Nan Yan, Zheng Fang, Qing-Quan Liu, Xiao-Hong Guo and Xiang-Guo Hu
Org. Biomol. Chem., 2016,14, 3469-3475
DOI:
10.1039/C6OB00063K,
Paper
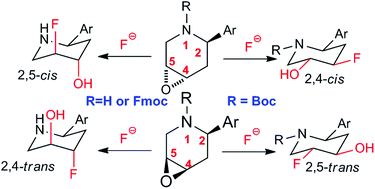
Utilizing molecular conformation as a controlling factor, epoxide-containing 2-aryl-piperidines can be ring-opened with the reagent combination of tetrabutylammonium fluoride (TBAF) and potassium bifluoride (KHF2) in a regioselective and divergent fashion. Four different types of hydroxylated fluoro-piperidines, valuable building blocks in drug development, were readily synthesized using this method. The basic nature of the reagent combination allowed a one-pot deprotection/ring opening process, which increased the efficacy of this transformation.