Dynamic NMR for coordination chemistry
Abstract
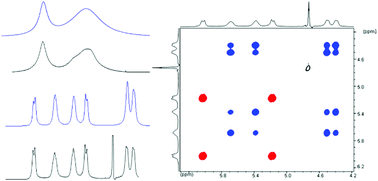
The emphasis of this mini review is on the representation of dynamic NMR methods to obtain kinetic and mechanistic information on the equilibria of metal complexes in aqueous solution. A brief discussion of the basic aspects of NMR relaxation and the effect of chemical exchange on that providing information on the dynamics of equilibrium is given. The experimental and computational tools for extracting kinetics from dynamic data are also described, underlining the importance of a multinuclear approach. It reviews some theoretical background citing useful books and papers on the chemical applications of the most often used dynamic NMR methods. It focuses on the use of these methods for elucidating reaction pathways, and mechanisms and for determination of kinetic parameters, through examples, rather than on the technical and theoretical details or on an exhaustive survey of the present literature. The usefulness of the multinuclear NMR approach in this context is illustrated quoting cases when it has been applied. Most of the concrete experimental data refer to the authors own work on the kinetics of lanthanide, uranium and thallium complexes; however, the general aspects are always emphasized. The aim of this paper is to encourage coordination chemists to employ NMR and discover that the dynamic aspects of this very powerful technology are exactly made for coordination chemistry.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...