A composite of Co nanoparticles highly dispersed on N-rich carbon substrates: an efficient electrocatalyst for Li–O2 battery cathodes†
Zhang
Zhang
,
Liwei
Su
,
Mei
Yang
,
Meng
Hu
,
Jie
Bao
,
Jinping
Wei
and
Zhen
Zhou
*
Tianjin Key Laboratory of Metal and Molecule Based Material Chemistry, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Institute of New Energy Material Chemistry, Synergetic Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, China. E-mail: zhouzhen@nankai.edu.cn; Fax: +86 22 23498941; Tel: +86 22 23503623
First published on 11th November 2013
Abstract
In this work, we present a facile sol–gel method to prepare a composite of Co nanoparticles highly dispersed on N-rich carbon substrates (Co–C composite). The assembled Li–O2 batteries with the composite as a cathode catalyst showed lower overpotential and better cyclability, and the improved performance may be attributed to the superior electrocatalytic activity of the Co–C composite.
Rechargeable Li–O2 batteries have been attracting much attention due to their extremely high theoretical specific energy density of ∼5200 W h kg−1 (including O2) compared with other energy storage systems.1 However, high overpotentials during charge lead to poor round-trip efficiency and cyclability.2 Also, the instability of organic electrolytes is a barrier for the rechargeable Li–O2 batteries, and it is imperative that we search for a suitable electrolyte for rechargeable Li–O2 batteries.3,4 Frequently-used carbonate or linear-chain ether electrolytes cannot satisfy the demand for practical Li–O2 batteries along with the incomplete discharge products (Li2CO3, HCO2Li, and CH3CO2Li).5–7 Tetraethylene glycol dimethyl ether (TEGDME) can be employed as the electrolyte solvent due to its relatively high stability towards superoxide ions (O2−).8
Even though an ideal stable electrolyte has been realized, the cathode catalysts still remain a big challenge. Much attention has been paid to exploring cathode catalysts to improve their energy storage efficiency. Traditional catalysts include porous carbon materials, transition metal oxides and nitrides, precious metals,9–13 and perovskite- or pyrochlore-based compounds.14–17 Very recently, a TiC-based cathode has emerged for rechargeable aprotic Li–O2 batteries.18 The charge voltage can be remarkably reduced when adding an efficient catalyst to the cathode.
As for carbon materials, Ketjen black (KB) showed decent performances of oxygen reduction reactions (ORR) in non-aqueous electrolytes. Graphene and carbon nanotubes have also been identified as excellent electrocatalysts in the ORR and OER (oxygen evolution reaction) processes due to their large specific area and high electronic conductivity.19,20 Especially, when hierarchical porous graphene was applied to Li–O2 batteries, an extraordinary discharge capacity of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mA h g−1 (carbon) was obtained.21
000 mA h g−1 (carbon) was obtained.21
Transition metal oxides are also promising catalysts for ORR and OER processes because of their low cost and environmental friendliness.22 Nazar and coworkers utilized Co3O4 on reduced graphene oxide (Co3O4/RGO) as the electrocatalyst, which delivered a cut-off capacity of 6000 mA h g−1 (carbon) and continued for 7 cycles.2 Dong's group reported that MnO2 directly coated on graphene showed predominant performance with a low charge voltage (3.9 V) and a cut-off capacity of 2900 mA h g−1 (carbon) for 25 cycles.12 Although the transition metal oxides displayed good performance, the decrease of the overpotential should be further enhanced to the degree comparable to precious metals (platinum–gold nanoparticles).13 Therefore, it is still a big challenge to explore new catalysts that significantly increase the coulombic efficiency and cyclic stability of Li–O2 batteries.
In this work, we prepared a composite with Co nanoparticles highly dispersed on N-rich carbon substrates (including carbon nanosheets and nanotubes) through a simple and economical sol–gel method (Co–C composite). The composite has good electrocatalytic activity in the cathodes of Li–O2 batteries in non-aqueous electrolytes. We demonstrated that the Co/C electrocatalyst could significantly reduce the overpotential and enhance the cyclic stability of rechargeable Li–O2 batteries.
The morphology of the as-prepared Co–C composite was characterized using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The sample consists of large carbon nanosheets (Fig. 1a). The high-magnification SEM image (Fig. 1b) shows an interesting structure where hundreds of “carpenterworms”, carbon nanotubes, overspread the carbon nanosheets. From the energy dispersive X-ray spectra (EDX, Fig. S1, ESI†), it can be seen that the carbon nanosheets contain abundant Co. Also, the carbon nanotubes contain even more Co (inset in Fig. S1, ESI†). The nano-sized Co particles are shown in Fig. 1c; they are uniformly dispersed on the carbon nanosheets, with a size of 20–60 nm. Notably, the Co nanoparticles are partly embedded into the carbon nanotubes, and the EDX also verifies the phenomenon. The high-magnification TEM image clearly presents the lattice fringes of the (220) plane of the Co nanoparticles, corresponding to the d-spacing of 1.25 nm (Fig. 1d). Around the Co nanoparticles are disordered carbon and few-layered graphene.
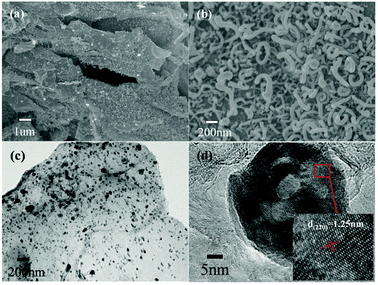 | ||
| Fig. 1 SEM images (a, b) with different magnifications of Co–C composite. Low magnification (c) and high magnification (d) TEM images of Co–C composite. | ||
The crystal structure of the Co–C composite was examined using X-ray diffraction (XRD). Fig. 2a shows the XRD patterns of the composite. Aside from the peak of the standard graphite, three broad peaks at 2θ around 44.2°, 51.5° and 75.8° are observed, and are well assigned to the (111), (200), and (220) planes of cubic Co (JCPDS 1-1255). The composite was further characterized by using the N2 adsorption–desorption experiments (Fig. 2b). The specific surface area reaches 325.5 m2 g−1. The pore size distribution can be observed in the inset. The formation of pores may be caused by the release of mixed gas (CO2, H2O, and NH3). The sol–gel method is economical and environmentally benign without any surfactants or toxic materials, and urea plays a crucial role in the formation of highly-dispersed Co nanoparticles on the carbon substrates. Notably, due to the high-temperature treatment with the release of gases, the Co nanoparticles can be highly dispersed on the porous substrate. This feature is critical to composite catalysts. Interestingly, the carbon materials also contained rich N from the decomposed urea (Fig. S1, ESI†). X-ray photoelectron spectroscopy (XPS) confirms the existence of N and C elements. Different C-containing groups can be characterized by the presence of three peaks at 284.8 eV, 285.8 eV, and 288.6 eV, well assigned to the sp2 C–sp2 C, N–sp2 C, and N–sp3 C bonds, respectively (Fig. 2c). The N1s spectra demonstrate the coexistence of two N species: pyridinic N at 398.3 eV and pyrrolic N at 400.3 eV (Fig. 2d).23 N-doping would benefit the homogeneous dispersion of metal nanoparticles.24 This method may provide us a new strategy to optimize the composition and structure of the carbon cathode, and then improve its reactivity in Li–O2 batteries.
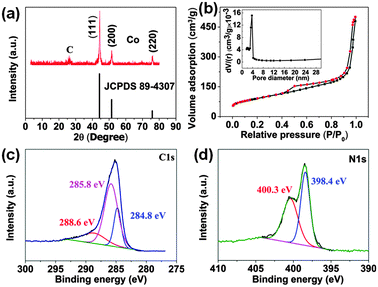 | ||
| Fig. 2 (a) XRD patterns of Co–C composite. (b) N2 adsorption–desorption isotherms, and inset is the pore-size distribution of Co–C composite. (c) C1s and (d) N1s XPS of N-containing carbon materials. | ||
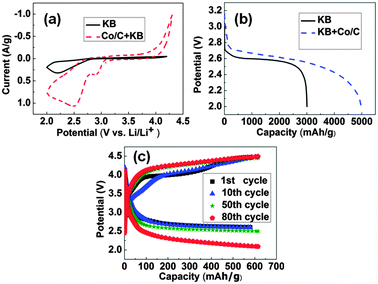
Firstly, the electrocatalytic activity of the composite was investigated in the cathode of Li–O2 batteries, compared with the KB electrode without any catalyst. Both the applied current density (mA g−1) and achieved capacity (mA h g−1) were calculated on the total mass of KB and catalysts (if any). We utilized cyclic voltammetry (CV) to explore the ORR catalytic activity of the Co/C catalyst in the TEGDME electrolyte from 4.3 to 2 V (Fig. 3a). Compared with the electrode without the catalyst, the Co/C-based electrode exhibited a higher ORR onset potential (∼2.9 V) and a more positive current peak. The typical charge–discharge profiles are shown in Fig. 3b. The discharge capacity of the Co/C electrode was ∼5000 mA h g−1, which is much higher than that of the KB electrode (∼3000 mA h g−1) at the current density of 300 mA g−1. The cyclability is one of the most important indices in non-aqueous Li–O2 batteries, which could be promoted when utilizing a stable electrolyte and an effective catalyst. For the cyclic tests, the cells were discharged and charged with the cut-off capacity of 600 mA h g−1 at a current density of 200 mA g−1, and the cells showed good performances over 80 cycles with stable discharge–charge voltage platforms (Fig. 3c). It is difficult to compare the Co–C composite with other catalysts, such as Fe–N–C composites,25 platinum–gold nanoparticles,13 Co oxides,26 especially, and nitrogen-doped graphene catalysts (with carbon nanotubes and Co9S8),27 due to different measurement systems. Compared with the KB electrode under the same conditions (Fig. S2, ESI†), the Co/C-based electrode showed little apparent increase in overpotentials up to 50 charge–discharge cycles, demonstrating much better cyclability. To further explore and gain insight into discharge and charge processes of Li–O2 batteries with Co/C based cathodes, we used XRD and SEM to analyze the discharged and subsequent charged products during the 5th cycle. According to the XRD patterns (Fig. S3a, ESI†), the peaks of Li2O2 could be clearly observed in the discharged electrode. After charging to 4.3 V, the Li2O2 characteristic peaks nearly disappeared, indicating the reversibility of most Li2O2 formation and decomposition. However, there still existed some obscure Li2O2 diffraction peaks after the charging process, probably because some lithium compounds such as Li2CO3 and LiOH accumulated at the cathode and reduced the catalytic activity, leading to the existence of Li2O2 in the cathode.28,29 Before electrochemical tests, the Co/C catalyst and KB particles were observed in the as-prepared electrode (Fig. S3b, ESI†). After discharge, many small particles aggregated on the surface of the cathode, which are considered to be nanocrystalline Li2O2 (Fig. S3c, ESI†). When the electrode was recharged, a relatively clean cathode was observed, and the cathode recovered to the pristine morphology (Fig. S3d, ESI†). Nevertheless, the presence of the Co/C catalyst benefits the formation and decomposition of Li2O2, leading to the improved cycle performance.
The excellent electrochemical performances of the Co–C composite are attributed to the highly-dispersed Co nanoparticles on N-rich carbon substrates. The Co nanoparticles are in tight contact with the carbon substrates, and the ultrathin carbon nanosheets offer favorable support to activate the catalytic activity of the Co nanoparticles in the discharge–charge processes. The existence of N in carbon substrates can enhance the dispersion of Co nanoparticles, and might also have some individual catalytic activity, which is being investigated separately.
In summary, Co–C composites were synthesized through a facile and convenient sol–gel route. Li–O2 batteries with Co–C composites as cathode electrocatalysts exhibited improved performances. We believe that the composites based on Co nanoparticles and N-rich carbon substrates are promising electrocatalysts for Li–O2 batteries.
This work was supported by the Research Fund for the Doctoral Program of Higher Education of China (20120031110008).
Notes and references
- K. M. Abraham and Z. Jiang, J. Electrochem. Soc., 1996, 143, 1 CrossRef CAS PubMed.
- R. Black, J. H. Lee, B. Adams, C. A. Mims and L. F. Nazar, Angew. Chem., Int. Ed., 2013, 52, 392 CrossRef CAS PubMed.
- F. Li, T. Zhang, Y. Yamada, A. Yamada and H. Zhou, Adv. Energy Mater., 2012, 3, 532 CrossRef.
- D. Xu, Z. L. Wang, J. J. Xu, L. L. Zhang, L. M. Wang and X. B. Zhang, Chem. Commun., 2012, 48, 11674 RSC.
- T. Ogasawara, A. Debart, M. Holzapfel, P. Novak and P. G. Bruce, J. Am. Chem. Soc., 2006, 128, 1390 CrossRef CAS PubMed.
- S. A. Freunberger, Y. Chen, Z. Peng, J. M. Griffin, L. J. Hardwick, F. Barde, P. Novak and P. G. Bruce, J. Am. Chem. Soc., 2011, 133, 8040 CrossRef CAS PubMed.
- S. A. Freunberger, Y. Chen, N. E. Drewett, L. J. Hardwick, F. Barde and P. G. Bruce, Angew. Chem., Int. Ed., 2011, 50, 8609 CrossRef CAS PubMed.
- B. D. McCloskey, D. S. Bethune, R. M. Shelby, G. Girishkumar and A. C. Luntz, J. Phys. Chem. Lett., 2011, 2, 1161 CrossRef CAS.
- H. D. Lim, K. Y. Park, H. Song, E. Y. Jang, H. Gwon, J. Kim, Y. H. Kim, M. D. Lima, R. O. Robles, X. Lepro, R. H. Baughman and K. Kang, Adv. Mater., 2013, 25, 1348 CrossRef CAS PubMed.
- R. R. Mitchell, B. M. Gallant, C. V. Thompson and Y. Shao-Horn, Energy Environ. Sci., 2011, 4, 2952 CAS.
- A. Debart, A. J. Paterson, J. Bao and P. G. Bruce, Angew. Chem., Int. Ed., 2008, 47, 4521 CrossRef CAS PubMed.
- Y. Cao, Z. Wei, J. He, J. Zang, Q. Zhang, M. Zheng and Q. Dong, Energy Environ. Sci., 2012, 5, 9765 CAS.
- Y. C. Lu, Z. Xu, H. A. Gasteiger, S. Chen, K. Hamad-Schifferli and Y. Shao-Horn, J. Am. Chem. Soc., 2010, 132, 12170 CrossRef CAS PubMed.
- F. Li, R. Ohnishi, Y. Yamada, J. Kubota, K. Domen, A. Yamada and H. Zhou, Chem. Commun., 2013, 49, 1175 RSC.
- J. J. Xu, D. Xu, Z. L. Wang, H. G. Wang, L. L. Zhang and X. B. Zhang, Angew. Chem., Int. Ed., 2013, 52, 3887 CrossRef CAS PubMed.
- W. Yang, J. Salim, S. Li, C. Sun, L. Chen, J. B. Goodenough and Y. Kim, J. Mater. Chem., 2012, 22, 18902 RSC.
- S. H. Oh, R. Black, E. Pomerantseva, J. H. Lee and L. F. Nazar, Nat. Chem., 2012, 4, 1004 CrossRef CAS PubMed.
- M. M. Ottakam Thotiyl, S. A. Freunberger, Z. Peng, Y. Chen, Z. Liu and P. G. Bruce, Nat. Mater., 2013, 12, 1050 CrossRef CAS PubMed.
- Y. Li, J. Wang, X. Li, D. Geng, R. Li and X. Sun, Chem. Commun., 2011, 47, 9438 RSC.
- G. Q. Zhang, J. P. Zheng, R. Liang, C. Zhang, B. Wang, M. Hendrickson and E. J. Plichta, J. Electrochem. Soc., 2010, 157, A953 CrossRef CAS PubMed.
- J. Xiao, D. Mei, X. Li, W. Xu, D. Wang, G. L. Graff, W. D. Bennett, Z. Nie, L. V. Saraf, I. A. Aksay, J. Liu and J. G. Zhang, Nano Lett., 2011, 11, 5071 CrossRef CAS PubMed.
- A. Débart, J. Bao, G. Armstrong and P. G. Bruce, J. Power Sources, 2007, 174, 1177 CrossRef PubMed.
- Z. H. Sheng, L. Shao, J. J. Chen, W. J. Bao, F. B. Wang and X. H. Xia, ACS Nano, 2011, 5, 4350 CrossRef CAS PubMed.
- L. Su, Z. Zhou and P. Shen, J. Phys. Chem. C, 2012, 116, 23974 CAS.
- J. L. Shui, N. K. Karan, M. Balasubramanian, S. Y. Li and D. J. Liu, J. Am. Chem. Soc., 2012, 134, 16654 CrossRef CAS PubMed.
- W. Yang, J. Salim, C. Ma, Z. Ma, C. Sun, J. Li, L. Chen and Y. Kim, Electrochem. Commun., 2013, 28, 13 CrossRef CAS PubMed.
- G. Wu, N. H. Mack, W. Gao, S. Ma, R. Zhong, J. Han, J. K. Baldwin and P. Zelenay, ACS Nano, 2012, 6, 9764 CrossRef CAS PubMed.
- Y. Chen, S. A. Freunberger, Z. Peng, F. Barde and P. G. Bruce, J. Am. Chem. Soc., 2012, 134, 7952 CrossRef CAS PubMed.
- R. Black, S. H. Oh, J. H. Lee, T. Yim, B. Adams and L. F. Nazar, J. Am. Chem. Soc., 2012, 134, 2902 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, EDX of carbon nanosheets and carbon nanotubes, XRD and SEM of the discharged and charged cathode during the 5th cycle. See DOI: 10.1039/c3cc47149g |
| This journal is © The Royal Society of Chemistry 2014 |