Multi-functional mesoporous β-Ga2O3: Cr3+ nanorod with long lasting near infrared luminescence for in vivo imaging and drug delivery†
Xin-Shi Wanga,
Wei-Shuo Lia,
Jun-Qing Situa,
Xiao-Ying Yinga,
Hui Chena,
Yi Jin*b and
Yong-Zhong Du*a
aCollege of Pharmaceutical Sciences, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, P.R. China
bNational Pharmaceutical Engineering Center for Solid Preparation in Chinese Herbal Medicine, Jiangxi University of Traditional Chinese Medicine, Nanchang 56 Yangming Road, 330006, P.R. China. E-mail: duyongzhong@zju.edu.cn; jinyizju@hotmail.com; Fax: +86 571 88208439; Tel: +86 571 88208439
First published on 6th January 2015
Abstract
The long lasting luminescent β-Ga2O3: Cr3+ nanorod allows detection in deep organs hours after injection based on the fact that it exhibits more than 72 h afterglow in the wavelength range of 650–850 nm after the cessation of ultraviolet light irradiation. Moreover, its mesoporous structure provides a reservoir for anticancer drugs.
Due to the development of sensitive optical sensors and powerful probes such as semiconductor nanocrystals,1–4 fluorescent proteins,5,6 and near-infrared fluorescentmolecules,7–9 optical imaging is an emerging tool for in vivo studies. However, it faces numerous disadvantages. The first is the auto-fluorescence10 from tissue organics resulting in a poor signal-to-noise ratio. In addition, deep tissue imaging is difficult because of intrinsic tissue signal attenuation.
Multi-functional nanoparticles combining imaging with drug delivery are being developed to improve the outcome of drug therapy. Notably, these multi-functional drug deliveries usually consist of more than one component, combined physically or chemically. However, in the fabrication of a multi-component composite nanosystem,11,12 multistep synthetic procedures and sometimes stringent synthetic conditions are involved. Some toxic surfactants or solvents are also employed in some reaction systems, which limits the application of a final material with significant toxicity.13 Moreover, a composite structure commonly results in the performance degradation of individual components and inhomogeneity of the morphology and properties of the constructed system.12
To address these difficulties, we have developed a uniform, mesoporous, nanoscale β-Ga2O3: Cr3+ by a universal and convenient method. The β-Ga2O3: Cr3+ nanorod can be optically excited before in vivo local or systemic injection and its long-lasting afterglow can eliminate background noise originating from in situ excitation. Also, the mesoporous nature of β-Ga2O3: Cr3+ has been firstly exploited to serve as a reservoir for anticancer drug storage and controlled drug release.
Herein, the β-Ga2O3: Cr3+ nanorod was synthesized by the hydrothermal process followed by calcination. The GaOOH: Cr3+ was first synthesized through an improved hydrothermal process, in which not only reaction temperature but also the time decreased, and also PEG400 was employed as a template to orient the attachment of the GaOOH: Cr3+. After calcination of the obtained GaOOH: Cr3+, the mesoporous β-Ga2O3: Cr3+ nanorod was synthesized. The crystal structure of the GaOOH: Cr3+ was confirmed by XRD (see Fig. S1A, ESI†); also, the transmission electron microscope (TEM) image of the GaOOH: Cr3+ (see Fig. 1A) showed a rod-like shape of approximately 500 nm length and 250 nm width, whose hydrodynamic diameter was measured at 360 ± 87 nm (see Fig. S2, ESI†). Notably, the calcination after which the GaOOH: Cr3+ transformed into β-Ga2O3: Cr3+, imparted the β-Ga2O3: Cr3+ with a mesoporous structure (see Fig. 1B) without any changes to its original shape and size (see Fig. 1B), which provides the possibility for the final β-Ga2O3: Cr3+ to be applied in vivo.
Similarly, β-Ga2O3: Cr3+ was confirmed by XRD (see Fig. S1B, ESI†). The weight content of Cr3+ doped in β-Ga2O3 was 0.24% (see Fig. S3, ESI†), and there was a weight loss during the conversion from GaOOH: Cr3+ to β-Ga2O3: Cr3+ of 14.05% (see Fig. S4, ESI†), as analyzed by thermogravimetry. The excitation spectrum and emission spectrum of the β-Ga2O3: Cr3+ nanorod are shown in Fig. 1C. The emission spectrum band was quite large (650–850 nm) in the near infrared range. The mesoporous structure of the β-Ga2O3: Cr3+ was confirmed by nitrogen adsorption–desorption isotherm (see Fig. 1D). The isotherm can be classified as a type IV, which is the characteristic of a mesoporous structure.
To investigate the long-lasting near infrared luminescence of the synthesized β-Ga2O3: Cr3+, the nanorod was excited by UV. Subsequently the long-lasting luminescence signal was collected by the in vivo imaging system after ceasing the UV. As shown in Fig. 2, the persistent near infrared luminescence of the nanorod could be detected even at 72 h post-excitation, and, furthermore, the material could be excited repeatedly with the same emission intensity.
 | ||
| Fig. 2 Time dependence of the luminescence intensity of the β-Ga2O3: Cr3+ nanorods and the afterglow image of β-Ga2O3: Cr3+ re-excited with white light. | ||
In order to study the cytotoxicity of the β-Ga2O3: Cr3+ nanorod, a Cell Counting Kit (CCK-8) assay was performed on the L929 and MCF-7 cell lines. As shown in Fig. 3A, the viabilities of the two kinds of cells were all above 85% when the concentration of β-Ga2O3: Cr3+ reached 800 μg ml−1.
Furthermore, the different levels of nitric oxide (NO, which is one of the pro-inflammatory mediators produced by macrophages, and which plays an important role in patho-physiological responses, including inflammation,14 infection,15 and neurodegenerative disorders16) produced by macrophage RAW 264.7 cells after exposure to different concentrations of β-Ga2O3: Cr3+ nanorod were detected. Fig. 3B showed that the levels of NO had no significant change among all the treatment groups, and were nearly the same as the control group, which presented more evidence for the low toxicity and considerable biocompatibility of the β-Ga2O3: Cr3+ nanorod.
The persistent near-infrared luminescence of the β-Ga2O3: Cr3+ nanorod in vivo was further studied. After being exposed to UV for 3 min, the suspension of β-Ga2O3: Cr3+ nanorod was subcutaneously injected into three different locations on the back of a mouse at different doses (from head to tail: 300 μg, 200 μg, 100 μg). As shown in Fig. 4A, the afterglow of the β-Ga2O3: Cr3+ nanorods at different doses was observed at 1 h post-injection (exposure time: 2 min) and the intensity of the signal increased with the increase in dose. However, when the nude mouse was exposed to the in vivo imaging system under the fluorescence mode, the auto-fluorescence of the nude mouse was so strong that it severely interfered with the targeted fluorescence (see Fig. S5, ESI†), which testified the superiority of the afterglow in bio-imaging.
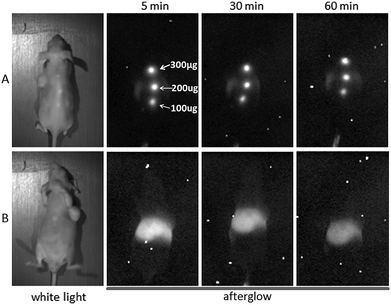 | ||
| Fig. 4 In vivo near infrared persistent luminescence images acquired by the in vivo imaging system after a subcutaneous injection (A) and after an intravenous injection (B). | ||
To testify whether the near infrared afterglow signal in the deep tissue can be easily monitored or not, a suspension of the β-Ga2O3: Cr3+ nanorod was intravenously injected. As shown in Fig. 4B, at 1 h-post i.v. injection, the β-Ga2O3: Cr3+ mainly accumulated in the liver, resulting in an intense afterglow, and at 48 h-post i.v. injection, as a result of the EPR effect, the β-Ga2O3: Cr3+ gradually accumulated into the tumour and an afterglow signal was observed in the tumour from the result of the ex vivo imaging (see Fig. 5).
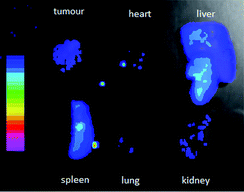 | ||
| Fig. 5 Afterglow image of the isolated organs and tumour from a mouse bearing Hela tumour at 48 h post i.v. injection of β-Ga2O3: Cr3+ re-excited by white light. | ||
To investigate the potential of the mesoporous β-Ga2O3: Cr3+ nanorod as a drug reservoir, DOX, which is a model anticancer drug, was chosen to evaluate the drug loading rate and release kinetics. After stirring with DOX solution in PBS for 12 h, the DOX-loaded β-Ga2O3: Cr3+ nanorod was collected by centrifugation, followed by washing with PBS. The loading rate was 8.0%. The burst and sustained release kinetics of DOX from the β-Ga2O3: Cr3+ nanorod were shown in Fig. 6A. The profiles clearly showed that the pH of the medium had a strong effect on the DOX release rate from the β-Ga2O3: Cr3+ nanorod. The DOX release rate in pH 5.5 was higher than that in pH 7.4, which demonstrated that in the physiological environment, DOX was released slowly from the nanorod. When the nanorod was uptaken by the tumor cells, the intracellular acidic environment accelerated the drug release.
 | ||
| Fig. 6 (A) Release profiles of DOX from the β-Ga2O3: Cr3+ nanorod under different pH. (B) Vibrations of cells viabilities against DOX-loaded β-Ga2O3: Cr3+ nanorod concentrations. | ||
The pharmacological effect of the drug-loaded nanorod against L929 and MCF-7 cells was assessed by a CCK-8 assay. As shown in Fig. 6B, the cytotoxicity of the DOX-loaded β-Ga2O3: Cr3+ nanorod was significantly improved compared with the blank β-Ga2O3: Cr3+ nanorod (see Fig. 3A). This may be explained by the fact that DOX was released from the carriers to kill the tumour cells.
Conclusions
In summary, uniform and nanosized β-Ga2O3: Cr3+ was synthesized via a convenient and ground-saving method. The long-lasting luminescent β-Ga2O3: Cr3+ nanorod allowed detection in rather deep organs hours after injection. Moreover, its mesoporous structure provided a reservoir for anticancer drugs and could realize a pH-dependent drug release. The nanosized mesoporous β-Ga2O3: Cr3+ fabricated herein is full of potential as a multifunctional drug delivery.Acknowledgements
This work was supported by the Scientific Research Fund of Ministry of Health – Medical Science Major Technology Fund Project of Zhejiang Province (no. WKJ2012-2-023), National Nature Science Foundation of China (no. 81373345), and the Nature Science Foundation of Zhejiang province(no. LZ13H300001).Notes and references
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS PubMed.
- B. Dubertret, P. Skourides, D. J. Noriis, V. Noireaux, A. H. Brivanlou and A. Libchader, Science, 2002, 298, 1759 CrossRef CAS PubMed.
- M. K. So, C. Xu, A. M. Loening, S. S. Gambhir and J. Rao, Nat. Biotechnol., 2006, 24, 339 CrossRef CAS PubMed.
- B. Ballou, B. C. Lagerholm, L. A. Ernst, M. P. Bruchez and A. S. Waggoner, Bioconjugate Chem., 2004, 15, 79 CrossRef CAS PubMed.
- S. Bhaumik and S. S. Gambhir, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 377 CrossRef CAS PubMed.
- C. H. Contag and M. H. Bachmann, Annu. Rev. Biomed. Eng., 2002, 4, 235 CrossRef CAS PubMed.
- Z. Cheng, J. Levi, Z. Xiong, O. Gheysens, S. Keren, X. Chen and S. S. Gambhir, Bioconjugate Chem., 2006, 17, 662 CrossRef CAS PubMed.
- R. Weissleder, C. H. Tung, U. Mahmood and A. Bogdanov, Nat. Biotechnol., 1999, 17, 375 CrossRef CAS PubMed.
- A. Becker, C. Hessenius, K. Licha, B. Ebert, U. Sukowski, W. Semmler, B. Wiedenmann and C. Grötzinger, Nat. Biotechnol., 2001, 19, 327 CrossRef CAS PubMed.
- J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626 CrossRef CAS PubMed.
- D. Shi, H. Cho, Y. Chen, H. Xu, H. Gu and J. Lian, Adv. Mater., 2009, 21, 217 CrossRef.
- Z. Zhelev, H. Ohba and R. Bakalova, J. Am. Chem. Soc., 2006, 128, 6324 CrossRef CAS PubMed.
- C. Zhang, C. Li, C. Peng, R. Chai, S. Huang and D. Yang, Chem.–Eur. J., 2010, 16, 5672 CrossRef CAS PubMed.
- Y. Kobayashi, J. Leukocyte Biol., 2010, 88, 1157 CrossRef CAS PubMed.
- L. A. Perrone, J. A. Belser, D. A. Wadford, J. M. Katz and T. M. Tumpey, J. Infect. Dis., 2013, 207, 1576 CrossRef CAS PubMed.
- R. A. Roberts, D. L. Laskin, C. V. Smith, F. M. Robertson, E. M. Allen, J. A. Doorn and W. Slikker, Toxicol. Sci., 2009, 112, 4 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental, XRD, size distribution and TGA of GaOOH: Cr3+; XRD and EDS of β-Ga2O3: Cr3+; bioimaging of nude mouse with β-Ga2O3: Cr3+ fluorescence. See DOI: 10.1039/c4ra14348e |
| This journal is © The Royal Society of Chemistry 2015 |


