Synthesis and self-assembly of partially sulfonated poly(arylene ether sulfone)s and their role in the formation of Cu2S nanowires†
Jeyoung Park ac,
Changjun Parka,
Byoung Tak Yima,
Myungeun Seo
ac,
Changjun Parka,
Byoung Tak Yima,
Myungeun Seo *b and
Sang Youl Kim*a
*b and
Sang Youl Kim*a
aDepartment of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 305-701, Korea. E-mail: kimsy@kaist.ac.kr
bGraduate School of Nanoscience and Technology, KAIST, Daejeon 305-701, Korea. E-mail: seomyungeun@kaist.ac.kr
cCenter for Industrial Chemical Biotechnology, Korea Research Institute of Chemical Technology (KRICT), Ulsan 681-230, Korea
First published on 10th June 2015
Abstract
Partially sulfonated amphiphilic poly(arylene ether sulfone)s (PSPAESs) were synthesized by one-step nucleophilic aromatic substitution copolymerization. A 4-fluoro-4′-hydroxydiphenyl sulfone potassium salt was used as a hydrophobic monomer, and 5-((4-fluorophenyl)sulfonyl)-2-hydroxybenzenesulfonic acid as a hydrophilic monomer bearing a sulfonic acid group was synthesized from the hydrophobic monomer via selective sulfonation. 1H and 13C nuclear magnetic resonance spectroscopy analysis of PSPAESs indicated formation of statistical amphiphilic copolymers with control over the degree of sulfonation by varying the feed. Dynamic light scattering and transmission electron microscopy analysis indicated that PSPAESs self-assembled into spherical micelles in aqueous solutions. Interestingly, the micellar solution of PSPAESs prepared by dialysis was found to grow Cu2S nanowires on a Cu grid under ambient conditions. Formation of Cu2S nanowires on various substrates including a Si wafer and graphene was demonstrated in the presence of Cu and a sulfur source. UV-vis spectroscopy and X-ray photoelectron spectroscopy data suggests PSPAESs assist dissolution of metallic Cu into Cu(II) enabling the formation of Cu2S nanowires.
Introduction
Amphiphilic polymers consisting of both hydrophilic and hydrophobic segments self-assemble in aqueous solutions to form polymeric micelles or vesicles, which have been of great interest for applications such as drug delivery and templating nanomaterials.1,2 Most amphiphilic polymers are amphiphilic block polymers typically synthesized by controlled polymerization of an initial monomer and subsequent polymerization of a second monomer from the chain end.3–8 While the block architecture provides control over the lengths of hydrophilic and hydrophobic segments and a sharp interface between them, syntheses of amphiphilic block polymers are often tedious and costly because they require multi-step copolymerization.Amphiphilic random copolymers consisting of randomly distributed hydrophilic and hydrophobic repeating units in the polymer backbone are another class of amphiphilic polymers but much rarer than amphiphilic block polymers.9–22 However, amphiphilic random copolymer has distinct synthetic advantages over amphiphilic block polymers because they can be synthesized by one-step copolymerization of hydrophilic and hydrophobic monomers avoiding multi-step processes. Similar to amphiphilic block copolymers, formation of spherical micelles, multi-micellar aggregates, and vesicles of amphiphilic random copolymers in aqueous media has been observed and their encapsulation capability of hydrophobic guests has been investigated.13–22
In this study, we explored synthesis of partially sulfonated poly(arylene ether sulfone)s (PSPAES) with various degrees of sulfonation by one-step copolymerization of a hydrophobic diphenyl sulfone monomer and a hydrophilic monomer consisting of sulfonic acid derived from the hydrophobic monomer. Poly(arylene ether sulfone)s are high-performance engineering plastics synthesized via nucleophilic aromatic substitution (SNAr) polymerization.23 Particularly, sulfonated poly(arylene ether sulfone) has attracted recent attention as a proton exchange membrane polymer for fuel cell application due to its stability at high temperature.24,25 Surprisingly, sulfonated poly(arylene ether sulfone)s have not been realized as amphiphilic polymers and their self-assembling behaviors has not been documented in the literature. To this end, we investigated self-assembly of PSPAESs with different counter cations in aqueous solution and observed formation of spherical aggregates. Interestingly, we found that PSPAESs facilitate dissolution of metallic Cu into Cu(II) in aqueous solution and promote formation of copper sulfide (Cu2S) nanowires26–28 on a substrate under ambient conditions. We show that PSPAESs mediate facile growth of Cu2S nanowires on silicon or carbonaceous surfaces in the presence of copper and sulfur sources.
Results and discussion
Synthesis of partially sulfonated poly(arylene ether sulfone)s
We synthesized PSPAES by one-step copolymerization of a hydrophobic and a hydrophilic diphenyl sulfone monomers via SNAr reaction (Scheme 1). While postsulfonation of poly(arylene ether sulfone) has been more popular in synthesis of sulfonated poly(arylene ether sulfone)s, direct incorporation of sulfonic acid groups by copolymerization of monomers bearing sulfonic acid groups are advantageous for accurate control over the degree of sulfonation and the position of sulfonic acid groups.For the synthesis of PSPAES, commercially available 4-fluoro-4′-hydroxydiphenyl sulfone (1) was selected as a hydrophobic AB type monomer.29–31 From the monomer 1, 5-((4-fluorophenyl)sulfonyl)-2-hydroxybenzenesulfonic acid (2) was synthesized by selective sulfonation at the ortho position of the hydroxyl group and used as a hydrophilic monomer (Fig. 1 and Fig. S1†). The monomers were converted into potassium salts prior to polymerization, and polymerization was conducted in sulfolane at 10 wt% monomer concentration in the presence of 18-crown-6 as an additive. After heating for 120 h at 160 °C, PSPAES was obtained by precipitation in aqueous KCl solution.
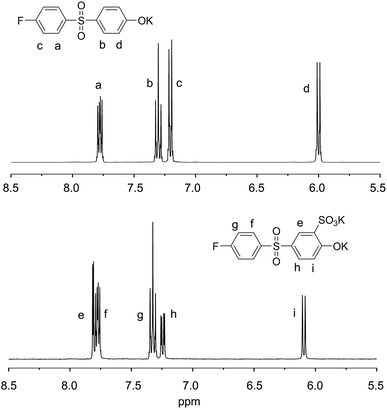 | ||
| Fig. 1 1H NMR spectra of hydrophobic monomer 1 (top) and hydrophilic monomer 2 (bottom) (400 MHz, DMSO-d6, 20 °C). | ||
We synthesized several PSPAESs using different feed ratios of 1 and 2 and summarized their characterization data in Table 1. PSPAESs synthesized in this study were designated as Pn–X where n and X denote the percentage of the monomer 2 in PSPAESs and the counter cation of sulfonic acid group, respectively. 1H nuclear magnetic resonance (NMR) spectroscopy of the PSPAESs (SO3K form (Pn–K)) was used to determine fraction of the monomers 1 and 2 incorporated in PSPAESs by integration. Fig. 2 shows that PSPAESs contained both monomers and the amount of 2 incorporated in the polymer (i.e., degree of sulfonation) could be controlled by varying the feed ratio. Size exclusion chromatography (SEC) analysis of PSPAESs (Pn–K) were conducted using N,N′-dimethylformamide (DMF) containing LiBr and phosphoric acid as an eluent to homogeneously dissolve amphiphilic PSPAESs. From the SEC traces, number average molecular weights of PSPAESs were determined as ca. 4 kg mol−1 with dispersity of 1.4–1.8 based on linear polystyrene standards (Fig. S2†).
| Entry | Feed ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Incorporation ratioa (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Mnb | Mwb | Đb |
|---|---|---|---|---|---|
| a Determined by 1H NMR analysis (Fig. S3).b Determined by DMF-GPC with UV detector using linear polystyrene standards. | |||||
| P15–K | 66.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.3 33.3 |
85.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.7 14.7 |
4850 | 8730 | 1.79 |
| P25–K | 50.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.0 50.0 |
75.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.7 24.7 |
3830 | 5620 | 1.47 |
| P36–K | 33.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66.7 66.7 |
64.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35.8 35.8 |
4100 | 5790 | 1.41 |
Integration of the 1H NMR spectra also indicated the monomer 2 was less incorporated than the feed (Fig. S3†). We posit that highly hydrophilic PSPAESs containing the monomer 2 as the major fraction may be lost during precipitation in KCl (aq) increasing population of the monomer 1 in PSPAESs obtained by precipitation (note that homopolymer of the monomer 2 was soluble in KCl (aq)). Also, higher temperature required for homopolymerization of the monomer 2 (160 °C) than that of the monomer 1 (120 °C) indicated that the monomer 2 is less reactive than the monomer 1. Using 13C NMR spectroscopy, we estimated reactivity of the fluorine leaving groups in the monomer 1 and 2 based on the peak position of a doublet corresponding to the carbon adjacent to fluorine and did not observe a significant difference (164.6 ppm for the monomer 1 vs. 164.8 ppm for the monomer 2 in cases of potassium salts) (Fig. S1†). Thus we attribute incorporation of the negatively charged sulfonic acid group at the ortho position to the lowered reactivity of the phenoxide group of the monomer 2.
13C NMR spectra of PSPAESs showed existence of chain ends consisting of fluorine groups from both monomers (Fig. S4†). This is consistent with a statistical copolymerization scheme rather than block copolymerization where the more reactive monomer 1 would be consumed first and then the monomer 2 is polymerized to produce chain ends only from the less reactive monomer 2. We also performed a control experiment by homopolymerizing the monomer 2 in the presence of presynthesized homopolymer of the monomer 1, which was synthesized via chain-growth condensation polymerization and contained only fluorine end groups.30 A 13C NMR spectrum of the resulting polymer showed fluorine chain ends from both monomers, indicating that the monomer 2 was inserted into polymer chains of the monomer 1 via transetherification (Fig. S5†). Consequently, PSPAESs were expected to possess statistical distribution of the monomer units aided by sequence scrambling via transetherification. The lowered reactivity of the phenoxide group of the monomer 2 and occurrence of transetherification were also attributed to formation of rather low molecular weight copolymers.
Self-assembly of PSPAESs in aqueous solution
Self-assembly of PSPAESs was induced by dialyzing 10 wt% DMF solution of PSPAES (Pn–K) in deionized water at room temperature for 1 day. The final concentration was adjusted to 2 wt% by adding more water after dialysis. Dynamic light scattering analysis (DLS) of the resulting solutions indicated the existence of micelles (Table 2 and Fig. 3). While P15–K and P25–K formed micelles with a mean effective diameter of 10.3 and 9.6 nm, P36–K formed much larger aggregates with a mean effective diameter of 77.7 nm suggesting aggregation of micelles. Direct dissolution of P25–K in deionized water by heating also yielded micellar solution without dialysis (P25–KND in Table 2), but rather larger aggregates with diameter of 65.0 nm were obtained presumably due to incomplete dissolution of PSPAES (Fig. S6†).Micelles of Pn–K were visualized by field-emission transmission electron microscopy (FE-TEM). Samples were prepared by dropping the solution on a nickel grid, blotting and drying and then imaged without staining. TEM images of P25–K showed spherical micelles with the diameter of 30–300 nm (Fig. 4).
By changing deionized water into H2SO4 solution (0.5 M) and CuCl2 solution (0.2 M) for dialysis, micelles of Pn–H (SO3H form) and Pn–Cu ((SO3)2Cu form) were also obtained. When the size of Pn–Cu was compared with that of Pn–K, we did not observe significant variation except for P36–Cu which showed much smaller mean effective diameter of 13.2 nm compared to 77.7 nm of P36–K.
Growth of Cu2S nanowires assisted by PSPAESs
Interestingly, uniform nanowires with high aspect ratios over several microns were found over a large area when TEM samples of Pn–K aqueous solutions were prepared on a formvar/carbon coated copper grid (Fig. 5). Diameters of wires were varied from 5 nm to 100 nm irrelative to the type of polymers. High magnification images of the nanowires clearly showed lattice fringes with spacing of 0.34 nm, suggesting highly crystalline nanowires were formed (Fig. 5b). Energy dispersive X-ray spectroscopy (EDX) analysis of the nanowires indicated large fraction of C, Cu, and S, which was confusing due to possible interference from the grid.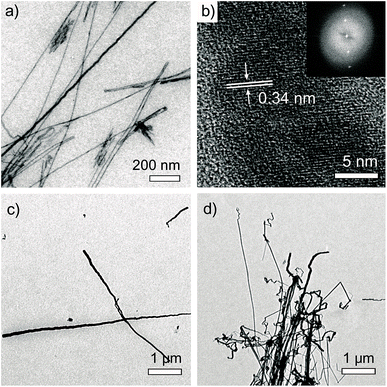 | ||
| Fig. 5 FE-TEM images of Cu2S nanowires grown on a formvar/carbon TEM Cu grid by dipping in aqueous solution of (a and b) P25–K (inset of (b): FFT image), (c) P15–K, and (d) P36–K for 12 h. | ||
We realized that identical nanowires could be produced on silicon wafers with different surface properties (n-type, HF-treated, piranha-treated, hexamethyldisilazane-treated), highly ordered pyrolytic graphite (HOPG), or graphene in the presence of Cu foil suggesting a critical role of Cu in formation of nanowires (Fig. 6 and S7–S9†).32 To accurately determine chemical composition of the nanowires, we dispersed nanowires formed on a silicon wafer into water by sonication and transferred them onto a holey Mo grid by dipping the grid in the solution. EDX analysis of the nanowires on the Mo grid revealed that the nanowires were mainly composed of Cu and S with the atomic ratio of Cu/S = 2.16–2.34, suggesting Cu2S (Fig. S10†). Amounts of C and O in the nanowires were negligible. Based on selected-area electron diffraction (SAED) analysis, we identified hexagonal-phase chalcocite structure of Cu2S (Fig. S11†).33,34
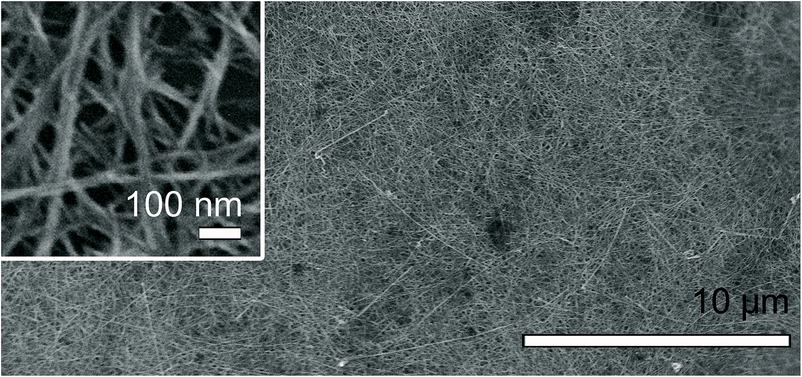 | ||
| Fig. 6 FE-SEM image of Cu2S nanowires grown on HF treated Si wafer by dipping with Cu foil in aqueous solution of P25–K for 3 days. | ||
Cu2S nanocrystals are p-type semiconducting material potentially useful for photovoltaic,35–38 electrochemical,39–41 optical,42–45 and catalytic46,47 applications. Particularly, synthetic pathways to Cu2S nanowires has been widely investigated including homogeneous solution reaction of Cu(II) salt and gas–solid reaction39,48–53 of H2S gas on Cu substrate. The latter produces Cu2S nanowires on Cu surface at mild conditions avoiding high temperature reactions. Anionic surfactants such as sodium dodecyl sulfate and sodium bis(2-ethylhexyl) sulfosuccinate have been often utilized to assist controlled formation of nanowires, although the mechanism has not been clearly elucidated.26–28 Compared to the gas–solid reaction methodologies, our approach also employs ambient synthetic condition with aid of amphiphilic polymer (PSPAES) but has a distinct advantage; it allows formation of Cu2S nanowires on various surfaces, not restricted to Cu.
One big question to us was the sulfur source for formation of Cu2S. We did not add any particular sulfur-containing chemicals except for PSPAES, and it was obvious that sulfur in Cu2S nanowires cannot come from PSPAES where sulfur atoms are covalently bonded to carbon and oxygen atoms. Because commercial dialysis packs are known to contain a little amount of sulfide contaminants,54 we postulated PSPAES micelles may adventitiously encapsulate sulfide during dialysis and release it in the presence of Cu to form Cu2S. Indeed, aqueous solution of P25–K prepared without dialysis (by direct dissolution in deionized water by heating) did not produce any nanowires on a formvar/carbon coated copper grid. Addition of small amount of aqueous dispersion of sulfur to the same solution did produce nanowires, supporting our hypothesis.
We also found PSPAESs facilitated etching of metallic copper into Cu(II). UV/vis spectroscopy showed that P25–K aqueous solution with dipped Cu foil for 3 days developed an absorption band at 406 nm, which was identical to the absorption band of Cu(II) in P25–Cu solution (Fig. 7a). X-ray photoelectron spectroscopy (XPS) also supported leaching of Cu into Cu(II) in P25–K aqueous solution (Fig. 7b). A mixture of the monomers 1 and 2 did not show such etching ability. Thus we speculate that cooperative binding of multiple sulfonic acid groups along the chain of PSPAES would induce dissolution of Cu into solution, and the resulting Cu(II) bound at the periphery of the micelle would react with sulfur source encapsulated in the micelle to form Cu2S nanowires. Participation of Cu(II) ions in formation of Cu2S is also consistent with the fact that Pn–Cu solutions can form Cu2S nanowires in the presence of sulfur source (i.e., after dialysis) without Cu foil.
Conclusions
We synthesized PSPAESs as new amphiphilic random polymers by one-step SNAr copolymerization of hydrophobic and hydrophilic diphenyl sulfone monomers. Amphiphilic copolymers consisting of statistically distributed sulfonic acid groups were obtained, and amount of sulfonic acid groups was directly controlled by varying the feed. Spherical micelles of PSPAES in aqueous solution were found to play a key role in formation of Cu2S nanowires on a substrate. Our observation suggests that the micelles dissolve Cu into Cu(II) and promote reaction of Cu(II) with encapsulated sulfur source. Facile growth of Cu2S nanowires assisted by PSPAES under ambient conditions demonstrates importance of new polymers for nanomaterial synthesis and suggests promise of this methodology.Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) funded by the Korean Government (MSIP) under Project Number ERC, Active Polymer Center for Pattern Integration 2007-0056091.Notes and references
- I. F. Uchegbu and A. G. Schatzlein, Polymers in Drug Delivery, Taylor & Francis Group, New York, 2006 Search PubMed.
- A. Harada, Supramolecular Polymer Chemistry, Wiley-VCH, Weinheim, 2012 Search PubMed.
- P. Alexandridis and B. Lindman, Amphiphilic Block Copolymers: Self-Assembly and Applications, Elsevier, Amsterdam, 2000 Search PubMed.
- M. Lazzari, G. Liu and S. Lecommandoux, Block Copolymers in Nanoscience, Wiley-VCH, Weinheim, 2006 Search PubMed.
- A. H. E. Müller and O. Borisov, Self Organized Nanostructures of Amphiphilic Block Copolymers, Springer, Heidelberg, 2011 Search PubMed.
- S. Forster and M. Antonietti, Adv. Mater., 1998, 10, 195 CrossRef.
- A. Blanazs, S. P. Armes and A. J. Ryan, Macromol. Rapid Commun., 2009, 30, 267–277 CrossRef CAS PubMed.
- S. J. Holder and N. Sommerdijk, Polym. Chem., 2011, 2, 1018–1028 RSC.
- N. Suzuki, Y. Morishima, S. Arimori and T. Endo, Polym. J., 2007, 39, 187–191 CrossRef CAS.
- H. M. L. Lambermont-Thijs, R. Hoogenboom, C. A. Fustin, C. Bomal-D'Haese, J. F. Gohy and U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 515–522 CrossRef CAS PubMed.
- H. Morinaga, H. Morikawa, Y. Wang, A. Sudo and T. Endo, Macromolecules, 2009, 42, 2229–2235 CrossRef CAS.
- Y. Oda, S. Kanaoka, T. Sato, S. Aoshima and K. Kuroda, Biomacromolecules, 2011, 12, 3581–3591 CrossRef CAS PubMed.
- X. Y. Liu, J. S. Kim, J. Wu and A. Eisenberg, Macromolecules, 2005, 38, 6749–6751 CrossRef CAS.
- Y. B. Li, Y. H. Deng, X. L. Tong and X. G. Wang, Macromolecules, 2006, 39, 1108–1115 CrossRef CAS.
- F. Tian, Y. Y. Yu, C. C. Wang and S. Yang, Macromolecules, 2008, 41, 3385–3388 CrossRef CAS.
- K. Dan, N. Bose and S. Ghosh, Chem. Commun., 2011, 47, 12491–12493 RSC.
- N. Li, Y. B. Li and X. G. Wang, Macromolecules, 2011, 44, 8598–8606 CrossRef CAS.
- K. Feng, N. Xie, B. Chen, L. P. Zhang, C. H. Tung and L. Z. Wu, Macromolecules, 2012, 45, 5596–5603 CrossRef CAS.
- A. Honglawan and S. Yang, Soft Matter, 2012, 8, 11897–11904 RSC.
- N. Li, Y. B. Li and X. G. Wang, Polymer, 2012, 53, 3975–3985 CrossRef CAS PubMed.
- K. Dan, P. Rajdev, J. Deb, S. S. Jana and S. Ghosh, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4932–4943 CrossRef CAS PubMed.
- H. Wu, J. Dong, C. Li, Y. Liu, N. Feng, L. Xu, X. Zhan, H. Yang and G. Wang, Chem. Commun., 2013, 49, 3516–3518 RSC.
- J. B. Rose, Polymer, 1974, 15, 456–465 CrossRef CAS.
- M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla and J. E. McGrath, Chem. Rev., 2004, 104, 4587–4611 CrossRef CAS.
- K. Nakabayashi, K. Matsumoto and M. Ueda, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3947–3957 CrossRef CAS PubMed.
- S. H. Wang and S. H. Yang, Adv. Mater. Opt. Electron., 2000, 10, 39–45 CrossRef CAS.
- S. H. Wang and S. H. Yang, Chem. Phys. Lett., 2000, 322, 567–571 CrossRef CAS.
- S. Gorai, D. Ganguli and S. Chaudhuri, Mater. Chem. Phys., 2004, 88, 383–387 CrossRef CAS PubMed.
- J. Park, M. Moon, M. Seo, H. Choi and S. Y. Kim, Macromolecules, 2010, 43, 8304–8313 CrossRef CAS.
- J. Park, M. Seo, H. Choi and S. Y. Kim, Polym. Chem., 2011, 2, 1174–1179 RSC.
- J. Park, J. Kim, M. Seo, J. Lee and S. Y. Kim, Chem. Commun., 2012, 48, 10556–10558 RSC.
- Other various substrates such as indium tin oxide (ITO), gold and mica were not effective.
- X. S. Du, Z. Z. Yu, A. Dasari, J. Ma, Y. Z. Meng and Y. W. Mai, Chem. Mater., 2006, 18, 5156–5158 CrossRef CAS.
- X. S. Du, M. S. Mo, R. K. Zheng, S. H. Lim, Y. Z. Meng and Y. W. Mai, Cryst. Growth Des., 2008, 8, 2032–2035 CAS.
- T. J. Coutts and J. D. Meakin, Current Topics in Photovoltaics, Academic Press, Orlando, FL, 1985 Search PubMed.
- R. C. Neville, Solar Energy Conversion: The Solar Cell, 2nd edn, Elsevier, Amsterdam, 1995 Search PubMed.
- H. Lee, S. W. Yoon, E. J. Kim and J. Park, Nano Lett., 2007, 7, 778–784 CrossRef CAS PubMed.
- Y. Wu, C. Wadia, W. L. Ma, B. Sadtler and A. P. Alivisatos, Nano Lett., 2008, 8, 2551–2555 CrossRef CAS PubMed.
- J. Chen, S. Z. Deng, N. S. Xu, S. H. Wang, X. G. Wen, S. H. Yang, C. L. Yang, J. N. Wang and W. K. Ge, Appl. Phys. Lett., 2002, 80, 3620–3622 CrossRef CAS PubMed.
- S. C. Liufu, L. D. Chen, Q. Yao and F. Q. Huang, J. Phys. Chem. C, 2008, 112, 12085–12088 CAS.
- C. H. Lai, K. W. Huang, J. H. Cheng, C. Y. Lee, B. J. Hwang and L. J. Chen, J. Mater. Chem., 2010, 20, 6638–6645 RSC.
- Y. B. Chen, L. Chen and L. M. Wu, Chem.–Eur. J., 2008, 14, 11069–11075 CrossRef CAS PubMed.
- Y. X. Zhao, H. C. Pan, Y. B. Lou, X. F. Qiu, J. J. Zhu and C. Burda, J. Am. Chem. Soc., 2009, 131, 4253–4261 CrossRef CAS PubMed.
- Y. Wang, Y. X. Hu, Q. Zhang, J. P. Ge, Z. D. Lu, Y. B. Hou and Y. D. Yin, Inorg. Chem., 2010, 49, 6601–6608 CrossRef CAS PubMed.
- J. M. Luther, P. K. Jain, T. Ewers and A. P. Alivisatos, Nat. Mater., 2011, 10, 361–366 CrossRef CAS PubMed.
- Y. Kim, K. Y. Park, D. M. Jang, Y. M. Song, H. S. Kim, Y. J. Cho, Y. Myung and J. Park, J. Phys. Chem. C, 2010, 114, 22141–22146 CAS.
- Z. Li, W. H. Chen, H. L. Wang, Q. Ding, H. W. Hou, J. M. Zhang, L. W. Mi and Z. Zheng, Mater. Lett., 2011, 65, 1785–1787 CrossRef CAS PubMed.
- N. Wang, K. K. Fung, S. Wang and S. Yang, J. Cryst. Growth, 2001, 233, 226–232 CrossRef CAS.
- S. H. Wang and S. H. Yang, Chem. Mater., 2001, 13, 4794–4799 CrossRef CAS.
- S. H. Wang, S. H. Yang, Z. R. Dai and Z. L. Wang, Phys. Chem. Chem. Phys., 2001, 3, 3750–3753 RSC.
- S. H. Wang, L. Guo, X. G. Wen, S. H. Yang, J. Zhao, J. Liu and Z. H. Wu, Mater. Chem. Phys., 2002, 75, 32–38 CrossRef CAS.
- C. X. Lai, Q. B. Wu, J. Chen, L. S. Wen and S. Ren, Nanotechnology, 2010, 21, 215602 CrossRef PubMed.
- S. Z. Zhao, G. Y. Han and M. Y. Li, Mater. Chem. Phys., 2010, 120, 431–437 CrossRef CAS PubMed.
- The specification data provided by the manufacturer indicates the sulfur content is less than 0.1% of the membrane, which corresponds to approximately 5 μM of S in our experimental condition.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, figures showing 13C NMR spectra of the monomers, 1H NMR spectrum of P25–K used in the calculation of the % incorporation ratio of 2, 13C NMR spectra of P25–K and control experiment, DLS correlation functions, FE-SEM images of Cu2S NWs on various substrates, and ED analysis data. See DOI: 10.1039/c5ra05563f |
| This journal is © The Royal Society of Chemistry 2015 |





