 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The life-cycle environmental performance of producing formate via electrochemical reduction of CO2 in ionic liquid†
Andrea
Paulillo
 *,
Martina
Pucciarelli
,
Fabio
Grimaldi
and
Paola
Lettieri
*,
Martina
Pucciarelli
,
Fabio
Grimaldi
and
Paola
Lettieri
Department of Chemical Engineering, University College London, UK. E-mail: andrea.paulillo@ucl.ac.uk
First published on 10th August 2021
Abstract
Carbon capture and utilisation provide a means to mitigate climate change caused by anthropogenic greenhouse gas emissions by delaying carbon emissions via temporary storage in goods. This article presents a comprehensive Life Cycle Assessment (LCA) study of a novel process that generates formate via electrochemical reduction of CO2 in ionic liquid. We performed a scenario analysis, covering uncertain parameters like the recycling rate of unreacted reagents and the market price of CO2, and compared the environmental performance of the carbon utilisation system with that of the conventional process, which relies on fossil sources. Inventory data is obtained from a mix of literature sources and commercial LCA databases. Our analysis indicates that (i) the system needs to attain a 99.9% recycling rate to be competitive with the conventional process; (ii) a future negative market price of CO2 would substantially reduce the environmental impacts associated with formate; (iii) there are significant environmental trade-offs between the carbon utilisation system and the conventional process, with the former outperforming the latter in 6/8 out of the 14 impact categories investigated. It should be noted that our results are conservative because inventory data for the electrochemical reduction process is obtained from laboratory experiments.
1 Introduction
The reduction of global greenhouse gas emissions to avoid catastrophic effects of climate change is arguably humanity's most challenging task of this century. The latest IPCC report indicates that to achieve the stricter target set by the Paris Agreement,1i.e. to keep the global temperature rise below 1.5 °C from pre-industrial levels, anthropogenic emissions of greenhouse gases need to fall by 45% from 2010 levels by 2030 and to reach net-zero by 2050.2 This effort will require a rapid and far-reaching transition to low-carbon alternatives in all sectors of the economy, and a wide portfolio of mitigation options; these must include technologies for capturing CO2 from flue gas, and either sequestering it in long-term sinks such as geological formations or utilising it as a solvent or as a feedstock for producing valuable products like chemicals and fuels.3–5 Carbon capture and storage (CCS) avoids CO2 emissions and, when applied to biogenic carbon, enables the permanent removal of CO2 from the atmosphere that is essential for achieving the Paris Agreement target. On the other hand, carbon capture and utilisation (CCU) delays CO2 emissions by temporary storing carbon in goods. The duration of the storage, and therefore the effectiveness in mitigating climate change, is dependent on the product; for example, it is typically shorter for fuels than for chemicals.4 An additional benefit of carbon utilisation is that it reduces the depletion of resources and environmental emissions by providing intermediate feedstocks.6 Traditional fuels as well as the majority of bulk chemicals – especially organic chemicals, alcohol and olefins – are in fact almost exclusively produced from fossil feedstock like natural gas and oil.7A multitude of technologies to utilise CO2 – including homogeneous, heterogeneous, photochemical and electrochemical catalytic conversion – have been developed to various (but mostly low) technological readiness levels.4,5,8,9 The electrochemical reduction (ER) of CO2 presents numerous advantages; a notable one is that it can be implemented at atmospheric temperature and pressure because the driving force of the reaction is controlled via the applied potential.10,11 The technology is typically compact and easily scalable (because of its intrinsic modular design),12 and can be used as a means to store excess electricity from intermittent energy sources like sunlight and wind.13,14 ER has been investigated using aqueous and non-aqueous molecular solvents and ionic liquids (ILs). The latter, in particular, has attracted increasing interests in recent years because of a number of properties, including negligible vapour pressures (which is also important for carbon capture applications), high CO2 solubility and intrinsic ionic conductivity.4,10,15 Notably, these properties can be tailored to specific applications via synthetic alteration of anions and cations independently.4 Some of them also provide the opportunity to regenerate the solvent at various ranges of temperature and pressure, which is not available for conventional solvent alternatives.4
In this article, we focus on an electrochemical reduction process that uses a novel IL – trihexyltetradecylphosphonium 1,2,4-triazolide ([P66614][124Triz]) – for the production of formate, the anionic form of formic acid. [P66614][124Triz] is a super-basic room temperature IL that can chemisorb CO2 in nearly equimolar quantities and that requires low overpotentials to reduce CO2 compared to other ILs.15,16 Formate can in turn be easily converted into formic acid, a widely used chemical in numerous applications such as de-icing (as salt of formic acid), cleaning (descaling and cleaning bathroom surfaces and toilet), pH regulator (in the bleaching operations of the textile industry and in the dyeing and tanning of the leather sector).17 Formic acid can also be used to generate electricity for low-power appliances in fuel cells, typically known as Direct Formic Acid Fuel Cells;18 notably, formic acid holds many advantages over methanol for this application, including easier transports and storage conditions.19 In 2018, global trade of formic acid had a value of $430 million; it grew by nearly 60% between 2017 to 2018,20 and is projected to further grow at a compound annual rate of ∼4% from 2019 to 2024.21
The objective of this study is to quantify the environmental performance of the novel IL-based ER process for producing formate, in particular in comparison with the conventional alternative that uses fossil feedstock, using Life Cycle Assessment (LCA). LCA is a widely adopted and ISO standardised22,23 methodology for quantifying the environmental impacts of products in a holistic manner, which entails adoption of a life-cycle perspective and coverage of a wide variety of environmental issues that include but are not limited to climate change. This holistic perspective enables identification of trade-offs, and thus make LCA a robust tool to support decision and policy-making. The LCA methodology has been increasingly applied to assess the environmental performance (primarily focusing on carbon emissions) of carbon utilisation technologies,24,25 including that of electrochemical reduction of CO2 and for the production of formate. To the best of the authors’ knowledge, no LCA study in the literature has investigated the environmental performance of an IL-based ER process to produce formate. The general approach to implement LCA for assessing CCU technologies, including common pitfalls, is discussed by von der Assen.26,27 Dominguez-Ramos and co-authors11 quantified the carbon intensity of formate production via electrochemical reduction in aqueous solution using data from laboratory experiments and simulations models. In 2019, Rumayor et al.12 investigated the effects of the cathode's lifetime on the environmental performance using data from Dominguez-Ramos et al. They compared the environmental performance of producing formate via electrochemical reduction of CO2 and via homogenous catalysis of CO2 and H2.28 These studies demonstrated that producing formate from electrochemical reduction can be competitive, and under optimistic conditions even advantageous, compared with the carbon footprint of the conventional process. Ahn et al.,29 which investigated the environmental performance of formate production via CO2 and H2, presents one of the few studies focusing on several environmental categories other than climate change.
The remainder of this article is organised as follows: section 2 describes the ER process and introduces the LCA study in terms of goal and scope, inventory data and impact assessment method; section 3 presents the LCA results, which are discussed in section 4; the key conclusions are summarised in section 5.
2 Methods
2.1 System description
Fig. 1 reports a schematic diagram of the novel system that utilises CO2via electrochemical reduction (ER) for producing formate. The ER process, which uses the super-basic room temperature ionic liquid (IL) [P66614][124Triz], is based on the experimental set-up developed by Hollingsworth et al.16 This envisages driving an electric current with an applied potential of 0.7 V through a platinum and a silver electrode immersed in 8 ml solution of acetonitrile, IL [0.1 M] and water [5.6 M], while CO2 is bubbled at a flow rate of 15 ml min−1. (The experimental set-up also included a reference electrode [Ag/Ag+], against which potentials were measured.) In these conditions, formate is produced with a faradaic efficiency of 95%.16 | ||
| Fig. 1 Schematic diagram of the system investigated that produces formate via electrochemical reduction in ionic liquid of CO2 captured from natural gas power plant. | ||
We assume that the resulting solution is fed into a purification phase where formate is separated from unreacted reagents and wastes, and concentrated. Unreacted reagents are re-circulated and mixed with fresh synthesis solution; wastes, which we assume include unseparated reagents as well as ionic liquid that may be degraded, are treated as hazardous waste and incinerated.30 Of note, the low applied potential may not lead to degradation of IL, but, to the best of the Authors’ knowledge, this is yet to be experimentally confirmed. In the absence of data in the literature, we model the purification phase based on that developed by Dominguez-Ramos et al.11 for a water-based ER process, which envisages a gas/liquid separation unit and a distillation column.
Although ionic liquids are widely considered an advantageous alternative sorbent for CO2 capture,4 the effects of impurities on the ER process are yet to fully understood. For this reason, we assume that CO2 is obtained from the exhaust gas of a combined-cycle power plant that uses natural gas as feedstock. The CO2 is captured in a post-combustion capture unit based on chemical absorption using mono-ethanolamine (MEA) as solvent. The MEA-based capture technology, which can easily be retrofitted to existing plants without making substantial changes to their design, is assumed to achieve a capture efficiency of 90%, producing a nearly pure (typically >99%) CO2 stream;31 this is because the technology also captures others pollutants, including SO2, NO2, HCl and NH3. For the purpose of this study, we make the simplifying assumption that the captured-CO2 stream does not require any purification process.
2.2 Goal and scope
The goal of this study is to evaluate the environmental performance of producing formate via electrochemical reduction of captured CO2. We perform a scenario analysis to investigate the effect of modelling assumptions and uncertain parameters and to identify the highest environmental performance that could be achieved under optimistic conditions. We identify environmental hot-spots to suggest improvements in eco-efficiency, and compare the environmental performance of the carbon utilisation system with that associated with the conventional process for producing formic acid (of note, formate can be easily converted into formic acid, and vice versa); this envisages synthesis via hydrolysis of methyl formate and hinges on fossil fuels, primarily natural gas as feedstock.17We adopt a prospective perspective and an attributional approach. The former implies evaluation of the environmental impacts that will occur in the future, when the process is implemented at scale. The latter entails that the analysis does not account for the environmental consequences – including direct substitution of products and price rebound mechanisms – that could follow implementation.32–35 The functional unit corresponds to the production of 1 kg of formate. The system boundaries, which are schematically reported in Fig. 2, are “cradle-to-gate”: they include all activities from the extraction of raw materials to the production of formate, excluding the use phase of formate and its final disposal.
 | ||
| Fig. 2 Schematic diagram of the “cradle-to-gate” system boundaries that are considered in this study. | ||
2.3 Scenarios
The scenario analysis covers two operational parameters – the recycling rate of unreacted reagents and thermal energy requirements of the purification phase – the market value of CO2 and therefore the resulting allocation strategy, and current and prospective electricity mixes; the parameters that are investigated in the scenario analysis are reported in Table 1. The scenarios covering the operational parameters reflect the uncertainty of the process when implemented at scale. The recycling rate is dependent on the proportion of degraded ionic liquid (if any) and the efficiency of the purification phase, and affects the amounts of unreacted reagents (i.e., acetonitrile and ionic liquid) that are recycled and of hazardous wastes that are sent for disposal. We defined four recycling rates: 95%, 99%, 99.5% and 99.9%. A 95% recycling rate implies that 95% of the solution that remains after purification is recycled, with 5% being disposed of as hazardous waste. The thermal energy consumption in the purification phase represents another important operational parameter. We investigated three scenarios using literature data from Dominguez Ramos et al.;11 these represent a commercial-scale operation (35 MJ kg−1 of formate), laboratory-based conditions (150 MJ kg−1) and optimal operating conditions where the thermal consumption corresponds to the latent heat of vaporization of formate (0.483 MJ kg−1).| Parameter | Values | |||
|---|---|---|---|---|
| Recycling rate | 95% | 99% | 99.5% | 99.9% |
| Thermal energy consumption (purification) | 150 MJ kg−1 of formate | 35 MJ kg−1 of formate | 0.483 MJ kg−1 of formate | |
| Electricity mix (EU-28) | 2020 | 2030 | 2050 | |
| Market value of CO2 and partitioning factors | Nil market value. | Negative market value (CO2 as a waste). | Positive market value (CO2 as a product). | |
| No partitioning factors. | Partitioning factors: 80%; 90%; 95%. | Partitioning factors: 10%; 50%; 90%. | ||
The market value of CO2 affects the allocation strategy and therefore the estimated environmental performance of the carbon utilisation system. Allocation is a procedure used in LCA to apportion the environmental impacts among the functions of multi-functional processes. Our scenario analysis covers three possibilities: CO2 with a market value higher, lower and equal to zero; the resulting allocation of the environmental impacts is schematically represented in Fig. 3. When the CO2 has a negative market value, it is considered a waste; this entails that the ER process is multi-functional -i.e., it produces a valuable product, formate, whilst managing a waste, CO2 – and that its environmental impacts need be allocated between its functions. Likewise, CO2 becomes a product when it has a positive market value. In this case, the environmental impacts of the carbon capture unit are to be allocated between its two functions: managing a waste, the flue gas, whilst producing a product, a pure CO2 stream. The case of a nil market value requires no allocation and assumes that the CO2 that is used in the electrochemical reduction process is burden-free, i.e. it has no associated environmental impacts.
 | ||
| Fig. 3 Schematic of the approach to allocating environmental impacts based on the market value of CO2. The yellow area identifies the portion of environmental impacts that is allocated to formate. | ||
In this study, we implement the allocation procedure using partitioning factors based on economic value,26i.e. the environmental impacts of multi-functional processes are distributed according to the revenues (estimated as the product of price and quantity) associated with the relevant products. We calculated the partitioning factors based on the price of formic acid,36 current and forecasted prices of CO237,38 and carbon taxes;39–42 these are reported in Table 2. Notably, we made the simplifying assumption that the carbon tax values are representative of the cost of disposing of CO2 when this has a negative market value. The resulting partitioning factors are reported in Table 1, and represent the proportion of the environmental impacts of the relevant process that are allocated to formate. For example, in the case of a negative market value of CO2, a partitioning factor of 80% means that 80% of the environmental impacts associated with the ER process are allocated to the function of formate production, with the remainder 20% to the function of waste management. On the other hand, when the CO2 has a positive market value, a partitioning factor of 10% entails that 10% of the environmental impacts of the carbon capture unit are allocated to the production of CO2.
The last parameter covered by the scenario analysis is the electricity grid mix. ER is inherently an energy-intensive process; this implies that the environmental performance of formate production can be significantly affected by the source of electricity and therefore by the electricity grid mix. Our analysis covers three electricity mixes that are representative of the average electricity grid mix in the European Union in 2020, 2030 and 2050.
2.4 Life cycle inventory
The inventory for the foreground system is based on a mix of literature and laboratory experiment data, whilst the background system is modelled using data from commercial databases including Sphera (previously Thinkstep) professional database, service package 3643 and Ecoinvnent version 3.5.44 We assume that the production of formate occurs in Europe. The transportation of goods, unless not accounted for in the relevant datasets, was not explicitly considered.Table 3 reports inventory data for the electrochemical reduction of CO2 and for the subsequent purification phase, which was obtained from Hollingsworth et al.15,16 and Dominguez-Ramos et al.,11 respectively (see section 2.1). The amounts of reagents (i.e., water, ionic liquid, acetonitrile) and wastes are dependent on the recycling rate, and are reported according to the scenarios defined in section 2.3. We did not consider the potential degradation of the working electrode: this is believed to act as a catalyst for the reduction of CO2, and experiments did not show any evidence of degradation (A. Greer, personal communication, 2018).
| Quantity | Unit | ||
|---|---|---|---|
| Input flows | Captured carbon dioxide (gas) | 9.80 × 10−1 | kg |
| Ionic liquid- make up based on different recycling rates (RT) | RT 99.9%: 1.00 × 10−2 | kg | |
| RT 99.5%: 6.00 × 10−1 | |||
| RT 99%: 1.20 × 10−1 | |||
| RT 95%: 6.00 × 10−1 | |||
| Acetonitrile- make up based on different recycling rates (RT) | RT 99.90%: 3.00 × 10−2 | kg | |
| RT 99.50%: 4.30 × 10−1 | |||
| RT 99.00%: 9.30 × 10−1 | |||
| RT 95.00%: 4.900 × 101 | |||
| Water | 2.8 × 10 | kg | |
| Silver electrode | 4.10 × 10−6 | kg | |
| Platinum electrode | 8.40 × 10−6 | kg | |
| Electricity - stirring and heating | 4.51E × 101 | MJ | |
| Electricity- applied potential | 5.00 × 10−2 | MJ | |
| Thermal energy-separation of formate form ionic liquid | 3.60E × 101 | MJ kg−1 | |
| 1.50E × 102 | |||
| 4.83 × 10−1 | |||
| Output flows | Hazardous wastes – output | RT 99.90%: 1.10 × 10−1 | kg |
| Based on the different recycling rates (RT) | RT 99.50%: 5.60 × 10−1 | ||
| RT 99.00%: 1.11 × 10 | |||
| RT 95.00%: 5.60 × 10 | |||
| Formate | 1.00 × 10 | kg | |
As noted in section 2.1, we modelled the purification phase only on the basis of the amount of thermal energy required; the relevant data was obtained from Dominguez-Ramos et al.11 for the case of a water-based electrochemical reduction process. Data for ionic liquid production [P66614][124Triz] is obtained from Cuéllar-Franca et al.;45 Table S1 in the ESI† reports electricity consumption and amounts of primary precursors. Inventory data for the carbon capture unit is based on multiple literature sources;31,46–48 key operational data is reported in Table 4, whilst material requirements for construction and capture performances are included in Tables S2, S3 and S4 in the ESI.† It must be noted that we considered that the portion of flue gases that is not captured (and therefore that is released into the atmosphere) represents an emission of the carbon capture unit, which is allocated according to the strategies described in section 2.3. The electricity grid mixes are obtained from Sphera professional database (see Fig. S1†), whilst the conventional process for formate production in the UK is modelled using both Ecoinvent and Sphera datasets.
| Quantity | Unit | References | ||
|---|---|---|---|---|
| Input flows | Carbon dioxide in flue gas | 1.10E × 10 | kg | Rao and Rubin (2002)31 |
| Sodium hydroxide | 3.46 × 10−6 | kg | Rao and Rubin (2002)31 | |
| Monoethanolamine | 6.22 × 10−5 | kg | Koorneef et al. (2008); Veltman et al. (2010)46,47 | |
| Activated carbon | 1.99 × 10−6 | kg | Rao and Rubin (2002)31 | |
| Electricity | 3.40 × 10−1 | MJ | Koornneef et al. (2008)46 | |
| Output flows | Hazardous wastes | 6.38 × 10−5 | kg | Koornneef et al. (2008)46 |
| Captured carbon dioxide | 1.00E × 10 | kg | Rao and Rubin (2002)31 | |
2.5 Life cycle impact assessment
Environmental impacts are calculated using the ILCD/PEF version 1.09 method.49Table 5 reports the environmental categories that have been analysed. The product system was modelled with GaBi software.50| Impact category | Impact Category Indicator units |
|---|---|
| Acidification | mol H+ equivalent |
| Climate change | kg CO2 equivalent |
| Ecotoxicity for aquatic freshwater | CTUe (Comparative Toxic Unit for ecosystems) |
| Eutrophication, aquatic | Fresh water: kg P equivalent |
| Marine: kg N equivalent | |
| Eutrophication, terrestrial | mol N equivalent |
| Human toxicity, cancer effects | CTUh (Comparative Toxic Unit for humans) |
| Human toxicity, non- cancer effects | CTUh (Comparative Toxic Unit for humans) |
| Ionising radiation, human health effects | kg U235 equivalent (to air) |
| Ozone depletion | kg CFC-11 equivalent |
| Particulate matter/respiratory inorganics | kg PM2.5-eq per kg (intake fraction for fine particles) |
| Photochemical ozone formation | kg NMVOC (Non-Methane Volatile Organic Compounds) equivalent |
| Resource depletion, mineral, fossil | kg antimony (Sb) equivalent |
| Resource depletion, water | m3 water use related to local scarcity of water |
3 Results
3.1 Scenario analysis
Fig. 4 reports the effect of the recycling rate parameter on the environmental performance of formate production via electrochemical reduction of CO2 in the ionic liquid [P66614][124Triz]. We adopt a baseline scenario that includes commercial-scale estimate for thermal energy consumption in the purification phase (35 MJ per kg formate), the 2020 EU electricity grid mix and a nil market value for CO2. The environmental impacts are compared with those of the conventional process of formate production (see section 2.1), and expressed as percentage difference; for example, a value of 10% means that the carbon utilisation system has an environmental impact that is 10% greater than that of the conventional process. Numerical values are provided in the ESI.† With a recycling rate of 95%, the system yields environmental impacts that are between 2.5 and 50 times higher than those of the conventional process. The chart demonstrates the profound importance of the recycling rate parameter: the environmental performance improves significantly with increasing recycling rates. With a 99% recycling rate, the system outperforms the conventional process only in terms of water consumption, whilst underperforming by more than 100% in all other categories except for ozone depletion. At 99.5% the system achieves lower environmental impacts than the conventional process also in the ozone depletion category, whilst underperforming in two other environmental categories by less than 100%. At 99.9% the system outperforms the reference process by 30–70% in six environmental categories: freshwater ecotoxicity, freshwater eutrophication, human toxicity (cancer and non-cancer effects) ozone depletion and water consumption. In the remaining categories, the system has higher impacts, from 30% in the category respiratory inorganics and up to five times in the category ionising radiation.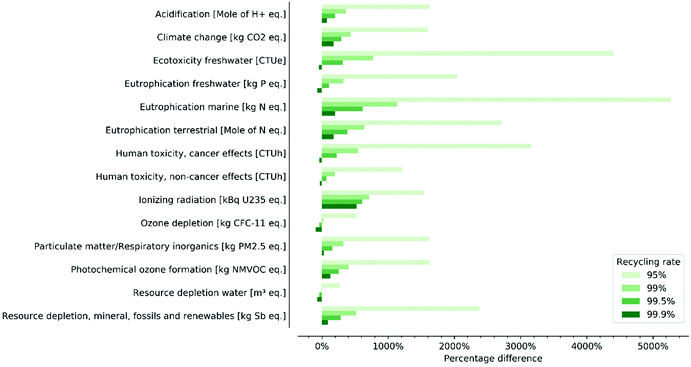 | ||
| Fig. 4 Percentage difference between environmental impacts of formate produced via electrochemical reduction and via the conventional alternative for four recycling rates. | ||
Fig. S2 and S3 in the ESI† report hot-spot analyses for 95% and 99.9% recycling rates. For the former, a substantial portion of the environmental impacts are associated with either manufacturing or end-of-life of the reagents; this explains why the recycling rate parameter is so important in determining the environmental performance of the carbon utilization system. With a 99.9% recycling rate, the environmental impacts associated with the reagents are substantially reduced; the dominant source of environmental impacts becomes the electrochemical process, with the majority of impacts originating from electricity requirements for stirring and from production of platinum for the cell's electrode (Fig. S4†).
We note that our recycling rate scenarios are purely speculative and do not reflect actual operating conditions or achieved performances, rather they were devised to identify minimum operating conditions that would make the carbon utilisation system at least comparable with the conventional process. For this reason, we adopt a 99.9% recycling rate as part of the baseline scenario for the remainder of this article. The other recycling rates yield too low performances for the carbon utilisation system to be environmentally competitive with the conventional process.
Fig. 5 shows results for the scenarios focusing on the thermal energy requirements of the purification phase. The environmental impacts are expressed as percentage difference compared with the baseline scenario that assumes a 99.9% recycling rate and a thermal energy consumption of 35 MJ per kg of formate, which is representative of a commercial scale operation. The chart highlights significant changes in six environmental categories, including climate change, eutrophication terrestrial and freshwater, photochemical ozone formation and respiratory inorganics; notably, these categories correspond those for which the thermal energy has non-negligible contributions (see Fig. S3 in the ESI†). The scenario representing laboratory-based conditions (150 MJ kg−1) yields increased environmental impacts compared to the baseline scenario, ranging from 20% in the category particulate matter/respiratory inorganics and up to 90% in climate change impacts. On the other hand, the best-case scenario where the thermal energy requirements correspond to the latent heat of vaporization of formate (0.483 MJ kg−1) reduces the environmental impacts between 5% and up to 20% in the same categories.
 | ||
| Fig. 5 Percentage difference between two scenarios for thermal energy consumption in purification compared to the baseline scenario (with a thermal energy consumption of 35 MJ kg−1 of formate). | ||
In Fig. 6 we investigate the impact of different allocation strategies and partitioning factors that are associated with alternative market values for CO2, compared to the baseline scenario where the CO2 is assumed to have a nil market value (i.e., no associated environmental impacts). The comparative analysis demonstrates that the environmental impacts of the carbon utilisation system are significantly reduced when the CO2 has a negative market value (i.e., it is a waste), ranging from 5% and up to 20% depending on the partitioning factor. Notably, the extent of the reduction is equal in all categories and corresponds to the complement (to 100) of the partitioning factor, i.e. a partitioning factor of 90% yields a reduction of 10%. This is expected: when the CO2 is considered a waste, only a portion of the environmental impacts of the ER process is allocated to formate (see section 2.3). On the other hand, the scenario where the CO2 has a positive market value (i.e., it is modelled as a product) yields higher environmental impacts, but the changes are less significant than the case when the market value is negative. The increase in impacts remains below 5% for the majority of the remaining categories; they are above 10% only in the categories human toxicity, cancer effects and water depletion for a partitioning factor of 90%. The only exception is represented by the ozone depletion category, which increases up to 20% when the partitioning factor equals 90%.
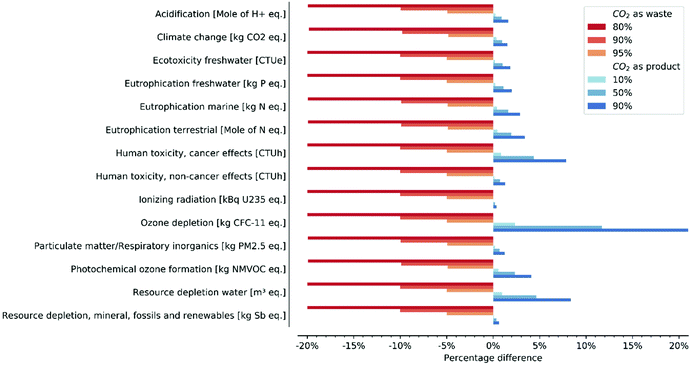 | ||
| Fig. 6 Percentage difference between different allocation strategies and partitioning factors compared to the baseline scenario that assumes CO2 is burden-free (i.e., nil market value). | ||
In Fig. 7 we analyse the effect of different electricity mixes compared to the baseline scenario corresponding to the EU electricity mix in 2020. The chart shows that both 2030 and 2050 electricity mixes yield substantial – and increasing – environmental benefits in six environmental categories and minor benefits in four categories, whilst yielding higher environmental impacts in three categories. The highest environmental benefits (>30%) are found for the categories acidification, climate change, marine and terrestrial eutrophication, particulate matter/respiratory inorganics and photochemical ozone formation; that is, categories that are strictly related to fossil-based electricity generation. On the other hand, the ionizing radiation category is that most negatively affected by future electricity mixes (up to 25% for the electricity mix at 2050); this is due to the projected increased adoption of nuclear power. The importance of the electricity grid mix is explained by the hot-spot analysis (see Fig. S2 and S3 in the ESI†) that shows that most of the environmental impacts of the carbon utilization system with a recycling rate of 99.9% originate from the electrochemical reduction, and in particular from the consumption of electricity.
 | ||
| Fig. 7 Percentage difference of two prospective scenarios for the EU electricity grid mix compared to the baseline scenario that is based on the 2020 mix. | ||
3.2 Comparative analysis
In Fig. 8 we compare the environmental performance of producing formate via electrochemical reduction of CO2 and via the conventional fossil-based process (see section 1). We include two scenarios for the carbon utilisation system: the baseline scenario which includes a 99.9% recycling rate (see section 3.1) and a best-case scenario that assumes a negative market value for CO2 and the lowest partitioning factor (80%), the 2050 electricity mix and the lowest thermal energy consumption in the purification phase (0.483 MJ kg−1 of formate). The results are reported as percentage difference between the environmental impacts of the two scenarios for the carbon utilisation system and those associated with the conventional process. Numerical values are provided in the ESI.† | ||
| Fig. 8 Percentage difference between baseline and best-case scenarios for the carbon utilisation system compared with conventional process of producing formate. | ||
The best-case scenario yields significant improvements in the categories where the baseline scenario underperforms the conventional process, whilst benefits for the remaining categories are diminished. In only two categories – climate change and particulate matter/respiratory inorganics – the extent of environmental impacts reduction makes the best-case scenarios environmental advantageous compared to the conventional process. Overall, our analysis shows that no option is environmentally advantageous across the full spectrum of environmental categories. The scenarios for carbon utilisation system outperform the conventional process in 6–8 categories (out of the 14 considered), including human and environmental toxicity, freshwater eutrophication, ozone depletion, water consumption and, for the best-case scenario, climate change and respiratory inorganics. In particular, formate production via carbon utilization yields climate change impacts that are 179% higher in the baseline scenario but 7% lower in the best-case one.
4 Discussion
Our study attempts to characterize the potential environmental performance of a carbon utilization system that produces formate from CO2 captured from a natural gas power plant using via electrochemical reduction in a novel ionic liquid ([P66614][124Triz]). Because the analysis relies on laboratory-scale data, primarily obtained from literature sources, we investigate different potential scenarios, covering the recycling rate of unreacted reagents, the electricity grid mix, the consumption of thermal energy in the purification phase, and various allocation strategies and partitioning factors that are associated with potential market values of CO2. We find that the recycling rate is the most significant parameter in determining the environmental performance of the system. This is because with a 95% recycling rate, most of the environmental impacts are associated with the manufacturing of the reagents and/or their incineration as hazardous waste (Fig. S2†). Our analysis indicates that the process needs to achieve a recycling rate around 99.9% to be environmentally competitive with the conventional process of formic acid production, which is based on the hydrolysis of methyl formate obtained from fossil sources. Recycling rates lower than 99.9% yield environmental impacts that are many orders of magnitude higher than those of the conventional process, making impossible to reduce the gap via process optimization and scaling-up. The recycling rate scenarios reflect the efficiency of the separation process as well as the portion of unreacted reagents that may be degraded. We are not aware of any lab-scale studies that investigated these issues, and therefore the feasibility of reaching such high recycling rate is uncertain and remains to be tested.The scenarios on allocation strategies and associated partitioning factors reflect the short- and long-term uncertainty regarding the market price of captured CO2. The current literature reports positive market prices in the order of 10–20 USD per ton38 with the potential to raise up to 450 USD per ton.37 However, the rising global pressure to reduce carbon emissions will make available increasing quantities of captured CO2 to the market, which may drive market prices to below zero.51,52 In the absence of better literature data, we approximated the potential negative market prices of CO2 with current and projected carbon tax values; the underlying rationale is that it would be uneconomical (and illogical) to pay a price higher than the carbon tax to dispose of a CO2-rich flue gas. This is a simplifying assumption that is nonetheless insightful: it demonstrates that negative market prices of CO2 deliver significant environmental benefits to the electrochemical reduction process, and by extension to any carbon utilisation system. Furthermore (and reassuringly) positive market values do not significantly increase the environmental impacts; this is because the environmental impacts of the carbon capture unit are minor compared to those of the electrochemical reduction process. It must be noted that, with any other product system, different allocation strategies may yield substantially different environmental results (e.g. in the case of a heat-and-power cogeneration plant53).
The scenarios on electricity grid mixes and thermal energy requirements in the purification phase also provide interesting insights. With a 99.9% recycling rate, the electricity consumption of the electrochemical reduction process is one of the major contributors to the environmental impacts of the carbon utilization system; this explains why the electricity grid mix is a significant parameter in determining the environmental performance, in particular in those categories that are typically associated with energy production from fossil fuels, such as climate change, acidification, particulate matter formation and photochemical oxidant formation. When moving from the electricity grid mix in 2020 to the forecasts in 2050, the impacts in these categories are substantially reduced and only marginally balanced by less marked increases in other categories (e.g. ionizing radiation and depletion of resources) that are associated with nuclear and renewable energy sources. Overall, our analysis shows that the projected decarbonization of the power generation sector in the EU will bring significant environmental benefits to the carbon utilisation system.
The scenarios on thermal energy requirements of the purification phase show significant changes in a limited number of categories, i.e. those for which this activity has non-negligible contributions (see Fig. S2†). Due to lack of literature data, we used data for a water-based ER process. The actual environmental impacts will be dependent on the specific technology that is appropriate for an IL-based ER process, which, as we noted above, needs to be investigated from a technical as well as an environmental perspective.
We complete our analysis by comparing the performance of the carbon utilisation system with that of the conventional process for producing formate. Our results are interesting in that they identify significant environmental trade-offs, with neither alternative systematically outperforming the other. The best-case scenario – which includes the most advantageous partitioning factors for the case of a negative market value, the 2050 electricity grid mix and the lowest thermal energy consumption in the purification phase – yields substantial environmental improvements, especially in the categories where the baseline scenario features low performances. However, the extent of these improvements makes the carbon utilisation system more advantageous than the conventional alternative only in two additional categories (respiratory inorganics and climate change). The high consumption of electricity represents the key limitation of the carbon utilisation system, but this may be overcome when scaling up from laboratory to commercial scale. For this reason, our results are conservative in that the environmental performance of the carbon utilisation system should be higher when the process is implemented at scale.
The underlying inventory represents the most important limitation of this study; this is mainly due to the low Technological Readiness Level (TRL) of the carbon utilisation system. The inventory for the electrochemical reduction process relies on laboratory-scale experiments, whilst that for the purification phase, which also covers commercial scale and a potential best case scenario (see section 2.4), is based on a water-based ER process. To address this limitation, we investigated the environmental performance of various scenarios. In this way, not only we accounted for the uncertainty of key parameters, but we also estimated the performance of a potential best-case scenario. Nevertheless, future studies should focus on assessing the technical and environmental performance of an optimised and scaled-up ER process based on IL for the production of formate. The key issues that should be addressed include modelling the purification phase specifically for an IL-based ER process (including the efficiency of separation and the portion of degraded reagents) and developing a scaled-up ER process with optimised electricity and thermal energy requirements. Of note, we don't expect that an IL-specific purification process will substantially affect the environmental performance of the system because of the typically negligible vapor pressure of ionic liquids.4
The inventory for the MEA-based carbon capture unit is more robust because the process has already been implemented at a commercial scale and because it is obtained from references widely adopted for LCA purposes, though substantially older than other references that we used throughout the study. However, the carbon capture unit has minor contributions to the overall environmental impacts (Fig. 6); as such, we don't expect that the adoption of new inventories will significantly affect the results. For the same reason, we deem that the assumption that the captured-CO2 stream (which typically has purity greater than 99%) does not require any pre-treatment is reasonable. Future studies should investigate whether and to what extent impurities may impair the electrochemical reduction process, and therefore whether and what type of pre-treatment is necessary.
The poor availability of data also meant that our analysis did not account for the construction and decommissioning of the ER unit; however, these are expected to have a small or even negligible contributions to the environmental performance of the system.
5 Conclusions
This article presented a comprehensive Life Cycle Assessment (LCA) study of a novel carbon utilization system that converts CO2 into formate (which can be easily converted into formic acid) via electrochemical reduction using the ionic liquid [P66614][124Triz]. We assumed that the CO2 is obtained from the exhaust gas of a natural gas power plant using the MEA-based capture technology. Inventory data for the electrochemical reduction process mostly relies on laboratory experiments; for this reason, we performed a scenario analysis to investigate the effects of critical parameters and to identify a potential best-case scenario. Our analysis indicates that the recycling rate of unreacted reagents is the most significant parameter. The process needs to achieve a 99.9% recycling rate to be environmentally competitive with the conventional process of formic acid production, which relies on the hydrolysis of methyl formate obtained from fossil sources. The recycling rate depends on the efficiency of the separation process as well as on the amount of degraded ionic liquids; both aspects need to be investigated experimentally.The market price of CO2 represents another critical factor; this affects the allocation strategy that is based on the economic value of the co-products. The environmental performance of the carbon utilization system for the production of formate is substantially improved when the CO2 has a sub-zero market price; this could occur when the demand for captured CO2 is outstripped by its supply, which will be increasing in the coming years as a means to mitigate climate change. On the other hand, a positive market price decreases the performance of the system, but to a lesser extent. The other two parameters covered by the scenario analysis include the thermal energy consumption in the purification phase and the electricity grid mix. The former significantly affects the environmental performance in a limited number of categories, including climate change. The latter yields substantial improvements in the majority of environmental categories when moving from the 2020 mix to that forecast in 2050, which includes higher contribution of renewable and nuclear energy sources.
The comparative analysis highlights several environmental trade-offs between the carbon utilization system and the conventional process, with the former being preferable in 6–8 impact categories depending on the scenario considered. Notably, the carbon utilisation system yields slightly lower carbon emissions only in the best-case scenario. Our results are conservative because the TRL of the electrochemical reduction process is very low; this entails that the environmental performance of the system is expected to be higher when implemented at scale. Future works should focus on investigating the environmental performance of an optimized and scaled-up process.
Author contributions
Andrea Paulillo: conceptualization, methodology, investigation, formal analysis, writing – original draft, visualization, writing – review & editing. Martina Pucciarelli: conceptualization, methodology, investigation, software, writing – original draft, visualization, writing – review & editing. Fabio Grimaldi: conceptualization, methodology, investigation, software, visualization. Paola Lettieri: supervision, project administration, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The Authors acknowledge funding from the EPSRC under grant no. EP/N009533/1, a multidisciplinary approach to generating low carbon fuels, carried out in collaboration with the University of Manchester, Queen's University Belfast, Cardiff University, and University College London. The Authors would like to thank Dr Rebecca Taylor and Dr Adam Greer for their support in collecting inventory data.References
- UNFCCC, Adoption of the Paris Agreement. FCCC/CP/2015/L.9/Rev.1, 2015, vol. 21932 Search PubMed.
- V. Masson-Delmotte, P. Zhai, H.-O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor and T. Waterfield, Global warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strenghtening the global response to the threat of climate change, IPCC, 2018 Search PubMed.
- I. Mohsin, T. A. Al-Attas, K. Z. Sumon, J. Bergerson, S. McCoy and M. G. Kibria, Cell Rep. Phys. Sci., 2020, 1, 100104 CrossRef.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- F. M. Baena-Moreno, M. Rodríguez-Galán, F. Vega, B. Alonso-Fariñas, L. F. Vilches Arenas and B. Navarrete, Energy Sources, Part A, 2019, 41, 1403–1433 CrossRef CAS.
- H. Ostovari, A. Sternberg and A. Bardow, Sustainable Energy Fuels, 2020, 4, 4482–4496 RSC.
- G. Centi, E. A. Quadrelli and S. Perathoner, Energy Environ. Sci., 2013, 6, 1711–1731 RSC.
- S. M. Jarvis and S. Samsatli, Renewable Sustainable Energy Rev., 2018, 85, 46–46 CrossRef CAS.
- E. V. Kondratenko, G. Mul, J. Baltrusaitis, G. O. Larrazábal and J. Pérez-Ramírez, Energy Environ. Sci., 2013, 6, 3112–3135 RSC.
- L. Chen, S. X. Guo, F. Li, C. Bentley, M. Horne, A. M. Bond and J. Zhang, ChemSusChem, 2016, 11(9), 1271–1278 CrossRef PubMed.
- A. Dominguez-Ramos, B. Singh, X. Zhang, E. G. Hertwich and A. Irabien, J. Cleaner Prod., 2015, 104, 148–155 CrossRef CAS.
- M. Rumayor, A. Dominguez-Ramos and A. Irabien, Sustain. Prod. Consum., 2019, 18, 72–82 CrossRef.
- H. R. M. Jhong, S. Ma and P. J. Kenis, Curr. Opin. Chem. Eng., 2013, 2(2), 191–199 CrossRef.
- M. Gattrell, N. Gupta and A. Co, Energy Convers. Manage., 2007, 48(4), 1255–1265 CrossRef CAS.
- N. Hollingsworth, S. F. R. Taylor, M. T. Galante, J. Jacquemin, C. Longo, K. B. Holt, N. H. De Leeuw and C. Hardacre, Angew. Chem., Int. Ed., 2015, 54, 14164–14168 CrossRef CAS PubMed.
- N. Hollingsworth, S. F. R. Taylor, M. T. Galante, J. Jacquemin, C. Longo, K. B. Holt, N. H. De Leeuw and C. Hardacre, Faraday Discuss., 2015, 183, 389–400 RSC.
- J. Hietala, A. Vouri, P. Johnsson, I. Pollari, R. Werner and H. Kieczka, Ullmann's Encycl. Ind. Chem., 2016, 1–23 Search PubMed.
- W. Cai, L. Yan, C. Li, L. Liang, W. Xing and C. Liu, Int. J. Hydrogen Energy, 2012, 37(4), 3425–3432 CrossRef CAS.
- C. Rice, S. Ha, R. I. Masel, P. Waszczuk, A. Wieckowski and T. Barnard, J. Power Sources, 2002, 111(1), 83–89 CrossRef CAS.
- Datawheel, The Observatory of Economic Complexity, 2012 Search PubMed.
- Mordor Intelligence, Formic Acid Market - Growth, trends, and forecast (2019–2024), 2019.
- ISO 14040:2006, 2006.
- ISO 14044:2006, 2006.
- A. Sternberg, C. M. Jens and A. Bardow, Green Chem., 2017, 19, 2244–2259 RSC.
- N. Thonemann and M. Pizzol, Energy Environ. Sci., 2019, 12, 2253–2263 RSC.
- N. Von Der Assen, J. Jung and A. Bardow, Energy Environ. Sci., 2013, 6, 2721–2734 RSC.
- N. Von Der Assen, P. Voll, M. Peters and A. Bardow, Chem. Soc. Rev., 2014, 43, 7982–7994 RSC.
- M. Rumayor, A. Dominguez-Ramos and A. Irabien, Appl. Sci., 2018, 8, 914 CrossRef.
- Y. Ahn, J. Byun, D. Kim, B. S. Kim, C. S. Lee and J. Han, Green Chem., 2019, 21, 3442–3455 RSC.
- Commission of the European Communities, Off. J. Eur. Communities, 2000, 2000D0532, 1–31 Search PubMed.
- A. B. Rao and E. S. Rubin, Environ. Sci. Technol., 2002, 36, 4467–4475 CrossRef CAS PubMed.
- H. L. Pesonen, T. Ekvall, G. Fleischer, G. Huppes, C. Jahn, Z. S. Klos, G. Rebitzer, G. W. Sonnemann, A. Tintinelli, B. P. Weidema and H. Wenzel, Int. J. Life Cycle Assess., 2000, 5, 21–30 CrossRef.
- J. B. Guinée, S. Cucurachi, P. J. G. Henriksson and R. Heijungs, Int. J. Life Cycle Assess., 2018, 6 Search PubMed.
- B. M. K. Manda, E. Worrell and M. K. Patel, Resour., Conserv. Recycl., 2015, 103, 47–57 CrossRef.
- B. A. Sandén and M. Karlström, J. Cleaner Prod., 2007, 15, 1469–1481 CrossRef.
- M. Pérez-Fortes, J. C. Schöneberger, A. Boulamanti, G. Harrison and E. Tzimas, Int. J. Hydrogen Energy, 2016, 41, 16444–16462 CrossRef.
- E. A. Quadrelli, G. Centi, J. L. Duplan and S. Perathoner, ChemSusChem, 2011, 4, 1194–1215 CrossRef CAS PubMed.
- P. Brinckerhoff, Accelerating the Uptake of CCS: Industrial Use of Captured Carbon Dioxide, 2011 Search PubMed.
- EMBER, Daily EU ETS carbon market price (Euros).
- S. Schjolset, “The MSR: Impact on market balance and prices Price forecast: Key assumptions”, Thomson Reuters, 2014 Search PubMed.
- High-level Commission on Carbon-Prices., High-Level Commission on Carbon Prices. 2017, 2017 Search PubMed.
- IEA, Real-world policy packages for sustainable energy transition., Paris, 2017 Search PubMed.
- T. Kupfer, M. Baitz, C. M. Colodel, M. Kokborg, S. Schöll, M. Rudolf, U. Bos, F. Bosch, M. Gonzalez, O. Schuller, J. Hengstler, A. Stoffregen and D. Thylmann, GaBi databases & modeling principles, 2020 Search PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- R. M. Cuéllar-Franca, P. García-Gutiérrez, S. F. R. Taylor, C. Hardacre and A. Azapagic, Faraday Discuss., 2016, 192, 283–301 RSC.
- J. Koornneef, T. van Keulen, A. Faaij and W. Turkenburg, Int. J. Greenhouse Gas Control, 2008, 2, 448–467 CrossRef CAS.
- K. Veltman, B. Singh and E. G. Hertwich, Environ. Sci. Technol., 2010, 44, 1496–1502 CrossRef CAS PubMed.
- B. Singh, A. H. Strømman and E. Hertwich, Int. J. Greenhouse Gas Control, 2011, 5, 457–466 CrossRef CAS.
- EC JRC, ILCD Handbook: Recommendations for Life Cycle Impact Assessment in the European context, 2011 Search PubMed.
- Sphera, https://gabi.sphera.com/.
- E. A. Quadrelli and G. Centi, ChemSusChem, 2011, 4, 1179–1181 CrossRef CAS PubMed.
- G. Centi and S. Perathoner, Greeenhouse Gas Sci. Technol., 2011, 21–35 CrossRef CAS.
- T. Tereshchenko and N. Nord, Appl. Therm. Eng., 2015, 76, 410–422 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1gc02306c |
| This journal is © The Royal Society of Chemistry 2021 |
