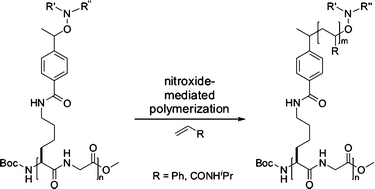
A series of peptides with an alternating sequence of alkoxyamine conjugated lysine and glycine residues were synthesized by classical solution phase peptide coupling. The resulting peptides containing up to eight alkoxyamine moieties were used as initiators in nitroxide-mediated polymerization (NMP) to obtain peptide–polymer conjugates with well defined linear peptide backbones and a defined number of polymeric side chains. Polymerization of styrene and N-isopropylacrylamide (NIPAM) occurred in a highly controlled fashion. Molecular weight and polydispersity index (PDI) were determined by gel permeation chromatography (GPC). Aggregation behaviour of these hybrid materials was investigated by dynamic light scattering (DLS) and atomic force microscopy (AFM). Depending on composition, number and length of the polymer side chains, the conjugates aggregate to different topologies. Whereas peptide–polystyrene conjugates may aggregate to so called honeycomb structures, peptide–poly-N-isopropylacrylamide conjugates show differentiated aggregation behaviour.