Open Access Article
Open Access ArticleSynthesis of P2C2O2 and P2CO via NHC-mediated coupling of the phosphaethynolate anion†
Robert J.
Gilliard
Jr.
 ab,
Riccardo
Suter
ab,
Riccardo
Suter
 b,
Erik
Schrader
b,
Erik
Schrader
 b,
Zoltán
Benkő
b,
Zoltán
Benkő
 c,
Arnold L.
Rheingold
c,
Arnold L.
Rheingold
 d,
Hansjörg
Grützmacher
d,
Hansjörg
Grützmacher
 *b and
John D.
Protasiewicz
*b and
John D.
Protasiewicz
 *a
*a
aDepartment of Chemistry, Case Western Reserve University, Cleveland, OH 44106, USA. E-mail: Protasiewicz@case.edu
bDepartment of Chemistry and Applied Biosciences, ETH Zurich, CH-8093 Zurich, Switzerland. E-mail: hgruetzmacher@ethz.ch
cBudapest University of Technology and Economics, H.111 Budapest Szent Gellért tér 4, Hungary
dDepartment of Chemistry, University of California, San Diego, La Jolla, CA 92093, USA
First published on 27th October 2017
Abstract
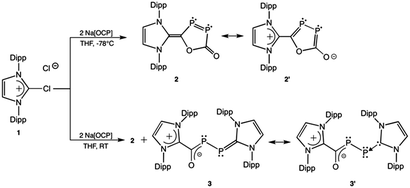
The reaction of the chloroimidazolium chloride salt, [NHC-Cl][Cl], NHC = C{N(2,6-iPr2C6H3)CH}2 (1) with two equivalents of sodium phosphaethynolate, Na[OCP]·(dioxane)2.5, results in the formation of NHC-{cyclo-(CO)-P2-C(O)} (2) and NHC-P2-C(O)-NHC (3). Notably, in the presence of free NHC ligand, compound 2 converts to compound 3via extrusion of CO at elevated temperatures. The nature of the bonding in these complexes was probed computationally and spectroscopically.
Over the past decade, a large number of main group atoms and units have been isolated using stable carbenes.1 These fascinating molecules have provided insight into fundamental issues of structure and bonding and have spawned new applications in main group chemistry. Prominent examples germane to this report include main group diatomics and unusual heterocycles containing CO functionalities. Indeed, Robinson and coworkers showed that P2 could be stabilized by potassium graphite reduction of an NHC-PCl3 adduct to afford NHC-P2-NHC A (Fig. 1).2 Compound A reacts with molecular oxygen to form a molecule containing a diphosphorus tetroxide unit, NHC-P(O)–P(O)-NHC.3 Bertrand et al. prepared bis-[cyclic(alkyl)amino carbene] diphosphorus complex B (CAAC-P2-CAAC) by the direct reaction of CAAC with P4 and demonstrated its reactivity in the formation of carbene-stabilized P2-radical cation and P2-dication.4 Recently, Braunschweig and coworkers utilized an NHC-stabilized diboryne5 to generate bis(boroketene) C.6 Compound C could be converted to the bicyclic bis(boralactone) D in the presence of CO.7 As CO coordination to main group elements is rare, the latter examples (C and D) serve as important contributions to the discussion of main group molecules that mimic bonding arrangements typically associated with transition metals.7,8
 | ||
| Fig. 1 Examples of NHC-stabilized main group species.2,4,6 | ||
Recently, we have been exploring the chemistry of sodium phosphaethynolate, Na[OCP], which can be viewed as a hybrid of three resonance structures, a phosphaethynolate [O–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) P]−, a phosphaketenide [O
P]−, a phosphaketenide [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P]− and a P-transfer reagent [O
P]− and a P-transfer reagent [O![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C→P]−. This remarkably stable synthon has been shown to afford cycloaddition chemistry, and, participate in salt metathesis reactions to provide facile routes to novel organophosphorus heterocycles and small molecules.9–25 Therefore, we reasoned that it would be possible to utilize Na[OCP] as a building block to study the coordination chemistry of compounds which contain the formal elements of P2 and CO. Herein, we report the synthesis, molecular structures, and computational studies of NHC-{cyclo-(CO)-P2-C(O)} (2) and NHC-P2-C(O)-NHC (3), NHC = C{N(2,6-iPr2C6H3)CH}2. Notably, the isolation of these highly reactive molecules represent unusual examples of P–P coupled materials derived from the phosphaethynolate anion, [OCP]−.
C→P]−. This remarkably stable synthon has been shown to afford cycloaddition chemistry, and, participate in salt metathesis reactions to provide facile routes to novel organophosphorus heterocycles and small molecules.9–25 Therefore, we reasoned that it would be possible to utilize Na[OCP] as a building block to study the coordination chemistry of compounds which contain the formal elements of P2 and CO. Herein, we report the synthesis, molecular structures, and computational studies of NHC-{cyclo-(CO)-P2-C(O)} (2) and NHC-P2-C(O)-NHC (3), NHC = C{N(2,6-iPr2C6H3)CH}2. Notably, the isolation of these highly reactive molecules represent unusual examples of P–P coupled materials derived from the phosphaethynolate anion, [OCP]−.
Compounds 2 and 3 were obtained in different ratios depending on the specific reaction conditions (Scheme 1; Dipp = 2,6-diisopropylphenyl). When two equivalents of Na(OCP)·(dioxane)2.5 is added to a suspension of [NHC-Cl][Cl] (1) in THF at −78 °C, 31P NMR of the reaction mixture indicates a single product (δ = 299.0 ppm, 74.6 ppm, 1Jp,p = 484 Hz), attributable to new red species 2. Compound 2 can be isolated in 34% yield via toluene extraction of the solid reaction mixture and subsequent washing with hexanes. If the reaction is performed at room temperature, compound 2 is isolated in significantly higher yield (62%) as well as a second green compound 3 in 13% yield. The characteristic 1H NMR imidazole resonance of 2 at δ = 6.03 ppm differs from the two resonances observed for 3 (δ = 5.89, 6.04 ppm), which indicate the presence of two chemically inequivalent NHC ligands. The proton-coupled 31P NMR spectrum of 3, exhibits two doublets at δ = 153.5 ppm and δ = −5.09 ppm (1Jp,p = 304 Hz), consistent with decreased P–P bond order compared to the heterocycle 2. These shifts differ significantly from the 31P NMR chemical shifts reported for bis-carbene-P2 adducts A (δ = −52.4 ppm)2 and B (δ = +54.4 ppm).4 Compound 2 is stable under inert atmosphere in a benzene-d6 or toluene solution at room temperature for ca. 2 weeks. However, compound 3 is unstable in solution at room temperature and significantly more prone to side reactions which produce free NHC. As compound 3 and free NHC ligand have very similar solubility, it has proved difficult to completely separate the two compounds on a preparative scale.
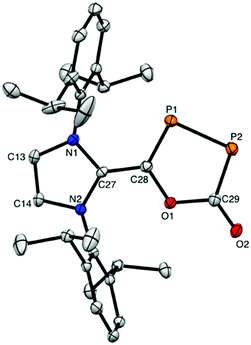
Red air- and moisture-sensitive needle-shaped crystals of 2 suitable for a single crystal X-ray diffraction study were grown from a THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene (5
toluene (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution at −35 °C (Fig. 2). The structure of 2 reveals two di-coordinate phosphorus atoms within a five-membered heterocyclic ring. Green air- and moisture-sensitive block-shaped crystals of 3 were isolated from a concentrated hexanes solution at −35 °C (Fig. 3). Structural analysis of 3 shows two di-coordinate phosphorus atomic centers with an open chain structure. The P–P bond distance in 2, 2.093 Å, is between a P–P single bond (∼2.20 Å)26 and a P
1) solution at −35 °C (Fig. 2). The structure of 2 reveals two di-coordinate phosphorus atoms within a five-membered heterocyclic ring. Green air- and moisture-sensitive block-shaped crystals of 3 were isolated from a concentrated hexanes solution at −35 °C (Fig. 3). Structural analysis of 3 shows two di-coordinate phosphorus atomic centers with an open chain structure. The P–P bond distance in 2, 2.093 Å, is between a P–P single bond (∼2.20 Å)26 and a P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P double bond (∼2.00 Å).27 Accordingly, the P–P bond distance in 2 is shorter than 3 (2.153 Å), which has more single bond character. Indeed, these values are in agreement with the P–P bond distances in NHC-P2-NHC A (2.205 Å),2 CAAC-P2-CAAC B (2.184 Å),4 and [NHC-P2-(NiPr2)][GaCl4] (2.061 Å).24 The C(29)–P(2) bond distance in 3 (1.799 Å) is comparable to the C–P bond in A (1.750 Å) which is slightly longer than in B (1.719 Å). Moreover, this data is in the range of the C(28)–P(1) [1.719 Å] and C(29)–P(2) [1.799 Å] bond distance of 2.
P double bond (∼2.00 Å).27 Accordingly, the P–P bond distance in 2 is shorter than 3 (2.153 Å), which has more single bond character. Indeed, these values are in agreement with the P–P bond distances in NHC-P2-NHC A (2.205 Å),2 CAAC-P2-CAAC B (2.184 Å),4 and [NHC-P2-(NiPr2)][GaCl4] (2.061 Å).24 The C(29)–P(2) bond distance in 3 (1.799 Å) is comparable to the C–P bond in A (1.750 Å) which is slightly longer than in B (1.719 Å). Moreover, this data is in the range of the C(28)–P(1) [1.719 Å] and C(29)–P(2) [1.799 Å] bond distance of 2.
Remarkably, at 55–60 °C in benzene-d6, and in the presence of free NHC ligand, compound 2 extrudes CO to form the more reactive compound 3. The reaction progress is easily monitored by 31P NMR spectroscopy, as shown in Fig. 4. An initial spectrum was taken of 2 and free NHC at room temperature (Fig. 4, red trace, a). After 15 min of heating, 2 and 3 can be observed in solution (Fig. 4, maroon trace, b). Complete conversion of 2 to 3 by 31P NMR is observed after 30 min (Fig. 4, green trace, c). Although the mechanism is unclear, the reaction may proceed via nucleophilic attack on phosphorus by the free carbene and subsequent loss of CO to form 3. It is noteworthy that heating compound 2 by itself (without free NHC) in benzene-d6 does not afford 3. If the temperature exceeds 60 °C, other products are produced, one of which was identified as NHC-P2-NHC (A).2 Attempts were made to convert 3 to Avia extrusion of CO, and though some of compound A was observed, there were a number of unidentifiable phosphorus-containing species. Based on these results, we believe that the formation of 3 from 1 in the room temperature reaction is due to the exothermic nature of the reaction in which some free NHC ligand is produced under highly reducing conditions, facilitating a reaction similar to that described in Fig. 4. Indeed, Bertrand reported that NHCs are incapable of capturing CO,25 therefore, unreacted NHC remains free in solution until it interacts with phosphorus to stabilize the P2C(O) moiety.
To gain a deeper insight into the bonding of these reactive molecules, density functional theory calculations at the ωB97XD/aug-cc-pVDZ level were performed on a simplified system where the Dipp groups have been replaced with methyl substituents. In 2M, the computational data is in agreement with the experimental results (for more details including atoms in molecules analysis see the ESI†). The Wiberg bond index (WBI) of the P–P bond in 2M is 1.45 and the computed bond distance is 2.088 Å, both supporting partial double bond character. This thereby suggests a moderate degree of π-interaction in the oxadiphosphole ring which is also reflected in the increased bond order of the C–P bond (WBI = 1.27) adjacent to the carbene. However, there is no indication of cyclic delocalization since the bonding parameters of the P–C bond is in the range of a typical P–C single bond. The (NHC)–C bond order in 2M is somewhat larger (1.419 Å, WBI = 1.26) than the similar (NHC)–C bond in 3M (1.501 Å, WBI = 1.01), indicating increased double bond character. Among the C–O bonds, the endocyclic ones can be described as single bonds, however, the exocyclic C–O bond is considerably shorter (WBI = 1.69). Based on these results, compound 2 is best represented as a hybrid of resonance structures 2 and 2′ with significant electron delocalization throughout the (NHC)–C–P–P fragment.
With regard to 3M, the bonding situation in the (NHC)–P fragment is similar to what is observed in known NHC–phosphinidene adducts NHC–PH26 and NHC–P2–NHC,2 which are described as a single bond with some double bond character (i.e., donation of an electron pair from the NHC and back-donation from the phosphorus lone pair). Indeed, the WBI of 1.28 for the (NHC)–P bond in 3M indicates a bond being somewhat stronger than a single bond. Furthermore, the NHC fragment attached to the P atom in 3M is almost neutral (the sum of partial charges over this moiety is +0.135 e), which implies that the contribution of a zwitterionic resonance structure [e.g. (NHC)+–P−] is negligible. The P–P bond in 3M corresponds to a single bond (WBI = 1.04) which is in accord with the WBI (1.04)2 of the P–P bond in A. In notable contrast, the P–C bond in the [PCO] fragment of 3 has double bond character, exhibited by the increased WBI of 1.35. Moreover, the P–C bond in 3M (1.754 Å) is shorter than the (NHC)–P bond (1.783 Å). The C–O bond of 3 is stronger than a normal single bond, which is indicated by the increased WBI (1.41) and by the shorter bond length in 3M (1.255 Å) compared to a prototypical single bond (1.419 Å in H3C–OH). Thus, the bonding properties of the P–C and C–O bonds unambiguously confirm a significant π-delocalization over the P–C–O moiety. As the (NHC)–C bond has been found to be a single bond (WBI = 1.04), there is no significant π-delocalization between the PCO unit and the attached carbene moiety. However, the partial charges clearly show that the second NHC unit (bound to the carbon atom) carries positive charge (sum of NPA charges in the NHC fragment is +0.479 e), while the O atom of the carbonyl group has a negative charge (−0.720 e). These results lead us to a conclusion that compound 3 is best represented as resonance structure 3′ shown in Scheme 1.
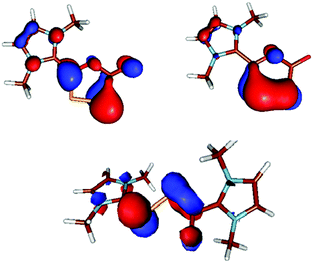
The structural assignments for 2 and 3 are also supported by the MO representations (Fig. 5). In 2, the HOMO reflects the resonance structure 2′ with the exocyclic O anion and some π-delocalization, while the HOMO−2 is mainly the PP π-bond (cf. mesomeric structure 2). In 3, the HOMO represents the proposed resonance structure 3′ (i.e., delocalization at the PPCO unit).
This work demonstrates the versatility of sodium phosphaethynolate as a synthon to access highly reactive, low-oxidation-state phosphorus species with unprecedented bonding modes. Reaction of salt [NHC-Cl][Cl] 1 with Na[OCP] affords 2, a heterocycle containing a P2 unit connected to two CO fragments, and 3, a P2 moiety bound to one CO, both stabilized by NHCs. Notably, CO can be extruded from 2 to form 3, which then can be partially converted to A. As 2 and 3 possess electron rich phosphorus centers, these complexes will be explored as ligands for main group and transition metal species.
We are grateful to the National Science Foundation (CHE-1464855), the ETH Zürich and the Swiss National Science Foundation (SNF) for funding. R. J. G. thanks the UNCF-Merck Fellowship program and the Ford Foundation for postdoctoral awards. Z. B. is grateful to the NKFIH (PD 116329) and the János Balyai Research Fellowship. We also appreciate Dr. Michael Wörle of the Small Molecule Crystallography Center at ETH Zürich for helpful discussions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For reviews on main group molecules isolated using stable carbenes: (a) Y. Wang and G. H. Robinson, Inorg. Chem., 2011, 50, 12326–12337 CrossRef CAS PubMed; (b) C. D. Martin, M. Soleilhavoup and G. Bertrand, Chem. Sci., 2013, 4, 3020–3030 RSC; (c) D. D. Wilson and J. L. Dutton, Chem. – Eur. J., 2013, 41, 13626–13637 CrossRef PubMed; (d) Y. Wang and G. H. Robinson, Inorg. Chem., 2014, 53, 11815–11832 CrossRef CAS PubMed; (e) M. Soleilhavoup and G. Bertrand, Acc. Chem. Res., 2015, 48, 256–266 CrossRef CAS PubMed; (f) M. Malaimi, R. Jazzar, M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2017, 56, 10046–10068 CrossRef PubMed.
- Y. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer, III, P. V. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2008, 130, 14970–14971 CrossRef CAS PubMed.
- Y. Wang, Y. Xie, P. Wei, H. F. Schaefer, III, P. V. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2013, 135, 19139–19142 CrossRef CAS PubMed.
- O. Back, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Nat. Chem., 2010, 2, 369–373 CrossRef CAS PubMed.
- H. Braunschweig, R. D. Dewhurst, K. Hammond, J. Mies, K. Radacki and A. Vargas, Science, 2012, 336, 1420–1422 CrossRef CAS PubMed.
- J. Böhnke, H. Braunschweig, T. Dellermann, W. C. Ewing, K. Hammond, J. O. C. Jimenez-Halla, T. Kramer and J. Mies, Angew. Chem., Int. Ed., 2015, 54, 13801–13805 CrossRef PubMed.
- H. Braunschweig, R. D. Dewhurst, F. Hupp, M. Nutz, K. Radacki, C. W. Tate, A. Vargas and Q. Ye, Nat. Chem., 2015, 522, 327–330 CAS.
- P. P. Power, Nature, 2010, 463, 171–177 CrossRef CAS PubMed.
- G. Becker, W. Schwarz, N. Seidler and M. Westerhausen, Z. Anorg. Allg. Chem., 1992, 612, 72–82 CrossRef CAS.
- F. F. Puschmann, D. Stein, D. Heift, C. Hendriksen, Z. A. Gal, H. F. Grützmacher and H. Grützmacher, Angew. Chem., Int. Ed., 2011, 50, 8420–8423 CrossRef CAS PubMed.
- D. Heift, Z. Benkő and H. Grützmacher, Dalton Trans., 2014, 43, 831–840 RSC.
- T. P. Robinson and J. M. Goicoechea, Chem. – Eur. J., 2015, 21, 5727–5731 CrossRef CAS PubMed.
- X. Chen, S. Alidori, F. F. Puschmann, G. Santiso-Quinones, Z. Benkő, Z. Li, G. Becker, H. F. Grützmacher and H. Grützmacher, Angew. Chem., Int. Ed., 2014, 53, 1641–1645 CrossRef CAS PubMed.
- D. Heift, Z. Benkő and H. Grützmacher, Chem. – Eur. J., 2014, 20, 11326–11330 CrossRef CAS PubMed.
- D. Heift, Z. Benkő and H. Grützmacher, Dalton Trans., 2014, 43, 831–840 RSC.
- L. Liu, J. Zhu and Y. Zhao, Chem. Commun., 2014, 50, 11347–11349 RSC.
- T. P. Robinson, M. J. Cowley, D. Scheschkewitz and J. M. Goicoechea, Angew. Chem., Int. Ed., 2015, 54, 683–686 CrossRef CAS PubMed.
- C. Camp, N. Settineri, J. Lèfevre, A. Jupp, J. M. Goicoechea, L. Maron and J. Arnold, Chem. Sci., 2015, 6, 6379–6384 RSC.
- A. R. Jupp and J. M. Goicoechea, J. Am. Chem. Soc., 2013, 135, 19131–19134 CrossRef CAS PubMed.
- R. Suter, Y. Mei, M. Baker, Z. Benkő, Z. Li and H. Grützmacher, Angew. Chem., Int. Ed., 2017, 56, 1356–1360 CrossRef CAS PubMed.
- M. Hansmann, D. Ruiz, L. Liu, R. Jazzar and G. Bertrand, Chem. Sci., 2017, 8, 3720–3725 RSC.
- J. M. Kieser, R. J. Gilliard, Jr., A. L. Rheingold, H. Grützmacher and J. D. Protasiewicz, Chem. Commun., 2017, 53, 5110–5112 RSC.
- L. N. Grant, B. Pinter, B. C. Manor, R. Suter, H. Grützmacher and D. J. Mindiola, Chem. – Eur. J., 2017, 23, 6272–6276 CrossRef CAS PubMed.
- A. Beil, R. J. Gilliard Jr. and H. Grützmacher, Dalton Trans., 2016, 45, 2044–2052 RSC.
- V. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller and G. Bertrand, Angew. Chem., Int. Ed., 2006, 45, 3488–3491 CrossRef CAS PubMed.
- A. M. Tondreau, Z. Benkő, J. R. Harmer and H. Grützmacher, Chem. Sci., 2014, 5, 1545–1554 RSC.
- J. D. Protasiewicz, M. P. Washington, V. B. Gudimetla, J. L. Payton and M. C. Simpson, Inorg. Chim. Acta, 2010, 364, 39–45 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1536982 (3) and 1536983 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc07654a |
| This journal is © The Royal Society of Chemistry 2017 |