Capsaicin presynaptically inhibits glutamate release through the activation of TRPV1 and calcineurin in the hippocampus of rats
Abstract
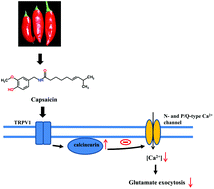
Capsaicin is the major ingredient in hot peppers of the plant Capsicum genus with neuroprotective effects in several preclinical models; its effect on glutamate release has been investigated in the rat hippocampus using isolated nerve terminals (synaptosomes) and brain slices. In a synaptosomal preparation, capsaicin dose-dependently reduced 4-aminopyridine-evoked Ca2+-dependent glutamate release, with an IC50 of approximately 11 μM. This inhibition was blocked by capsazepin, an antagonist of TRPV1, which was found to be colocalized with the vesicle marker protein synaptophysin in synaptosomes using double immunostaining. Capsaicin decreased 4-aminopyridine-evoked intrasynaptosomal Ca2+ concentration elevation and the capsaicin-mediated inhibition of glutamate release was prevented by the Cav2.2 (N-type) and Cav2.1 (P/Q-type) channel blocker ω-conotoxin MVIIC, but was not affected by the intracellular Ca2+-release inhibitors dantrolene and CGP37157. Furthermore, capsaicin increased the 4-aminopyridine-induced phosphorylation of protein phosphatase calcineurin and the calcineurin inhibitor cyclosporine A eliminated the inhibitory effect of capsaicin on evoked glutamate release. Additionally, capsaicin also reduced the frequency of miniature excitatory postsynaptic currents without affecting their amplitude in slice preparations. Together, these results suggest that capsaicin acts at TRPV1 present on hippocampal nerve terminals to increase calcineurin activation, which subsequently attenuates voltage-dependent Ca2+ entry to cause a decrease in evoked glutamate release.


 Please wait while we load your content...
Please wait while we load your content...