Understanding the structure–activity relationship between quercetin and naringenin: in vitro
Abstract
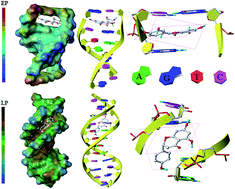
Quercetin and naringenin are polyphenolic flavonoids obtained from plant sources. A comparative study of their structure–activity relationships (SAR) has been carried out by multiple methods. Spectroscopy experiments show that quercetin intercalates into the double helix of deoxyribonucleic acid (DNA), while naringenin displays a groove binding mode. Molecular docking studies visually display different binding modes, interaction forces and binding regions while electrochemistry experiments indicate the main factor that effects the DNA–drug interaction during the electrochemical process. Antioxidant assay was performed to find out the difference in their anti-oxidative ability. The results obtained from all these experimentations illustrate that the presence of a double bond in ring A of quercetin is very important to maintain its structural planarity, which influences its binding mode, the main interaction force and the control step in electrochemistry experiments. Quercetin showed better antioxidant properties as compared to naringenin which may be attributed to the presence of a greater number of hydroxyl groups. These findings give an understanding of their structure–activity relationships which may be helpful in the design of analogs of these flavonoids and their application in drug and food industries.

 Please wait while we load your content...
Please wait while we load your content...