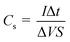Electrochemical fabrication of Ni(OH)2/Ni 3D porous composite films as integrated capacitive electrodes
Hong Jiangab,
Ying Guoa,
Tao Wanga,
Peng-Li Zhua,
Shuhui Yua,
Yan Yub,
Xian-Zhu Fu*a,
Rong Sun*a and
Ching-Ping Wongcd
aShenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, P. R. China. E-mail: xz.fu@siat.ac.cn; rong.sun@siat.ac.cn; Fax: +86-755-86392299; Tel: +86-755-86392151
bInstitute of Nano Science and Technology, University of Science and Technology of China, Suzhou 215123, P. R. China
cDepartment of Electronics Engineering, The Chinese University of Hong Kong, Hong Kong, China
dSchool of Materials Science and Engineering, Georgia Institute of Technology, Atlanta, GA 30332, USA
First published on 16th January 2015
Abstract
Ni(OH)2 coated on Ni porous films are facilely fabricated by anode oxidation of 3D hierarchical porous Ni films which are prepared through a hydrogen bubble template electro-deposition method. The highly porous nickel films function as effective 3D conductive network current collectors and scaffolds to in situ form a thin layer of Ni(OH)2 active material. The electrochemical capacitive performances are investigated by cyclic voltammetry (CV) and the galvanostatic charge–discharge technique in 6 M KOH electrolyte. The 3D porous Ni(OH)2/Ni integrated electrodes demonstrate a much higher specific capacity of 828 mF cm−2 than 126 mF cm−2 for the smooth Ni(OH)2/Ni electrode at a current density of 10 mA cm−2. The 3D porous Ni(OH)2/Ni integrated electrodes also display a good capacity retention of 95% after 1000 cycles. The superior capacitive properties of 3D porous Ni(OH)2/Ni electrodes might result from the thin layer Ni(OH)2 active materials in situ formed on the highly 3D porous Ni metallic current collector with large surface area, low contact resistance between Ni(OH)2 active material and Ni current collector, and fast electron/ion conduction.
Introduction
Supercapacitors, also named as electrochemical capacitors, are considered to be promising energy storage devices due to the advantages including high power density, long cycle life, fast charge–discharge process, and lower maintenance cost.1–4 Electrode materials with high performance and low cost are key for the practical application of supercapacitors. Transition metal oxides/hydroxides have attracted increasing attention as capacitive electrode materials in recent years owing to their large specific capacitance.5–7 Among them, Ni(OH)2 (nickel hydroxide) has been widely used as the positive electrode material of nickel-based secondary batteries for many years.8 Ni(OH)2 is also identified as one of the most promising electrode materials for supercapacitors because of its high theoretical specific capacitance, easy synthesis, low cost, environmental compatibility, and good rate capability.9–11 Unfortunately, Ni(OH)2 still suffers from lower capacity especially at high rate compared to the theoretical value owing to its too poorly conducting to support fast electron transport.12 Furthermore, the traditional strategy to fabricate electrodes used conductive agent, polymer binder and active materials to coat on the nickel foam, which would decrease the contact area of active material to the current collector and electrolyte solution, hamper the electron/ion transport rate, increase the total electrode mass and decrease the capacity.13,14Growth of Ni(OH)2 directly on the conductive substrates is an effective method to address the above problems. Lots of conductive materials have been developed to support Ni(OH)2 active materials, such as carbon nanotubes,15 graphene nanosheets,16 3D graphene foams,17 mesoporous carbon,18 graphite sheets,19 graphite foam,20 and Ni foams.5–7 These conductive substrate materials can improve the electrical conductivity of the composites and shorten the electron and ion diffusion pathways thus to efficiently utilize the Ni(OH)2 active materials and improve the electrochemical performance.21 Especially, highly conductive 3D continuous porous matrixes could provide high surface area for loading Ni(OH)2 active materials, greatly facilitate the fast electron transport from Ni(OH)2 active materials to the current collectors. Moreover, it would not need conductive agent and polymer binder for the integrated electrodes of coating of Ni(OH)2 active materials on the conductive current collector.22 3D porous metal films prepared by a hydrogen bubble template have attracted much interest for their high surface area, open porous structures and facile fabrication.23–28 But there are few reports about hydrogen bubble template to prepare 3D porous metallic current collectors supported capacitive materials, especially 3D porous Ni(OH)2/Ni capacitive electrodes.29
Herein, we fabricate binder-free integrated porous Ni(OH)2/Ni film capacitive electrodes through a simple in situ anode oxidation of 3D porous Ni film current collector which prepared by hydrogen bubble template electrochemical deposition. The 3D porous Ni(OH)2/Ni electrodes demonstrate greatly higher specific capacitance of 828 mF cm−2 than that of smooth Ni(OH)2/Ni electrode (126 mF cm−2). And the 3D porous Ni(OH)2/Ni electrodes also exhibit outstanding capacitance retention of 95% after 1000 charge–discharge cycles at a current density of 10 mA cm−2.
Experimental
Preparation of 3D porous Ni film current collectors
The 3D porous Ni films were prepared by a hydrogen bubble template electrochemical deposition.23 All the reagents were analytical grade and were used without further purification, and all solutions were prepared with secondary deionized (DI) water. Nickel foil was cleaned five minutes with 1 M HCl, ethanol and DI water. Then it was used as the cathode for porous Ni film deposition, and a large area of nickel foil was processed as above as the anode. The distance between cathode and anode was kept at 2 cm. Porous Ni was deposited on the surface of the Ni substrate under a constant current density of 5 A cm−2 for 60 s by using DC power PSW80-13.5 with electrolyte consisting of 0.2 M NiCl2 and 2 M NH4Cl at room temperature. After the electrochemical deposition, the porous Ni was thoroughly rinsed with DI water in order to remove the electrolyte solution from inside the pores for five times, dried first with an airflow and then in a vacuum oven at 50 °C for 5 h.Preparation of 3D porous Ni(OH)2/Ni integrated electrodes
A three-electrode cell was used in preparing the porous Ni(OH)2/Ni film, containing the as-prepared porous Ni film or smooth Ni foil with 1 cm × 1 cm surface area as the working electrode, a large area platinum foil as the counter electrode, and a Hg/HgO electrode as the reference electrode. Potentials were reported with respect to the Hg/HgO reference electrode. The electrochemical deposition experiments were performed in a freshly prepared 1 M KOH solution under quiescent conditions by a ZahnerZennium electrochemical workstation (Germany). Electrochemical anode oxidation of porous Ni to in situ form outside Ni(OH)2 thin layer was carried out potential dynamic method by subjecting the working electrode to potential cycling in the potential range of −0.1–0.6 V at a scan rate of 25 mV s−1 for 50 cycles. Then the films were rinsed with DI water in order to remove the electrolyte solution for five times and then dried with airflow for morphology and electrochemical characterization. The Ni(OH)2 film formed on the porous Ni film was called 3D porous Ni(OH)2/Ni, and Ni(OH)2 film formed on the smooth Ni foil was named smooth Ni(OH)2/Ni.Characterization and electrochemical measurement
The crystalline phase of samples were characterized by X-ray powder diffraction (XRD) using a Rigaku X-ray Diffractometer with Cu-Kα (1.5418 Å) radiation from 10 to 90° at a scanning speed of 5° min−1 operating at 30 kV and 20 mA. The morphology was observed using a Field Emission Scanning Electron Microscopy (FESEM, Nova NanoSEM 450). XPS analysis was recorder on a PHI 5800 XPS system, where Al Kα excitation source was used.Before electrochemical measurements were performed, all samples were immersed in 6 M KOH solution for 5 h. Electrochemical measurements were carried out with a ZahnerZennium electrochemical workstation (Germany) using the conventional three-electrode electrochemical cell with 6 M KOH solution as electrolyte at room temperature. The as-prepared electrode was used as the working electrode, the potential was referred to a Hg/HgO reference electrode and a large area platinum foil was set as the counter electrode.
Cyclic voltammetry was conducted on each sample at various scan rates over a range of 0.01–0.1 V s−1 in a potential range of 0–0.5 V. Galvanostatic charge–discharge experiments were carried out at different current densities from 1 mA cm−2 to 10 mA cm−2. The charge–discharge cycle performance was examined at a current density of 10 mA cm−2 for 1000 cycles.
Results and discussion
Fig. 1 illustrates the fabrication process of 3D porous Ni film current collector by a hydrogen bubble template electrochemical deposition. As a large current density of 5 A cm−2 is applied on the cathode of Ni substrate, numerous H2 bubbles would be generated on the cathode substrate for the water electrolysis. At the same time, the Ni2+ ions in the electrolyte would be also electrochemically reduced and deposited on the cathode substrate. The deposited metal Ni atoms can only grow between H2 bubbles then build 3D porous three-dimensional metal skeleton connected to the Ni substrate as the electrochemical reaction progress. | ||
| Fig. 1 Schematics illustrating the fabrication process of 3D porous Ni film current collector by hydrogen bubble template electrochemical deposition. | ||
Fig. 2 presents the typical XRD patterns of the porous Ni film and porous Ni(OH)2/Ni film. There are strong diffraction peaks of porous Ni film at 2θ = 44°, 52°, 76°, which are in good agreement with the values from the standard card (JCPDS no. 04-0850) of Ni metal. Except the strong Ni metal diffraction peaks, some new weak diffraction peaks appear at 2θ = 19.2°, 33°, 38.5°, 59°, 62.7° for porous Ni(OH)2/Ni film. These new weak peaks are indexed to Ni(OH)2 (JCPDS no. 14-0117), confirming the formation of Ni(OH)2 by anode oxidation of Ni substrate in the KOH solution. Ni is an active metal, which can be in situ electrochemically oxidized to form Ni(OH)2 outside coating layer in alkaline solution according to the previous report.30 In addition, the low intensity of Ni(OH)2 peaks relative to the remained Ni metal peaks indicate that the Ni(OH)2 layer is very thin on the surfaces of the porous nickel substrates.31 To investigate the surface Ni sate of the electrodeposited 3D porous Ni(OH)2/Ni film, the XPS analysis is performed, and measured results are shown in Fig. 3. The typical Ni 2p3/2 and 2p1/2 peaks of 3D porous Ni(OH)2/Ni film with two shake-up satellites (denoted as “sat.”) are obtained at 873.9 eV and 856.3 eV, respectively (Fig. 3a). The spin-energy separation of 17.6 eV indicates characteristic of the Ni(OH)2 phase, and in good agreement with previously reported.32,33 The peak at 531.7 eV that are assigned to O 1s photoelectrons (Fig. 3b), consisting of two oxygen bonds (533.4 eV and 531.6 eV), which can be associated with physi- and chemi-absorbed molecular water and Ni(OH)2. This result is also in consistent with the XRD analysis, confirming the in situ formation of Ni(OH)2 capacitive materials on the 3D porous Ni skeleton.
 | ||
| Fig. 3 (a) XPS peaks of Ni 2p of the 3D porous Ni(OH)2/Ni film electrode, and (b) XPS peaks of O 1s of the 3D porous Ni(OH)2/Ni film electrode. | ||
Fig. 4 shows the SEM images of the as-prepared Ni-based film electrodes. Comparing the smooth Ni film substrate (Fig. 4a), the gray villous morphology is retained and more or less evenly coat on the surface of Ni film after formation of Ni(OH)2 thin layer (Fig. 4b and c). SEM images in Fig. 4d–f clearly illustrate the as-prepared porous Ni film with 3D highly hierarchical porous network structure composed of numerous interconnected particles and interspaces. It can be observed in the large magnification SEM images (Fig. 4e and f) that there are lots of interconnected small pores and gaps in the surface and the large pore walls. The pore size of large pores is mainly in the range from 5 to 20 μm, whereas the small pores/gaps are usually less than 1 μm even 100 nm. After electrochemical deposited, the 3D porous structure is well maintained and shows no obvious change comparing to the original 3D porous Ni films as shown in Fig. 4g–i. It suggests the in situ formed Ni(OH)2 layer uniformly on the porous Ni scaffold. Comparing to the previous reported works about commercial low porous Ni foam due to large pore size (even more than 300 μm) and smooth wall for support Ni(OH)2 capacitive thin film,5–7 the as prepared Ni current collector skeleton with 3D highly and hierarchically porous architecture would be expected to exhibit excellent electrochemical performance due to the high surface area and open porous structure for easily electrolyte penetration/diffusion as well highly porous interconnected Ni metallic network for fast electron conduction.34 In addition, the Ni(OH)2 active material is in situ formed by anode oxidation of thin layer of outer 3D highly porous nickel skeleton itself while not obtained by deposition on Ni current collector, which might have better adhesion and low resistance between the Ni(OH)2 capacitive material and Ni current collector.
 | ||
| Fig. 4 SEM images of (a) smooth Ni substrate, (b and c) smooth Ni(OH)2/Ni films, (d–f) 3D porous Ni films and (g–i) 3D porous Ni(OH)2/Ni films. | ||
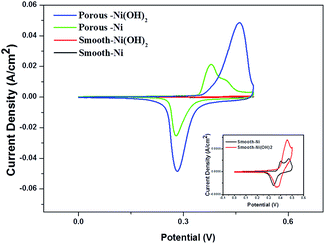
Fig. 5 presents the CV curves of the different Ni-based electrodes at the scan rate of 10 mV s−1 in the potential range from 0 V to 0.5 V in 6 M KOH electrolyte. In the first case, a pair of strong redox peaks is obviously observed for all the Ni-based electrodes. It is a typical pseudocapacitance faradaic redox behavior, which is very distinguished from the electric double layer capacitance that normally close to an ideal rectangular shape of CV curve.35 The redox peaks of the Ni(OH)2 electrodes correspond to the conversion between Ni(OH)2 and NiOOH, which can be simply expressed as follows:36
| Ni(OH)2 + OH− ⇔ NiOOH + H2O + e− |
The anodic peak is due to the oxidation of Ni(OH)2 to NiOOH, and the cathodic peak is for the reverse process.23 The symmetric characteristic of anodic peak and cathodic peak reveals the excellent reversibility of the Ni(OH)2 electrodes. The porous Ni and smooth Ni electrodes also illustrate similar CV curves to that of Ni(OH)2 since metallic Ni could be electrochemical oxidized to Ni(OH)2 in KOH solution as the in situ preparation of Ni(OH)2 layer on the porous Ni films. The 3D porous Ni(OH)2/Ni electrodes demonstrate stronger current response in comparison with the porous Ni metallic film electrodes, implying that the pseudocapacitance mainly results from the Ni(OH)2 film.31 Moreover, the peak currents of the 3D porous Ni(OH)2/Ni composite film electrodes are also much higher than that of the smooth Ni(OH)2/Ni electrodes, indicating superior capacitance of the porous Ni(OH)2/Ni electrodes due to the large area surface of Ni(OH)2 on the porous Ni metallic film current collectors.
Fig. 6a shows the cyclic voltammograms (CV) of the 3D porous Ni(OH)2/Ni composite film electrodes at scan rates from 10 to 100 mV s−1 in the range between 0 and 0.5 V. Apparently, the shape of all the curves revealed that the capacitance mainly resulted from the pseudocapacitive capacitance. The shapes of CV curves are not significantly influenced by the increasing of the scan rates, indicating the improved mass transportation and electron conduction and the stable electrochemical capacitive behavior of the 3D porous Ni(OH)2/Ni electrode. Moreover, the symmetry of two sets of redox peak reveals the good reversibility of the redox in the system. Furthermore, Fig. 6b shows the relation between oxidation/reduction peak current and the voltage scan rate of CV. The linear relationship between them reveals that the fast redox reaction of Ni(OH)2 is controlled by surface absorption while not diffusion limitation.24,25 It also indicates that the as-prepared porous Ni(OH)2 electrodes possess highly porous structure for electrolyte fast diffusion.
 | ||
| Fig. 6 (a) Cyclic voltammograms of 3D porous Ni(OH)2/Ni film electrode obtained at various scan rates, and (b) the relationship between the peak current and scan rate. | ||
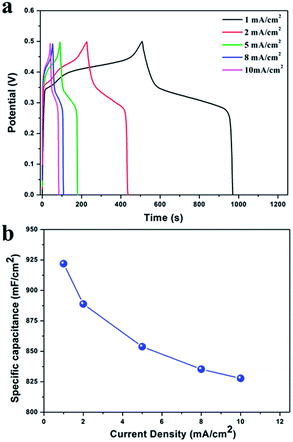
Fig. 7 illustrates the constant current charge–discharge curves of the 3D porous Ni(OH)2/Ni electrodes in 6 M KOH aqueous solution at various current densities within the potential window of 0 to 0.5 V. All the curves exhibit one couple of obvious charge and discharge plateaus, resulting from the transition between Ni(OH)2 and NiOOH, indicating a typical pseudocapacitance behaviour, which is consistent with the results of CV test.10 The specific capacitance can be calculated from the following equation:
To evaluate the long-term cyclic stability of 3D porous Ni(OH)2/Ni film electrodes for electrochemical capacitors, galvanostatic charge–discharge measurements were carried out for 1000 cycles at the current density of 10 mA cm−2. As shown in Fig. 8, the specific capacitance of the 3D porous Ni(OH)2/Ni film electrode gradually decreases in initial 200 cycles, and henceforth tends to level off with the electrochemical cycling. Finally, approximately 5% loss of specific capacitance after 1000 cycles indicates that the repetitive charge–discharges do not induce noticeable degradation of the 3D porous Ni(OH)2/Ni film electrodes. All the 3D porous Ni, smooth Ni(OH)2/Ni and Ni film electrodes demonstrate good cyclic stability, originating from the high redox reaction reversibility of Ni(OH)2/NiOOH. The 3D porous Ni(OH)2/Ni film electrodes exhibit greatly stable specific capacitance of about 828 mF cm−2 than those of 3D porous Ni film electrodes (about 569 mF cm−2), smooth Ni(OH)2/Ni film electrodes (about 126 mF cm−2) and smooth Ni foil electrodes (about 53 mF cm−2) although the 3D porous structure does not improve the cycle stability. It also should be noted that the capacitance of porous Ni film, smooth Ni(OH)2/Ni film, and smooth Ni foil electrodes increased with the continuous charge–discharge cycles in the staring stages. It might be originated from the metallic Ni current collector in situ conversion to Ni(OH)2 active materials in the electrochemical cycling.37
 | ||
| Fig. 8 Cycling performance of different electrodes at the current density of 10 mA cm−2 after 1000 cycles. | ||
The 3D network structure electrodes with high specific surface area and 3D conductive network scaffold to provide a highly conductive pathway for electrons and the catalyst of the conversion reaction to enhance the reverse reaction, meanwhile its interconnected porous network facilitate the rapid ion transport, shorten the solid-phase ion diffusion length to minimize the effect of sluggish solid-state ion transport and provide more electroactive sites for supercapacitor. The 3D porous structure plays a crucial role in optimizing superior capacitive performance of the Ni(OH)2 film, which is a good candidate as an electrode material for electrochemical capacitor. Thus the 3D porous Ni(OH)2/Ni film is a promising electrode for high performance supercapacitors.
Conclusions
3D porous Ni(OH)2/Ni integrated film electrodes are fabricated by simple electrochemical methods of hydrogen bubble template electrodeposition then in situ anode oxidation. The 3D porous Ni(OH)2/Ni integrated film electrodes demonstrate greatly higher stable specific capacitance of 828 mF cm−2 than that of smooth Ni(OH)2/Ni film electrodes (about 126 mF cm−2) at the current density of 10 mA cm−2. The 3D porous Ni(OH)2/Ni integrated film electrodes also exhibit excellent cyclic stability and rate capability. The high electrochemical capacitive performance of 3D porous Ni(OH)2/Ni integrated film electrodes might result from the high surface area of Ni(OH)2 layer in situ directly formed on the highly porous interconnected conductive Ni current collectors.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (no. 21203236, 21171015), Guangdong and Shenzhen Innovative Research Team Program (no. 2011D052, KYPT20121228160843692), Shenzhen Electronic Packaging Materials Engineering Laboratory (2012-372), Shenzhen Electronic Packaging and Device Assembly Key Laboratory (ZDSYS20140509174237196), the “1000 plan” from Chinese Government, program for New Century Excellent Talents in University (NCET), Sofjakovalevskaja award from Alexander von Humboldt Foundation.Notes and references
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- A. S. Arico, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4269 CrossRef CAS PubMed.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, 28–62 CrossRef PubMed.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- G. W. Yang, C. L. Xu and H. L. Li, Chem. Commun., 2008, 6537–6539 RSC.
- Y. Zhang, H. Feng, X. Wu, L. Wang, A. Zhang, T. Xia, H. Dong, X. Li and L. Zhang, Int. J. Hydrogen Energy, 2009, 34, 4889–4899 CrossRef CAS.
- U. M. Patil, K. V. Gurav, V. J. Fulari, C. D. Lokhande and O. S. Joo, J. Power Sources, 2009, 188, 338–342 CrossRef CAS.
- J. Li, F. Luo, X. Tian, Y. Lei, H. Yuan and D. Xiao, J. Power Sources, 2013, 243, 721–727 CrossRef CAS.
- H. Jiang, T. Zhao, C. Li and J. Ma, J. Mater. Chem., 2011, 21, 3818–3823 RSC.
- H. Wang, H. S. Casalongue, Y. Liang and H. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- F. Zhang, D. Zhu, X. A. Chen, X. Xu, Z. Yang, C. Zou, K. Yang and S. Huang, Phys. Chem. Chem. Phys., 2014, 16, 4186–4192 RSC.
- G. Hu, C. Li and H. Gong, J. Power Sources, 2010, 195, 6977–6981 CrossRef CAS.
- G. Zhang and X. W. Lou, Adv. Mater., 2013, 25, 976–979 CrossRef CAS PubMed.
- Z. Tang, C. H. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272–1278 CrossRef CAS.
- H. Chang, J. Kang, L. Chen, J. Wang, K. Ohmura, N. Chen, T. Fujita, H. Wu and M. Chen, Nanoscale, 2014, 6, 5960–5966 RSC.
- X. C. Dong, H. Xu, X. W. Wang, Y. X. Huang, M. B. Chan-Park, H. Zhang, L. H. Wang, W. Huang and P. Chen, ACS Nano, 2012, 6, 3206–3213 CrossRef CAS PubMed.
- U. Singh, A. Banerjee, D. Mhamane, A. Suryawanshi, K. K. Upadhyay and S. Ogale, RSC Adv., 2014, 4, 39875–39883 RSC.
- J. T. Zhang, S. Liu, G. L. Pan, G. R. Li and X. P. Gao, J. Mater. Chem. A, 2014, 2, 1524–1529 CAS.
- J. Ji, L. L. Zhang, H. Ji, Y. Li, X. Zhao, X. Bai, X. Fan, F. Zhang and R. S. Ruoff, ACS Nano, 2013, 7, 6237–6243 CrossRef CAS PubMed.
- X. Chen, K. Sun, E. Zhang and N. Zhang, RSC Adv., 2013, 3, 432–437 RSC.
- J. Xiao, S. Yang, L. Wan, F. Xiao and S. Wang, J. Power Sources, 2014, 245, 1027–1034 CrossRef CAS.
- H. C. Shin, J. Dong and M. L. Liu, Adv. Mater., 2003, 15, 1610–1614 CrossRef CAS.
- J. Yan, W. Sun, T. Wei, Q. Zhang, Z. Fan and F. Wei, J. Mater. Chem., 2012, 22, 11494–11502 RSC.
- X. Tian, C. Cheng, L. Qian, B. Zheng, H. Yuan, S. Xie, D. Xiao and M. M. F. Choi, J. Mater. Chem., 2012, 22, 8029–8035 RSC.
- M. S. Wu and J. F. Wu, J. Power Sources, 2013, 240, 397–403 CrossRef CAS.
- Y. Li, W. Z. Jia, Y. Y. Song and X. H. Xia, Chem. Mater., 2007, 19, 5758–5764 CrossRef CAS.
- Q. Q. Xiong, J. P. Tu, Y. Lu, J. Chen, Y. X. Yu, X. l. Wang and C. D. Gu, J. Mater. Chem., 2012, 22, 18639–18645 RSC.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, Y. J. Mai, X. L. Wang, C. D. Gu and X. B. Zhao, J. Phys. Chem. C, 2011, 115, 22662–22668 CAS.
- Z. Wu, X. L. Huang, Z. L. Wang, J. J. Xu, H. G. Wang and X. B. Zhang, Sci. Rep., 2014, 4, 3669–3677 Search PubMed.
- D. S. Kong, J. M. Wang, H. B. Shao, J. Q. Zhang and C. N. Cao, J. Alloys Compd., 2011, 509, 5611–5616 CrossRef CAS.
- J. Yan, Z. Fan, W. Sun, G. Ning, T. Wei, Q. Zhang, R. Zhang, L. Zhi and F. Wei, Adv. Funct. Mater., 2012, 22, 2632–2641 CrossRef CAS.
- J. W. Lee, T. Ahn, D. Soundararajan, J. M. Ko and J. D. Kim, Chem. Commun., 2011, 47, 6305–6307 RSC.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, Y. J. Mai, X. L. Wang, C. D. Gu and X. B. Zhao, J. Phys. Chem. C, 2011, 115, 22662–22668 CAS.
- D. D. Zhao, S. J. Bao, W. H. Zhou and H. L. Li, Electrochem. Commun., 2007, 9, 869–874 CrossRef CAS.
- H. Du, L. Jiao, K. Cao, Y. Wang and H. Yuan, ACS Appl. Mater. Interfaces, 2013, 5, 6643–6648 CAS.
- W. Xing, S. Qiao, X. Wu, X. Gao, J. Zhou, S. Zhuo, S. B. Hartono and D. Hulicova-Jurcakova, J. Power Sources, 2011, 196, 4123–4127 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |