Selective functionalisation of TNT for sensitive detection by SERRS†
Callum J.
McHugh
,
Ruth
Keir
,
Duncan
Graham
* and
W. Ewen
Smith
*
Department of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, UK G1 1XL. E-mail: duncan.graham@strath.ac.uk; Fax: 0141 552 0876; Tel: 0141 546 4701
First published on 18th February 2002
Abstract
Selective chemical functionalisation of 2,4,6-trinitrotoluene to a surface enhanced resonance Raman active species for sensitive detection.
Selective detection of explosives at ultra low concentration levels is a requirement for modern security systems.1–5 Raman spectroscopy provides a simple method of detecting and identifying explosives in bulk samples or as crystals under a microscope.6–10 The sharp spectra obtained enable the identification of individual components of mixtures without the use of separation procedures.7 However, the inherent lack of sensitivity of Raman scattering makes it less effective for the identification of low concentrations of target molecules in dispersed samples in solution or the vapour phase.2 In this study, we show how the technique of surface enhanced resonance Raman scattering, SERRS, enables the detection of 2,4,6-trinitrotoluene (TNT) at low concentration levels. In SERRS, an analyte which contains a chromophore is adsorbed onto a roughened metal (usually silver or gold) and the enhanced Raman scattering recorded from the surface.11–15
Surface enhanced Raman scattering, SERS, has been obtained from 2,4,6-trinitrotoluene and 2,4-dinitrotoluene previously.16,17 The sensitivity was insufficient for vapour detection of TNT, which led to the application of SERRS in this study. As TNT does not possess a chromophore in the visible region and does not adsorb efficiently on silver or gold surfaces, chemical modification of the explosive was used to obtain a highly coloured TNT derivative containing a functionality that enabled a strong interaction with the metal surface (Scheme 1).
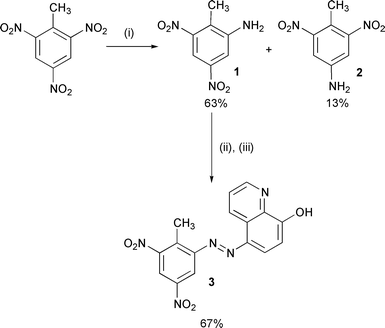 | ||
| Scheme 1 Reduction of TNT followed by azo dye formation (i) Fe/AcOH, (ii) HCl, NaNO2, (iii) NaOH, 8-HQ. | ||
There are a number of possible methods of modifying TNT18,19 but one way of producing a coloured species is to produce an azo dye by diazotisation of a reduced nitro group followed by coupling to a surface seeking species. There are three nitro groups present in TNT and in order to retain as much character of the original molecule as possible the aim was to selectively reduce only one of the nitro groups. Whilst, the reduction of mononitroaromatics is easy to achieve, the controlled reduction of polynitroaromatics such as TNT is more difficult.20 Reduction procedures have been reported previously for TNT and in each case mixtures of different reduction products were obtained.21–23 Therefore, a number of reducing systems were investigated to prepare the desired mono-amine derivative. The method chosen was that of Atkins and Wilson using iron in acetic acid,22 which gave complete reduction of TNT in 76% yield. Proton NMR of the crude reduction product revealed the presence of two species: 2-amino-4,6-dinitrotoluene [1] as 83% of the mixture and the remaining 17% yield as 4-amino-2,6-dinitrotoluene [2] (90% 2-amino and 10% 4-amino).22 Separation of the two isomers was simple. Recrystallization of the mixture resulted in the isolation of 2-amino-4,6-dinitrotoluene [1] as small yellow needles. Repeated fractional recrystallization of the remaining filtrate gave 4-amino-2,6-dinitrotoluene [2] again as yellow crystals. Thus, we could produce and isolate both mono-reduced forms of TNT in good yield by simple chemistry.
The diazotisation conditions for the 2-amino-4,6-dinitrotoluene [1] were investigated and diazotisation with HCl gave poor conversion to the diazonium salt. The yield was improved by dissolving the amine in hot HCl and then cooling rapidly in ice to precipitate the insoluble amine hydrochloride salt. Addition of aqueous nitrite at 0 °C caused dissolution of the hydrochloride to give a clear yellow solution of the diazonium salt which was added directly to the coupling component, without filtration, to produce good yields of dyes in very high purity.
Twenty two coupling reagents containing strong silver complexing groups and electron donating moieties for efficient diazo coupling, were examined. These included amine, pyridine, 1H-benzotriazole and quinoline derivatives. Thirteen novel TNT derived azo dyes were prepared, isolated and characterised. Each dye was assessed for ease of synthesis and efficiency of SERRS. We report the best coupling agent as being 8-hydroxyquinoline. It coupled rapidly with the diazotised aminodinitrotoluene in 10% NaOH solution to produce the sodium salt of 5-(2′-methyl-3′,5′-dinitrophenylazo)quinolin-8-ol [3] in 67% yield.
UV–visible spectrometry of the dye in methanol showed a strong absorption band at 408 nm (ε = 11000 dm3 mol−1 cm1) and a broad absorption shoulder at about 523 nm (ε = 3400 dm3 mol−1 cm1). The two bands corresponded to the azo and hydrazo forms of the dye respectively in a ratio of approximately 3∶1, indicating a predominance of the azo form under these conditions. The absorption spectra of the dye at pH 2, 6 and 8 were also examined. As expected at pH 2 the azo form of the dye was exclusively observed with a single λmax at 397 nm (ε = 9000 dm3 mol−1 cm1) and at pH 6 the azo form was still dominant with a strong absorption at 416 nm (ε = 7800 dm3 mol1 cm1). The hydrazo form of the dye was also observed at pH 6 as a broad shoulder at 515 nm (ε = 6060 dm3 mol−1 cm1). At pH 8 deprotonation of the phenol resulted in complete conversion to the keto-hydrazo form of the dye as observed by a single strong absorption at 523 nm (ε = 15700 dm3 mol−1 cm1)(Fig. 1).
 | ||
| Fig. 1 UV–vis spectra of the TNT dye at three different pH values. Dye dissolved in methanol/Hydrion buffer (1∶1). | ||
Based on the absorbance spectra, SERRS was acquired using a Renishaw microprobe with spectrometer and 3 mW of 514.5 nm radiation at the source, at neutral pH and at pH 9. (pH 9 was used as this was easier to achieve from a buffering point of view with respect to the colloid than pH 8 as used in the UV–visible studies.) The samples were analysed using a flow cell that has shown to deliver more reproducible signals due to control of the mixing of analyte, colloid and aggregating agent.24 Additionally, use of the flow cell for mixing the dye with colloid and aggregating agent is the first step in production of an automated system for TNT detection by SERRS (Fig. 2).
Clear differences were found between the spectra of the dye at different pH values. At neutral pH the major bands at 872, 1170, 1310, 1371, 1413 and 1559 cm−1 were assigned to nitro bending and stretching, hydrazo and azo vibrations and were detected at a concentration of 1 × 10−6 M. Thus, a mixture of both tautomers was present under neutral conditions. On moving to pH 9 the spectral intensity of the bands increased and the bands altered to reflect the change in tautomerism. The dominating bands in this spectrum are those from the hydrazo system at 1310 cm−1 and nitro at 1371 cm−1. Under these conditions the TNT dye could be easily detected at 1 × 10−9 M using a flow cell sampling device. This concentration is still well above the detection limit of SERRS and approaches the sensitivity needed for vapour detection of TNT.2
Thus, we have selectively derivatised trinitrotoluene and subsequently produced a SERRS active species in the form of an azo dye that can be easily detected at low concentration levels. Control of the chemistry and optimisation of the spectroscopy has provided a method capable of detecting TNT at ultra low concentrations which will be of real use in restricting the movement and deployment of explosives.
The authors wish to thank Dr Richard Lacey of the Home Office UK for funding to CM and RK and the BBSRC for the award of a David Phillips Fellowship to DG.
Notes and references
- J. Yinon and S. Zitrin, Modern Methods and Applications in the Analysis of Explosives, Wiley, New York, 1993 Search PubMed.
- P. Kolla, Angew. Chem., Int. Ed. Engl., 1997, 36, 800 CrossRef.
- P. Kolla, Anal. Chem., 1995, 67, 184A CAS.
- R. Speller, Radiation Phys. Chem., 2001, 61, 293 Search PubMed.
- K. G. Furton and L. J. Myers, Talanta, 2001, 54, 487 CrossRef CAS.
- T. Urbanski, Chemistry and Technology of Explosives (III), Pergamon Press, Oxford, 1965 Search PubMed.
- J. Akhaven, Spectrochim. Acta, 1991, 47A, 1247 Search PubMed.
- C. Cheng, T. E. Kirkbride, D. N. Batcheldor, R. J. Lacey and T. G. Sheldon, J. Forensic Sciences, 1995, 40, 31 Search PubMed.
- I. P. Hayward, T. E. Kirkbride, D. N. Batcheldor and R. J. Lacey, J. Forensic Sciences, 1995, 40, 883 Search PubMed.
- C. Passingham, P. J Hendra, C. Hodges and H. A. Willis, Spectrochim. Acta, 1991, 47A, 1235 Search PubMed.
- A. M. Stacy and R. P. Van Duyne, Chem. Phys. Lett., 1983, 102, 365 CrossRef CAS.
- P. Hildebrandt and M. Stockburger, J. Phys. Chem., 1984, 88, 5935 CrossRef CAS.
- C. Rodger, W. E. Smith, G. Dent and M. Edmondson, J. Chem. Soc., Dalton. Trans., 1996, 5, 791 RSC.
- K. Kneipp, H. Kneipp, I. Itzkan, R. R. Dasari and M. S. Feld, Chem. Rev., 1999, 99, 2957 CrossRef CAS.
- D. Graham, C. McLaughlin, G. McAnally, J. C. Jones, P. C. White and W. E. Smith, Chem. Commun., 1998, 1187 RSC.
- K. Kneipp, Y. Wang, R. R. Dasari, M. S. Feld, B. D. Gilbert, J. Janni and J. I. Steinfeld, Spectrochim. Acta, 1995, 51A, 2171 Search PubMed.
- J. M. Sylvia, J. A. Janni, J. D. Klein and K. M. Spencer, Anal. Chem., 2000, 72, 5834 CrossRef CAS.
- T. Urbanski, Chemistry and Technology of Explosives (I), Pergamon Press, Oxford, 1964 Search PubMed.
- T. Urbanski, Chemistry and Technology of Explosives (IV), Pergamon Press, Oxford, 1985 Search PubMed.
- M. Hudlicky, Reductions in Organic Chemistry, Ellis Horwood Limited, New York, 1984 Search PubMed.
- M. O. Terpko and R. F. Heck, J. Org. Chem., 1980, 45, 4992 CrossRef CAS.
- R. L. Atkins and W. S. Wilson, J. Org. Chem., 1986, 51, 2572 CrossRef CAS.
- A. T. Nielsen, R. A. Henry, W. P. Norris, R. L. Atkins, D. W. Moore and A. H. Lepie, J. Org. Chem., 1979, 44, 2499 CrossRef CAS.
- J. C. Jones, C. McLaughlin, D. Littlejohn, D. A. Sadler, D. Graham and W. E. Smith, Anal. Chem., 1999, 71, 596 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: full experimental details on the synthesis and analysis of the reported compounds. See http://www.rsc.org/suppdata/cc/b1/b110972c/ |
| This journal is © The Royal Society of Chemistry 2002 |

