Synthesis of formamidinium lead halide perovskite nanocrystals through solid–liquid–solid cation exchange†
Abstract
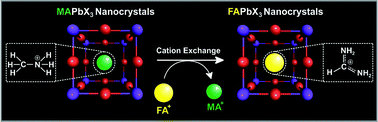
Hybrid organic–inorganic perovskites (HOIPs) have emerged as promising materials for applications in solar energy harvesting as well as in optoelectronic devices. Here, we report the first demonstration of cation exchange on HOIP nanocrystals (NCs). In this reaction, methylammonium cations are replaced in methylammonium lead halide (MAPbX3) NCs by formamidinium cations through a solid–liquid–solid cation exchange reaction. X-ray diffraction and optical characterizations allowed for the close monitoring of this process. Through this new type of cation-exchange reaction, formamidinium lead halide (FAPbX3) NCs with various halide compositions were synthesized by altering the starting material. This allowed for the formation of FAPbX3 HOIP NCs with a wide range of emissions spanning from 395 nm to 700 nm.
- This article is part of the themed collections: 2017 Journal of Materials Chemistry C HOT Papers and Journal of Materials Chemistry C Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...