A new tripodal-3-hydroxy-4-pyridinone for iron and aluminium sequestration: synthesis, complexation and in vivo studies†
Abstract
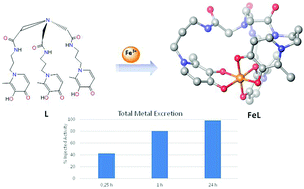
Elevated levels of iron and aluminium in the body can lead to tissue damage, organ failure and eventually death. To reduce complications of metal overload, specific chelating agents have been used. Following our recent developments in a series of hexadentate 3-hydroxy-4-pyridinones (3,4-HP) with high M3+ sequestering capacity, we herein present a novel tripodal homologue, whose design involves one methylene truncation on the linkers. This truncation aimed to reduce the ligand molecular weight and concomitant improvement in the membrane crossing ability and accessibility to cytoplasmic iron pools, but still retaining the capacity for the formation of a 1 : 1 (M3+ : L) complex with high thermodynamic stability and kinetic inertness. Besides the synthesis of a new ligand, solution studies have been performed to evaluate the acid–base properties and complexation capacity towards Fe(III) and Al(III) using potentiometry and a panoply of spectrometric techniques. The pFe and pAl values show some decrease, as compared with the non-truncated homologues, but still represent an improvement of, respectively, 7- and 4-orders of magnitude, as compared to the marketed drug deferiprone. The ability of this new ligand to facilitate metal-mobilization from the body was investigated using a mice model injected with 67Ga. The new chelator possessing high pM3+ values shows promising ability to remove Fe and Al under in vivo conditions.
- This article is part of the themed collection: Equilibrium Solution Coordination Chemistry


 Please wait while we load your content...
Please wait while we load your content...