Exploring the chelating capacity of 2-hydroxyphenyl-benzimidazole based hybrids with multi-target ability as anti-Alzheimer's agents
Abstract
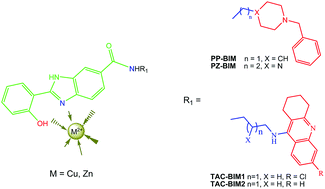
Alzheimer's disease (AD) is the most common (60–70%) form of dementia in the elderly population. Its complex and multifactorial nature requires the development of drugs capable of hitting several disease targets, such as cholinergic dysfunction, oxidative stress, deposits of amyloid-β (Aβ) and metal ion dyshomeostasis. Two series of hybrids, mimetics of donepezil (DNP) and tacrine (TAC), containing a 2-hydroxyphenyl-benzimidazole (BIM) chelating moiety (DNP-BIM and TAC-BIM), were formerly developed and found to exhibit multi-target ability as anti-AD compounds. Due to the recognized role of metal ions as age triggers of AD, namely responsible for oxidative stress and Aβ aggregation, the copper and zinc chelating capacity is herein evaluated for two DNP-BIM hybrids (PP-BIM and PZ-BIM) and one TAC-BIM (TAC-BIM1) hybrid, as well as the role of copper in their Aβ aggregation inhibitory capacity. The compounds exhibit good chelating capacity towards Cu(II) (pCu ∼ 11) and moderate towards Zn(II) (pZn ∼ 6) in a 50% w/w DMSO/water medium, with the formation of 1 : 1 (MHL) and 1 : 2 (MH2L2, ML2 and MH−1L2) complex species involving the phenolic oxygen and the imidazole nitrogen N(3) of the BIM moiety in the coordination shell. All hybrids are able to improve the inhibition of self-induced Aβ aggregation, probably by ligand intercalation between the β-sheets of Aβ fibrils, with markedly higher inhibitory capacity for the tacrine conjugates than for the donepezil conjugates. Nevertheless, the compounds do no’t seem able to retrieve Cu(II) from Aβ peptide and so they may have no relevant role in Cu(II)-induced-Aβ aggregation.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...