematological analysis or in the broncho-alveolar lavage fluid inflammatory markers and cell number, both at the end of the exposure and during the recovery period. However, alveolar macrophages with ingested GO were observed, although a gradual clearance was noted during the 21-day recovery period.24
Overall, the authors of three of five studies reported various levels of lung inflammation in Wistar or Sprague-Dawley rats after inhalation exposure to FLG, GNP or GO, not always reversible at the last observation time (ranging from 14 to 90 days). Although a NOAEL >1.88 mg m−3 of GNP was defined, based on the lack of inflammation and other lung pathologies, blood biochemical parameter changes and genotoxic effects, the presence of GNP in macrophages and in lung lymph nodes was recorded up to 90 days after inhalation exposure.22 Furthermore, it has to be underlined that inhalation exposure to GBMs was limited to 6 h or 1 week (5 days per week; 6 h day−1) and only in one study it was extended to 4 weeks (5 days per week; 6 h day−1), although the validated guidelines indicate 4 weeks or 13 weeks of exposure (5 days per week; 6 h day−1). No sub-chronic and chronic toxicity tests are available to evaluate other toxic effects and/or carcinogenicity, so far.
Oral exposure
Considering the exposure by ingestion, in vivo studies after oral administration are limited to GO and rGO, whose physicochemical properties are reported in Table 3. Male Sprague-Dawley rats (8–10 weeks-old) daily exposed by gavage to GO (10–40 mg per kg per day) for 5 days showed dose-dependent tissues signs of nephrotoxicity, tentatively mediated by oxidative stress.25 In contrast, no alteration of kidney and liver functions or hematochemical parameters was recorded in male C57BL/6 mice (6–8 weeks-old) after daily oral exposure by gavage, for 5 days, to 60 mg kg−1 of small or large sized rGO. On evaluating the mouse behavior, only a short-term decrease in locomotor activity and impaired neuromuscular coordination were initially observed, without effects on anxiety-like, exploratory, spatial learning and memory behaviors or tissue changes in the hippocampus and neuroglia cells in the brain. Moreover, after daily oral exposure by gavage, for 5 days, to 60 mg kg−1 of 125I-rGO, radioactivity was detected throughout the whole body after one day from the last exposure: in descending order in the kidney, stomach, liver, lung, and blood, indicating a considerable absorption of rGO. The majority of radioactivity decreased 15 or 60 days after the treatment.26A perinatal study on GO (0.05 and 0.5 mg ml−1 in drinking water, from day 1 to 21 after parturition) was carried out in female ICR mice (age/weight not specified) during the lactation period to verify the developmental effects on offspring. Significant perinatal toxic effects were observed and ascribed to the decreased maternal water consumption containing GO during lactation and the reduced milk production.27
In conclusion, after 5-day repeated oral exposure to 125I-rGO, the persistence of radioactivity significantly decreased after 15 or 60 days. A significant absorption was recorded in mice after 1 day from the last exposure, the kidney being the most involved organ.26 These data seem to be in agreement with the nephrotoxicity recorded in rats orally exposed to GO for 5 days.25 A reduced milk production, tentatively consequent to a decreased maternal mouse consumption of GO-containing water, induced significant perinatal effects. However, these studies, limited to GO and rGO, provide insufficient data for the GBMs’ hazard identification and characterization associated with oral exposure.
Cutaneous exposure
Regarding cutaneous toxicity, except for few in vitro studies showing the ability of FLG and GO to penetrate human primary keratinocytes and to exert low cytotoxic effects toward human HaCaT keratinocytes and CRL-2522 fibroblasts,28–31 no data are currently available on GBM effects at the skin level, one of the main barriers between the human body and the environment.The lack of these data hinders the characterization of GBMs’ effects at the skin level.
Ocular exposure
Concerning ocular toxicity, only two studies, limited to GO, were carried out. An acute eye irritation test in New Zealand white female rabbits (6 months-old), carried out according to the OECD TG 405, showed that dripping of GO on the conjunctival sac (10 μg per eye) did not cause local reactions. In contrast, after daily exposure of female Sprague-Dawley rats (3 weeks-old) to the same GO (25–200 ng per eye per day, for 5 days), reversible mild corneal opacity, conjunctival redness and corneal epithelium damage were noted at 100 and 200 ng per eye, which were mitigated by a topical treatment with reduced glutathione as an antioxidant agent.32 Another study in Japanese white rabbits (2–3 kg body weight, gender not specified) showed that single intravitreal injection of GO (100–300 ng per eye), which mimics a physical injury rather than exposure by eye contact, induced negligible effects on eyeball appearance, intraocular pressure, eyesight and electroretinogram up to 49 days, when histological analysis revealed no retinal alteration and a very small amount of residual GO.33 However, the physicochemical characterization of the tested materials is incomplete (Table 4).Although these findings indicate only minor ocular effects, they are not sufficient to draw any conclusion on GBMs’ effects at the ocular level.
Overall, some of these toxicity data suggest potential adverse effects for some materials, but general conclusions on GBMs’ effects cannot be drawn due to the limited solid available toxicity studies on laboratory animals. Consequently, these toxicological data are not sufficient as a starting point also to derive an occupational exposure limit (OEL) for GBMs in working places. Further toxicological studies, tentatively according to validated guidelines, have to be carried out to identify and characterize the hazard posed by GBMs. These studies should consider also other toxic effects, including genotoxicity and carcinogenicity as well as developmental and reproductive toxicity. In fact, although Kim et al. did not record genotoxic effects in the lung tissues of rats repeatedly exposed by inhalation to GNP (5 days, 6 h day−1),22 other in vivo studies after different exposure routes showed the genotoxic potential for some GBMs. In fact, El-Yamany et al.34 observed genotoxicity (DNA damage in the lung cells and chromosomal aberrations in the bone marrow) in male albino mice (strain not specified; 25 g) after the repeated intraperitoneal injection of GO (10–500 μg kg−1, once a week, the number of weeks not specified). A genotoxic effect (micronucleated polychromic erythrocytes) was also recorded after 5 days of repeated intravenous injections of GO to Kunming mice (25–30 g; gender not specified).35 Thus, the occurrence of genotoxic effects after long-term occupational exposure to GO or other GBMs cannot be excluded.
Concerning the developmental and reproductive toxicity, only two studies after oral and intratracheal exposure, respectively, are available. Perinatal toxicity in offspring after oral daily exposure (day 1–21 post parturition) of lactating mice to GO by drinking water (0.05 and 0.5 mg ml−1) was investigated, recording a significant retardation of the body weight, body length and tail length gain in the filial mice after exposure to 0.5 mg GO ml−1 (∼0.8 mg per mouse per day). Moreover, a delayed development of offspring and a decreased length of the intestinal villi were recorded during the lactation period. These effects could be ascribed to the reduced milk production due to the decreased GO-containing water consumption by maternal mice.27 In contrast, no reproductive toxicity in male mice was recorded after intratracheal instillation of GO, once a week for 7 consecutive weeks (18 μg per mouse per instillation; cumulative dose: 126 μg per mouse). No significant changes in epididymal sperm parameters, daily sperm production or testosterone levels were found after GO exposure, suggesting no reproductive toxicity in male mice.19
4. Occupational human exposure
Besides the potential hazard of GBMs, a crucial point of establishing OELs is the accurate and uniform evaluation of the human exposure in working places. Very limited data are currently available on airborne GBM concentrations in occupational settings, whose level depends on the production method and the measures aimed to reduce the exposure. For GNP, the airborne concentration during the collection of products from the discharge vessel was measured at 2.27 and 0.017 mg m−3.36 This concentration range is comparable to that not inducing signs of toxicity after 5 days of repeated nose-only exposure of rats to GNP (0.12–1.88 mg m−3)22 and even lower than that of GO (0.76–9.78 mg m−3).24 Very recently, experimental data, mimicking graphene production using chemical vapor deposition (CVD) in a working place, demonstrate no measurable risk of exposure to airborne graphene in the studied site and only a transient increase in graphene presence during the cleaning of the reactors.16 In another study, the exposure to graphene was monitored in two working places, one using graphite exfoliation and CVD, and the other growing graphene on a copper plate using CVD, which is then transferred to a polyethylene terephthalate sheet. The peak particle number concentration was lower than 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−3, with elemental carbon concentrations mostly below the detection limit, tentatively indicating a very low presence of graphene or of any other particles and very limited exposure.37 In another study, occupational exposure to GBMs by workers during the large-scale production of graphene was assessed. After 8 h average exposure, the particle concentration in air ranged from 909 to 6438 particles per cm3, equivalent to 0.38–3.86 μg cm−3.38 Comparable results were recently recorded for graphene within a study proposing a multi-metric approach based on the harmonized and tiered OECD methodology, in a research and development laboratory.39 All these concentrations are far lower than the inhalation exposure levels to GBMs provoking toxicity in animals, as assessed by inhalation studies (see above).
000 cm−3, with elemental carbon concentrations mostly below the detection limit, tentatively indicating a very low presence of graphene or of any other particles and very limited exposure.37 In another study, occupational exposure to GBMs by workers during the large-scale production of graphene was assessed. After 8 h average exposure, the particle concentration in air ranged from 909 to 6438 particles per cm3, equivalent to 0.38–3.86 μg cm−3.38 Comparable results were recently recorded for graphene within a study proposing a multi-metric approach based on the harmonized and tiered OECD methodology, in a research and development laboratory.39 All these concentrations are far lower than the inhalation exposure levels to GBMs provoking toxicity in animals, as assessed by inhalation studies (see above).
5. Data gap identification
During this literature survey, some gaps in knowledge to assess the safety of GBMs for human health, ranging from incomplete and not homogeneous physicochemical information of the studied materials to the actual human exposure, were identified.6 In particular, despite the use of standardized preparation procedures, GBMs with different physicochemical properties (i.e. lateral size, surface area, shape, aggregation, etc.) can be obtained. These differences are known to affect the toxicological properties of a GBM: for instance, the same material can induce different effects if tested as dispersed particles or as agglomerates, aggregates or agglomerated aggregates. Nevertheless, the physicochemical characterization of the investigated GBM is not reported through standardized parameters in all the toxicological studies (Tables 1–4). Therefore, a correct comparison of the effects recorded for the same type of GBM is not always allowed. Furthermore, some basic information on animals and experimental conditions, which can impair the value of the in vivo toxicological results (i.e. gender, age/weight, strain of animals, dark–light cycle, environmental temperature, etc.), are missing in some studies. It has to be underlined that besides the environmental conditions of the animal house, which could provoke hormonal variations affecting a toxic response, also the intrinsic animal characteristics (i.e. gender, strain, age, etc.) can affect the toxic response.These gaps can be overcome using validated guidelines for nanomaterials, if available (i.e. OECD TG 412 and 413, for inhalation exposure). Nevertheless, it has to be considered that the results obtained following validated guidelines for chemicals cannot always be directly applied to nanomaterials and, consequently, the toxicological evaluation has to be extrapolated with particular care.
Particular attention has to be paid to the exposure routes, focusing on the most suitable animal models mimicking real-life human exposure in occupational settings. For instance, studies after respiratory exposure should be carried out using head–nose or only-nose exposures models, avoiding non-physiological routes (i.e. intratracheal instillation or pharyngeal aspiration).
Further information on the Absorption, Distribution, Metabolism and Excretion (ADME) is also needed. In fact, only two animal studies on GBMs’ ADME related to occupational exposure are available: they are limited to respiratory or oral exposure to FLG, GNP or rGO.13,14,26 Other studies reporting ADME data were carried out after exposure routes not associated with an occupational scenario. In particular, after repeated intraperitoneal injection (8 injections in 4 weeks) in female Wistar/cmdb outbred rats (6 weeks-old), GO (4 mg kg−1) was accumulated as large agglomerates (up to 10 mm) along the injection site, as medium dots (around 2 mm) along the mesentery and as small dots (<1 μm) in the connective and fatty tissues of the liver serosa.40 After an acute intravenous injection of small or large 125I-GO (1 mg kg−1) in male ICR mice (age/weight not specified), a different distribution of the two materials was observed: small GO mainly accumulated in the liver, with few particles in the lungs and spleen, whereas the lungs became the main storage depot for large GO.41
Considering genotoxicity, few contradictory studies in rodents are reported: only one after respiratory exposure to GNP22 and two by intravenous or intraperitoneal injection of GO.34,35
Developmental toxicity should also be more deeply investigated, considering that oral administration of GO to maternal mice during lactation was shown to cause growth retardation in offspring.27 GO was shown to also induce malformations in the embryos of the aquatic vertebrate zebrafish, an alternative model to assess developmental toxicity, incubated in a medium containing GO at concentrations above 1 μg ml−1.42 Similarly, zebrafish embryos exposed to FLG (1–50 μg l−1) up to 96 hours showed significant mortality, delayed hatching, morphological defects, yolk sac edema and pericardial edema.43 Moreover, a single intravenous injection of small rGO or large rGO nanosheets (6.25 or 12.5 mg kg−1) to ICR female mice (6–8 weeks-old) caused maternal death or abortion during the late gestational stage,44 not giving any developmental toxicity indication due to the high administered doses.
In addition, reproductive toxicity data after the usual occupational exposure routes should be implemented, although no adverse effects were observed after repeated intratracheal instillations (one exposure per week for 7 consecutive weeks) of GO in NMRI male mice.19 Similarly, no reproductive adverse effects were observed in ICR male mice after single intravenous (6.25–25 mg kg−1) or 5 days of intraperitoneal injection (up to 60 mg kg−1 day−1) of small GO or large GO.45 No effects were recorded after the intravenous injection of small rGO or large rGO to ICR male and female 6–8 week-old mice (6.25–25.0 mg kg−1).44 No histopathological changes were recorded in the testes of BALB/c mice (6–8 weeks-old) after the intravenous injection of GO (200 μg per mouse)46 or FLG (20 mg kg−1) to Swiss albino mice (4–5 weeks-old).47 In contrast, the repeated intraperitoneal injection of GO (0.4, 2.0 and 10.0 mg kg−1 day−1, 7 or 15 repeated doses on alternate days for 15 or 30 days) to male Wistar rats (10–12 weeks-old) resulted in some adverse effects on the sperms: oxidative stress in the testes and, at the highest dose, also reduced sperm motility, total sperm count, morphological sperm abnormalities and tissues alterations in the testes. Anyway, structure and function alterations in the testes showed a significant recovery within 30 days of recovery period, while the fertility of male rats was not affected after the GO treatment.48 The physicochemical properties of the materials tested in these studies are reported in Table 5.
| Lateral size (nm) | Thickness | Surface area | Chemical composition | Impurity | Density | Aggregation | In vivo exposure route | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| nm | Layers | |||||||||
| iv = intravenous exposure; ip = intraperitoneal exposure. n/a = data not available.a The authors report that the GO suspension was stable for at least 1 month at room temperature. | ||||||||||
| FLG | 160 | 0.8 | 2–4 | n/a | n/a | n/a | n/a | n/a | iv | 47 |
| GO | 8–25 | n/a | n/a | 540–650 m2 g−1 | n/a | n/a | n/a | n/a | ip | 40 |
| 100–500 | 0.9 | 1 | n/a | n/a | n/a | n/a | n/a | iv | 41 | |
| 1000–5000 | n/a | n/a | n/a | n/a | n/a | |||||
| 156.4 | 0.7–1.5 | 1–2 | n/a | n/a | n/a | n/a | n/a | iv | 35 | |
| 300–1000 | 1 | n/a | n/a | n/a | n/a | n/a | Stablea | iv | 46 | |
| 55 | <4 | n/a | n/a | n/a | n/a | n/a | n/a | iv/ip | 45 | |
| 238 | n/a | n/a | n/a | n/a | n/a | n/a | ||||
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | ip | 34 | |
5000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.8–2 | 3–6 | >350 m2 g−1 | C 77.5% | n/a | 0.121 g mL−1 | n/a | ip | 48 | |
| O 16.0% | ||||||||||
| S 0.4% | ||||||||||
| H 1.2% | ||||||||||
| N 4.9% | ||||||||||
| rGO | 68 | n/a | 1–5 | n/a | n/a | n/a | n/a | n/a | iv | 44 |
| 659 | n/a | n/a | n/a | n/a | n/a | n/a | ||||
Furthermore, more data should be acquired on the possible impact of GBMs on the immune system. In fact, GBM accumulation in the macrophages and lung inflammation frequently recorded in rodents after airway exposure to these materials envisage an impact on the immune system. Moreover, several in vitro studies showed significant effects of GBMs on immune cells.49–54 Thus, in vivo studies should be carried out to elucidate the effects of GBMs on the immune system, also considering the use of these materials as biomedical tools.
Particular attention has to be paid to the potential carcinogenic effects of these materials, due to (i) their long persistence in the animal body recorded in the available ADME studies using radiolabeled materials;14,26,41 (ii) the inflammatory/fibrotic effects, observed in the lungs after acute intratracheal instillation or 5 days of repeated inhalation exposure, and the deposition in the lung macrophages, which appeared to be not reversible within 14/90 days recovery.11,13,14,20–24 On the basis of the current available data, carcinogenic effects induced by GBMs cannot be excluded, considering that related materials (some types of multiwalled carbon nanotubes) are classified by the International Agency for Research on Cancer (IARC) within the 2B group (possibly carcinogenic to humans). However, it should be clear that long, aggregated multiwalled carbon nanotubes have a completely different shape as well as mechanical properties, in comparison with graphene. Thus, considering the potential future market of GBMs, information on the potential carcinogenicity of these materials should be gathered for proper risk management to protect human health and environment, in compliance with specific regulations, such as the REACH (Registration, Evaluation, Authorisation and restriction of Chemicals) in the European Union. Sub-chronic and chronic studies following validated guidelines, suitable for regulatory purposes, are also necessary for the hazard identification and characterization of GBMs.
More data are also required for the occupational exposure assessment of GBMs through environmental monitoring studies carried out at the breathing area of the workers as well as by cutaneous dosimetry on the workers’ skin or clothes. Moreover, it has to be kept in mind that the exposure can be complex, involving more than one exposure route. Further exposure monitoring data should include the measurement of GBMs and/or toxicity biomarkers in workers exposed to these materials, until other human data (i.e. case reports, epidemiological studies etc.) are not available.55
All this information is important to define the OELs for GBMs, which could be further adjusted when workers health surveillance and/or other human data will be available.
6. Conclusions
The risk to human health posed by GBMs is associated mainly with an occupational scenario, during their industrial or small-scale production and waste discharge, which can occur mainly by inhalation, ingestion, cutaneous and ocular exposure. The inhalation toxicity data in laboratory animals, especially those obtained by toxicological studies, partially following the OECD guidelines, suggest that acute, 5 days and/or 4 weeks of repeated inhalation exposure to the tested GBMs (FLG, GO and GNP) might induce lung inflammatory/fibrotic reactions. However, these data are not sufficient to determine OELs since the relevant studies have been limited to a maximum of 5 days or 4 weeks of repeated exposure, so far. Anyway, based on the available data, airborne levels of GBMs in occupational settings seem to be lower than those inducing signs of toxicity in animal studies. Nevertheless, it is noteworthy that chronic and/or carcinogenicity studies are not yet available.On the other hand, no conclusions can be drawn for the oral, cutaneous and ocular exposure for which very scanty data, limited to GO or rGO are available, so far.
Thus, more data for the hazard identification and characterization should be acquired by robust sub-chronic and chronic toxicological studies, following the official guidelines for regulatory purposes. If available, validated guidelines specific for nanomaterials have to be used, since those validated for chemicals cannot always be applied to nanomaterials: in this case, the toxicological evaluation has to be extrapolated with particular care. In parallel, environmental monitoring to assess the actual occupational exposure to GBMs should be carried out in working places, in association with workers’ health surveillance. Once occupational exposure to GBMs and their impact on human health are clarified, we believe that identification of high-quality and safe GBMs, produced by the optimized standard procedures, will provide benefits to different industrial sectors and healthcare fields, in compliance with defined regulations, thus improving the possible negative public perception on nanotechnology.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the European Union H2020 Program under grant agreement no. 696656-Graphene Flagship Core1.References
- C. Klaassen, Casarett & Doull's Toxicology: the basic science of poisons, McGraw-Hill, Health Professions Division, New York, 2013 Search PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef PubMed.
- A. Bianco, Angew. Chem., Int. Ed., 2013, 52, 4986–4997 CrossRef PubMed.
- P. Wick, A. E. Louw-Gaume, M. Kucki, H. F. Krug, K. Kostarelos, B. Fadeel, K. A. Dawson, A. Salvati, E. Vázquez, L. Ballerini, M. Tretiach, F. Benfenati, E. Flahaut, L. Gauthier, M. Prato and A. Bianco, Angew. Chem., Int. Ed., 2014, 53, 7714–7718 CrossRef PubMed.
- M. V. D. Z. Park, E. A. J. Bleeker, W. Brand, F. R. Cassee, M. van Elk, I. Gosens, W. H. de Jong, J. A. J. Meesters, W. J. G. M. Peijnenburg, J. T. K. Quik, R. J. Vandebriel and A. J. A. M. Sips, ACS Nano, 2017, 11, 9574–9593 CrossRef PubMed.
- G. Reina, J. M. Gonzalez-Dominguez, A. Criado, E. Vázquez, A. Bianco and M. Prato, Chem. Soc. Rev., 2017, 46, 4400–4416 RSC.
- X. Guo and N. Mei, J. Food Drug Anal., 2014, 22, 105–115 CrossRef PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef PubMed.
- D. Moher, A. Liberati, J. Tetzlaff, D. G. Altman and The PRISMA Group, Ann. Intern. Med., 2009, 151, 264–269 CrossRef PubMed.
- M. Ema, M. Gamo and K. Honda, Regul. Toxicol. Pharmacol., 2017, 85, 7–24 CrossRef PubMed.
- M. C. Duch, G. R. Budinger, Y. T. Liang, S. Soberanes, D. Urich, S. E. Chiarella, L. A. Campochiaro, A. Gonzalez, N. S. Chandel, M. C. Hersam and G. M. Mutlu, Nano Lett., 2011, 11, 5201–5207 CrossRef PubMed.
- A. Schinwald, F. Murphy, A. Askounis, V. Koutsos, K. Sefiane, K. Donaldson and C. J. Campbell, Nanotoxicology, 2014, 8, 824–832 CrossRef PubMed.
- E. J. Park, G. H. Lee, B. S. Han, B. S. Lee, S. Lee, M. H. Cho, J. H. Kim and D. W. Kim, Arch. Toxicol., 2015, 89, 1557–1568 CrossRef PubMed.
- L. Mao, M. Hu, B. Pan, Y. Xie and E. J. Petersen, Part. Fibre Toxicol., 2016, 13, 7 CrossRef PubMed.
- J. R. Roberts, R. R. Mercer, A. B. Stefaniak, M. S. Seehra, U. K. Geddam, I. S. Chaudhuri, A. Kyrlidis, V. K. Kodali, T. Sager, A. Kenyon, S. A. Bilgesu, T. Eye, J. F. Scabilloni, S. S. Leonard, N. R. Fix, D. Schwegler-Berry, B. Y. Farris, M. G. Wolfarth, D. W. Porter, V. Castranova and A. Erdely, Part. Fibre Toxicol., 2016, 13, 34 CrossRef PubMed.
- S. Bengtson, K. B. Knudsen, Z. O. Kyjovska, T. Berthing, V. Skaug, M. Levin, I. K. Koponen, A. Shivayogimath, T. J. Booth, B. Alonso, A. Pesquera, A. Zurutuza, B. L. Thomsen, J. T. Troelsen, N. R. Jacobsen and U. Vogel, PLoS One, 2017, 12, e0178355 CrossRef PubMed.
- J. K. Lee, A. Y. Jeong, J. Bae, J. H. Seok, J. Y. Yang, H. S. Roh, J. Jeong, Y. Han, J. Jeong and W. S. Cho, Arch. Toxicol., 2017, 91, 667–676 CrossRef PubMed.
- R. Li, L. M. Guiney, C. H. Chang, N. D. Mansukhani, Z. Ji, X. Wang, Y. P. Liao, W. Jiang, B. Sun, M. C. Hersam, A. E. Nel and T. Xia, ACS Nano, 2018, 12, 1390–1402 CrossRef PubMed.
- A. Skovmand, A. Jacobsen Lauvås, P. Christensen, U. Vogel, K. Sørig Hougaard and S. Goericke-Pesch, Part. Fibre Toxicol., 2018, 15, 10 CrossRef PubMed.
- L. Ma-Hock, V. Strauss, S. Treumann, K. Küttler, W. Wohlleben, T. Hofmann, S. Gröters, K. Wiench, B. van Ravenzwaay and R. Landsiedel, Part. Fibre Toxicol., 2013, 10, 23 CrossRef PubMed.
- J. H. Shin, S. G. Han, J. K. Kim, B. W. Kim, J. H. Hwang, J. S. Lee, J. H. Lee, J. E. Baek, T. G. Kim, K. S. Kim, H. S. Lee, N. W. Song, K. Ahn and I. J. Yu, Nanotoxicology, 2015, 9, 1023–1031 CrossRef PubMed.
- J. K. Kim, J. H. Shin, J. S. Lee, J. H. Hwang, J. H. Lee, J. E. Baek, T. G. Kim, B. W. Kim, J. S. Kim, G. H. Lee, K. Ahn, S. G. Han, D. Bello and I. J. Yu, Nanotoxicology, 2016, 10, 891–901 CrossRef PubMed.
- S. G. Han, J. K. Kim, J. H. Shin, J. H. Hwang, J. S. Lee, T. G. Kim, J. H. Lee, G. H. Lee, K. S. Kim, H. S. Lee, N. W. Song, K. Ahn and I. J. Yu, BioMed Res. Int., 2015, 2015, 376756 Search PubMed.
- Y. H. Kim, M. S. Jo, J. K. Kim, J. H. Shin, J. E. Baek, H. S. Park, H. J. An, J. S. Lee, B. W. Kim, H. P. Kim, K. H. Ahn, K. Jeon, S. M. Oh, J. H. Lee, T. Workman, E. M. Faustman and I. J. Yu, Nanotoxicology, 2018, 1, 1–15 Search PubMed.
- A. K. Patlolla, J. Randolph, S. A. Kumari and P. B. Tchounwou, Int. J. Environ. Res. Public Health, 2016, 13, 380 CrossRef PubMed.
- D. Zhang, Z. Zhang, Y. Liu, M. Chu, C. Yang, W. Li, Y. Shao, Y. Yue and R. Xu, Biomaterials, 2015, 68, 100–113 CrossRef PubMed.
- C. Fu, T. Liu, L. Li, H. Liu, Q. Liang and X. Meng, Biomaterials, 2015, 40, 23–31 CrossRef PubMed.
- K. H. Liao, Y. S. Lin, C. W. Macosko and C. L. Haynes, ACS Appl. Mater. Interfaces, 2011, 3, 2607–2615 CrossRef PubMed.
- Y. Li, H. Yuan, A. von dem Bussche, M. Creighton, R. H. Hurt, A. B. Kane and H. Gao, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12295–12300 CrossRef PubMed.
- M. Pelin, L. Fusco, V. León, C. Martín, A. Criado, S. Sosa, E. Vázquez, A. Tubaro and M. Prato, Sci. Rep., 2017, 7, 40572 CrossRef PubMed.
- M. Pelin, L. Fusco, C. Martín, S. Sosa, J. Frontiñan-Rubio, J. M. Gonzalez-Dominguez, M. Duran, E. Vázquez, M. Prato and A. Tubaro, Nanoscale, 2018, 10, 11820–11830 RSC.
- W. Wu, L. Yan, Q. Wu, Y. Li, Q. Li, S. Chen, Y. Yang, Z. Gu, H. Xu and Z. Q. Yin, Nanotoxicology, 2016, 10, 1329–1340 CrossRef PubMed.
- L. Yan, Y. Wang, X. Xu, C. Zeng, J. Hou, M. Lin, J. Xu, F. Sun, X. Huang, L. Dai, F. Lu and Y. Liu, Chem. Res. Toxicol., 2012, 25, 1265–1270 Search PubMed.
- N. A. El-Yamany, F. F. Mohamed, T. A. Salaheldin, A. A. Tohamy, W. N. Abd El-Mohsen and A. S. Amin, Exp. Toxicol. Pathol., 2017, 69, 383–392 CrossRef PubMed.
- Y. Liu, Y. Luo, J. Wu, Y. Wang, X. Yang, R. Yang, B. Wang, J. Yang and N. Zhang, Sci. Rep., 2013, 3, 3469 CrossRef PubMed.
- W. A. Heitbrink, L. M. Lo and K. H. Dunn, J. Occup. Environ. Hyg., 2015, 12, 16–28 CrossRef PubMed.
- J. H. Lee, H. H. Han, J. H. Kim, B. Kim, D. Bello, J. K. Kim, G. H. Lee, E. K. Sohn, K. Lee, K. Ahn, E. M. Faustman and I. J. Yu, Inhalation Toxicol., 2016, 28, 281–291 CrossRef PubMed.
- A. Spinazzè, A. Cattaneo, D. Campagnolo, V. Bollati, P. A. Bertazzi and D. M. Cavallo, Aerosol Sci. Technol., 2016, 50, 812–821 CrossRef.
- F. Boccuni, R. Ferrante, F. Tombolini, D. Lega, A. Antonini, A. Alvino, P. Pingue, F. Beltram, L. Sorba, V. Piazza, M. Gemmi, A. Porcari and S. Iavicoli, Int. J. Mol. Sci., 2018, 19, E349 CrossRef PubMed.
- N. Kurantowicz, B. Strojny, E. Sawosz, S. Jaworski, M. Kutwin, M. Grodzik, M. Wierzbicki, L. Lipińska, K. Mitura and A. Chwalibog, Nanoscale Res. Lett., 2015, 10, 398 CrossRef PubMed.
- J. H. Liu, S. T. Yang, H. Wang, Y. Chang, A. Cao and Y. Liu, Nanomedicine, 2012, 7, 1801–1812 CrossRef PubMed.
- Y. Chen, X. Hu, J. Sun and Q. Zhou, Nanotoxicology, 2016, 10, 42–52 Search PubMed.
- B. Manjunatha, S. H. Park, K. Kim, R. R. Kundapur and S. J. Lee, Environ. Sci. Pollut. Res. Int., 2018, 25, 12821–12829 CrossRef PubMed.
- S. Xu, Z. Zhang and M. Chu, Biomaterials, 2015, 54, 188–200 CrossRef PubMed.
- S. Liang, S. Xu, D. Zhang, J. He and M. Chu, Nanotoxicology, 2015, 9, 92–105 CrossRef PubMed.
- G. Qu, X. Wang, Q. Liu, R. Liu, N. Yin, J. Ma, L. Chen, J. He, S. Liu and G. Jiang, J. Environ. Sci., 2013, 25, 873–881 CrossRef.
- A. Sasidharan, S. Swaroop, C. K. Koduri, C. Madathil Girish, P. Chandran, L. Panchakarla, V. H. Somasundaram, G. S. Gowd, S. Nair and M. Koyakutty, Carbon, 2015, 95, 511–524 CrossRef.
- N. K. Nirmal, K. K. Awasthi and P. J. John, Basic Clin. Pharmacol. Toxicol., 2017, 121, 202–210 CrossRef PubMed.
- M. Orecchioni, C. Ménard-Moyon, L. G. Delogu and A. Bianco, Adv. Drug Delivery Rev., 2016, 105, 163–175 CrossRef PubMed.
- M. Orecchioni, D. A. Jasim, M. Pescatori, R. Manetti, C. Fozza, F. Sgarrella, D. Bedognetti, A. Bianco, K. Kostarelos and L. G. Delogu, Adv. Healthcare Mater., 2016, 5, 276–287 CrossRef PubMed.
- M. Orecchioni, D. Bedognetti, F. Sgarrella, F. M. Marincola, A. Bianco and L. G. Delogu, J. Transl. Med., 2014, 12, 138 CrossRef PubMed.
- S. P. Mukherjee, K. Kostarelos and B. Fadeel, Adv. Healthcare Mater., 2018, 7, 1700815 CrossRef PubMed.
- S. P. Mukherjee, M. Bottini and B. Fadeel, Front. Immunol., 2017, 8, 673 CrossRef PubMed.
- J. Yan, L. Chen, C. C. Huang, S. C. Lung, L. Yang, W. C. Wang, P. H. Lin, G. Suo and C. H. Lin, Colloids Surf., B, 2017, 153, 300–309 CrossRef PubMed.
- E. Nielsen, G. Østergaard and J. C. Larsen, Toxicological risk assessment of chemicals. A practical guide, Informa Healthcare, New York, 2008 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |