The aqueous medium-dimethylsulfoxide conundrum in biological studies
Saeedeh Neginab,
Michael R. Gokela,
Mohit B. Patelbc,
Sergey L. Sedinkinab,
David C. Osborna and
George W. Gokel*abc
aCenter for Nanoscience, University of Missouri-St. Louis, St. Louis, Missouri, USA. E-mail: gokelg@umsl.edu; Fax: +1-314-516-5342; Tel: +1-314-516-5321
bDept. of Chemistry, University of Missouri-St. Louis, St. Louis, Missouri, USA
cDepartment of Biology, University of Missouri-St. Louis, St. Louis, Missouri, USA
First published on 15th December 2014
Abstract
A series of straight and branched chain pyrogallol[4]arenes was studied and found to be essentially nontoxic to two strains of E. coli. An apparent enhancement of potency observed for kanamycin against E. coli was found to be due to the presence of DMSO in the growth media. Quantitative studies are presented that assay the effect of DMSO concentration on the apparent potency of kanamycin against E. coli.
Introduction
Solubility is one of the most vexing problems in synthetic chemistry, in green chemistry, in medicinal chemistry, and in supramolecular chemistry. Techniques such as phase transfer catalysis1 have ameliorated some solubility issues in synthetic chemistry. A major effort has been made in recent years to emphasize atom economy and the use of non-polluting and non-toxic solvents.2 The conundrum in medicinal chemistry is how to achieve water solubility for drug delivery with molecules that must pass through bilayer membranes.3For much of the history of supramolecular chemistry, the goal was to design receptors that would interact selectively with certain guest molecules and the solvent in which this occurred was chosen for convenience. As supramolecular chemistry overlapped a broader range of more established fields, the importance of solubility, particularly in water, became a significant goal.4 Nature overcame this problem eons ago with water-soluble proteins that have internal cavities to accommodate specific guests or ligands. Early studies by Kubik and coworkers showed that strong binding and host solubility could be achieved by synthetic molecules in aqueous solutions.5 Other examples have emerged as the significance and desirability of water soluble receptors became more apparent.6
One recent direction in supramolecular chemistry is the study of such scaffolds as calixarenes, resorcinarenes,7 and pyrogallolarenes (Pgs) as biologically active agents or their development as medicaments. Calixarenes, in particular, have been elaborated and assayed for biological activity in the studies reported by Regnouf-de-vain and coworkers.8 We have also been interested in the biological properties of synthetic amphiphiles having supramolecular functionality such as the hydraphile synthetic cation transporters.9 These compounds show toxicity to bacteria,10 they augment the efficacy of certain antibiotics against bacterial strains,11 and they also alter the root morphology of the Arabidopsis thaliana plant.12
Our interest in assessing the inherent biological activity and potentially synergistic activity of pyrogallolarenes was hampered by their poor aqueous solubility. Even the pyrogallolarenes having relatively short side chains were relatively insoluble despite the presence of 12 hydroxyl groups on the upper perimeter of the macrocycle. We therefore resorted to the use of DMSO to enhance solubility in aqueous solution. There are several reports in the recent literature that disclose the use of DMSO in biological studies without specifying its concentration. In other cases, pure DMSO solutions were used as controls in biological studies.13 The need for aqueous solubility for biological studies and our general concern about the potential effect of DMSO on such studies caused us to examine the question in some detail with the results presented below.
The effect of DMSO on membrane lamellar phases has been studied in a concentration range of 10–70% with dipalmitoylphosphatidylcholine (DPPC) membrane monomers.14 More recently, changes in membrane lamellar spacings were studied by using small angle X-ray scattering of palmitoyloleoylphosphatidylethanolamine (POPE) in concentrations up to 40% by weight of DMSO.15 Williams and Barry state in a 2004 review16 titled “penetration enhancers” that a “vast array of literature describes the penetration enhancing activities of DMSO, and studies have shown it to be effective in promoting both hydrophilic and lipophilic permeants… The effects of the enhancer are concentration dependent and generally co-solvents containing >60% DMSO are needed for optimum enhancement efficacy.” A 2006 computational study addressing the molecular basis for the action of DMSO on membranes studied concentrations up to 12% of DMSO in DPPC membranes.17 The authors noted in an in silico analysis that at 27 mol%, a water pore was observed to form in the membrane. However, to our knowledge, there has been no report quantifying the effect of DMSO on the efficacy of antibiotics in a vital bacterial system.
The goals of this study were to assay the biological activity, if any, of pyrogallol[4]arenes having various side chains and to discover what limits there might be in adding DMSO to bring these amphiphiles into aqueous solution. The use of DMSO to enhance aqueous solubility as described in some published reports without specifying concentrations was a concern. This was especially the case considering the somewhat inconsistent previous reports noted above.
Results and discussion
Compounds used in the present study
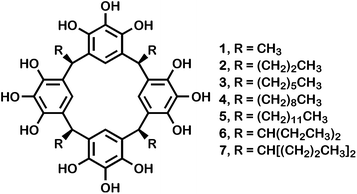
The preparation of the Pgs reported here was accomplished by heating pyrogallol and an appropriate aldehyde in aqueous, ethanolic hydrochloric acid solution. The product typically crystallized from the cooled reaction mixture and was isolated by filtration. Yields were usually in the 20–30% range, but the poor to modest yields are compensated by the simplicity of the reaction and the ease of isolation.Seven pyrogallol[4]arenes were prepared for the studies reported here. All of them are macrocyclic tetramers of pyrogallol and an aldehyde that have been previously described in the literature. In all cases, the side chains that derive from the aldehydes are aligned stereochemically in the products that crystallize. This configuration is called rccc, meaning that three of the chains are cis with respect to an arbitrarily designated reference chain, r. The compounds having n-alkyl chains are as follows: 1, R = methyl; 2, R = n-propyl; 3, R = n-hexyl; 4, R = n-nonyl; 5, R = n-dodecyl. The branched chain pyrogallol[4]arenes 6, R = 3-pentyl; and 7, R = 4-heptyl. The compounds are illustrated in Fig. 1.
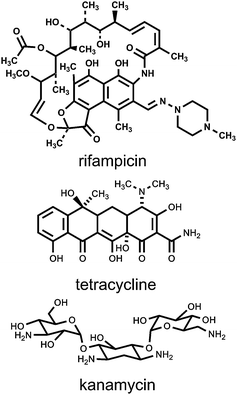
Several antibiotics were studied as part of this project. The antibiotics included rifampicin, tetracycline, and kanamycin [mixture of A, B, and C (Sigma-Aldrich)]. The structures are shown in Fig. 2. These antibiotics were chosen because they differ significantly in structure and because their modes of action are all known and different from each other. The antibiotic rifampicin binds to the β-subunit of RNA polymerase and thus inhibits RNA synthesis.18 The aminoglycoside kanamycin causes potassium ion leakage and also interferes with translation and cell respiration.19 The widely used antibiotic tetracycline prevents peptide elongation by binding to the 30S ribosome subunit.20 An important additional factor in our choice was that rifampicin and kanamycin are considered to be first and second line defenses, respectively, against the worldwide scourge of tuberculosis.
Antibacterial activity of pyrogallol[4]arenes and antibiotics
The initial phase of this study was to determine the toxicity, if any, of pyrogallol[4]arenes against one of two target strains of E. coli: DH5α or K-12. Antimicrobial activity was determined as the minimum inhibitory concentration (MIC) according to the protocols set forth in section M07-A9 of the Clinical and Laboratory Standards Institute compilation.21 A stock solution of the Pg was prepared in DMSO. An aliquot was added to the media and the total volume in each case was 1980 μL. Each sample tube was vortexed to ensure uniform dispersion throughout the solution. A 20 μL aliquot of the E. coli solution (OD600 = 0.6) was added to each tube, vortexed, and then maintained in an incubator/shaker for ∼24 h at 37 °C and 200 rpm. Note that MIC determinations are typically conducted by using serial dilutions that vary by a factor of two. When an approximate MIC is determined in this way, the dilution range is narrowed in order to obtain a final value. MIC values were determined for kanamycin and tetracycline against the K-12 strain of E. coli. The values were 35 μM and 5 μM, respectively (average of 6 replicates).Pyrogallol[4]arenes are phenolic compounds and it is well known that phenol (carbolic acid) has been used as an antiseptic for more than a century since its introduction in surgical practice by Lister. Notwithstanding the known biological activity for pyrogallol itself,22 to our knowledge the only MIC value reported in the literature is against Paenibacillus larvae, a microbe most commonly found in soil and plants. In this case, the MIC for pyrogallol is reported to be >128 μg mL−1 (∼1 mM).23 The Merck Index reports pyrogallol oral toxicity to rabbits at 1.6 g kg−1.24 To our knowledge no toxicity data are available in the literature for any pyrogallol[4]arene. Using the standard protocols noted above, we determined that for pyrogallol[4]arenes 1–7, in all cases, the MIC values were greater than 128 μM (3 or more replicates). A MIC value of 128 μM or greater is generally considered to be inactive against the microbe under study. By that standard, all of the Pgs tested were inactive against the DH5α and K-12 strains of E. coli used in this study.
Standard protocols require that the type of toxicity test referred to above should be conducted in aqueous solution. Pyrogallol[4]arenes, like many supramolecular or other amphiphiles, are poorly soluble in water and some DMSO was required to dissolve them. It is common knowledge that high concentrations of DMSO can affect membranes and therefore the organisms under study, but the sensitivity of such organisms to DMSO is variable, as shown below.
Co-administration of pyrogallol[4]arenes and antibiotics to E. coli
Numerous MIC determinations were made for E. coli in the presence of either kanamycin or tetracycline and Pgs. The studies involving either antibiotic were conducted at their half MIC values and various concentrations of Pgs up to 64 μM. An examination of the data obtained suggested that the Pgs, when administered at half MIC, approximately doubled the potency of kanamycin against E. coli. Because of solubility requirements, these experiments were conducted in the presence of 7–8 vol% of DMSO. Given the range of side chain structures present in the Pgs, this nearly identical behavior seemed implausible. We therefore became concerned that DMSO was exerting an effect on the experimental system. According to the growth curve studies conducted with DMSO and DH5α E. coli that are presented below, a concentration of 7–8% by volume of DMSO initially slowed bacterial growth, which was recovered after 24 hours.Effect of DMSO on bacterial growth
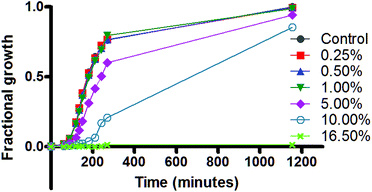
The growth of E. coli was monitored over an approximately 1200 min (∼20 h) time period in the presence of varied concentrations of DMSO. Given that the E. coli life cycle is ∼22–23 min, the results shown reflect more than 60 generations. Growth can be assessed by changes in the opacity (optical density, λ = 600 nm (OD600)) of a medium in which they are suspended. Data were recorded at approximately half hour intervals during the first ∼4 h and a final value was recorded at ∼20 h.The graph of Fig. 3 shows four growth regimes. At the top of the graph, the growth curve for the K-12 strain of E. coli (filled circles) is essentially superimposed on curves obtained in the presence of 0.25 (squares), 0.50 (triangles), or 1 vol% (inverted triangles) of DMSO in the media. The data show clearly that at these concentrations, the viability of E. coli is not affected. E. coli growth is compromised by the presence of 5 vol% (diamonds) of DMSO as shown in the trace that is third from the bottom. Even at this concentration, however, growth recovers to almost the same level as the control after 1200 min (20 h). Although a more dramatic slowing of growth is apparent initially when the DMSO concentration is 10 vol% (open circles), growth likewise recovers during 20 h. At 16.5% DMSO (crosses), no growth at all was observed in the 20 h time period. The 16.5%, rather than 15%, concentration was chosen for this experiment because it is the measured MIC for DMSO against the K-12 strain of E. coli.
Similar experiments were performed with the DH5α strain of E. coli. The growth profiles were not significantly different from those in Fig. 3 (data not shown). The volume of DMSO used in the kanamycin-C3Pg attempted synergy experiments was 7.5 vol%. The data in Fig. 3 show that at 7.5 vol% (intermediate between 5 and 10 vol%), bacterial growth is initially inhibited but recovers by the 20 h time point. The effect of DMSO in this case could be on the organism, per se, or on the combination of DMSO and the antibiotic in the presence of the organism.
Effect of DMSO on the antibiotics
The studies shown in Table 1 were done using half of the MIC concentration for each antibiotic, with the expectation that bacterial growth would not be inhibited by the antibiotic at this reduced concentration. The table records the presence or absence of bacterial growth by using the symbols + or −, which correspond either to growth or no growth, respectively. The data in the table reveal two findings concerning the effect of DMSO. First, even though the MIC of the antibiotic remained at half of its inhibitory level (half MIC), a bacteriostatic effect was observed when the only other agent present was DMSO. Second, the concentration effect of DMSO on bacteriostatic efficacy differed for the two antibiotics tested. We note that generally similar results were observed for rifampicin (data not shown).| DMSO present, Vol% | Antibiotic | ||
|---|---|---|---|
| Kanamycin MIC = 36 μM | Tetracycline MIC = 5 μM | Rifampicin MIC = 20 μM | |
| a Antibiotic concentration is half the measured MIC (see text and experimental for procedure).b The strain of E. coli is K-12. | |||
| 0 | + | + | + |
| 3.7 | + | + | + |
| 7.2 | + | + | + |
| 7.8 | − | + | + |
| 8.5 | − | + | + |
| 9.8 | − | + | + |
| 11.0 | − | + | + |
| 12.2 | − | − | − |
| 13.4 | − | − | − |
A study similar to the one using K-12, reflected in Table 1, was conducted with kanamycin and DH5α E. coli. As in Table 1, the concentration of antibiotic was held at half of its MIC value. The effect of DMSO was observed as before, but the concentration at which growth ceased was 6.9 vol%, rather than 7.8 vol%. These findings show that the DMSO effect varies not only with the antibiotic studied but with the subject organism – and even the strain of the organism – as well, at least for two strains of E. coli.
Scanning electron microscopy (SEM) of E. coli in the absence and presence of DMSO
As reported above, 0.5% by volume of DMSO had no effect on E. coli growth, whereas 5% by volume of DMSO showed a moderate lag in bacterial growth. To further define the DMSO-E. coli interaction, we observed the structure of E. coli cells under SEM, in the absence and presence of DMSO.Fig. 4 shows SEM images of DH5α on a nylon supporting background. The untreated E. coli cells, grown in LB medium were about 2 μm in length (panels A and B). The surface of the E. coli was corrugated, but remained intact as shown at the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification (panel B). Panels C and D show the bacteria after treatment with 0.5% by volume of DMSO. Within experimental error, bacterial length was unchanged and the only difference apparent was a slight swelling of the bacterial surface (see panel D). Essentially no further change was observed when the bacteria were exposed to 5% by volume of DMSO (panels E and F). In panel E, one apparently large bacterium is dividing.
000× magnification (panel B). Panels C and D show the bacteria after treatment with 0.5% by volume of DMSO. Within experimental error, bacterial length was unchanged and the only difference apparent was a slight swelling of the bacterial surface (see panel D). Essentially no further change was observed when the bacteria were exposed to 5% by volume of DMSO (panels E and F). In panel E, one apparently large bacterium is dividing.
The study reported above was conducted at concentrations of up to 5 vol% DMSO in panels A–F. Based on the growth curve results, we anticipated that microscopic images would likely show mostly expired bacteria at the 16.5 vol% concentration. In fact panels G and H show a bacterium that is becoming translucent. In an image that is not shown, an E. coli cell had a measured length of 1 μm, less than half the normal length for this species. There is a general understanding that “too much” or “excessive” DMSO present in aqueous solutions can have a deleterious effect on an assay using this solvent. On the contrary, generally unspecified “small amounts” or “low concentrations” of DMSO are thought to be inert. Although the images in Fig. 4 above show that the DH5α E. coli morphology is little altered when the amount of DMSO added does not exceed 5 vol%, the growth curve presented in Fig. 3 and panels G and H of Fig. 4 show clearly that bacterial growth is significantly affected in the initial phase of growth at the MIC value.
Conclusions
This quantitative work reports two major findings. The first is that a family of pyrogallol[4]arenes having both linear and branched side chains exhibits essentially no toxicity either to the DH5α or K-12 strain of E. coli. The Pgs show no synergistic activity of the type observed for the synthetic amphiphiles called hydraphiles.9 Second, we show by a series of comparative experiments that the effect of DMSO is concentration dependent and that the effect is variable depending on the experiment's components.A recent and apparently more successful effort than our own has been reported25 in which the diiodide salt of [N,N-bis((methylpyridinium-2-yl)methyl)dodecan-1-amine] was found to enhance the potency of erythromycin against E. coli and Enterobacter aerogenes. The study reports that the cells used were assayed for viability against DMSO, but the amount of co-solvent, if any, was unclear. The present study shows that controls must be conducted between DMSO and each of the individual components to be used in the experiment so that an inference of synergy will not be incorrectly drawn. In the work reported here, DMSO showed different effects depending on the identity of the organism and the antibiotic. The presence of pyrogallol[4]arenes, which are documented to be pore-formers, proved to be superfluous.
Experimental section
Kanamycin, rifampicin, and tetracycline were purchased from Sigma Aldrich Chemical Co. (St. Louis) and used as received.Tetramethylpyrogallol[4]arene, 1, was prepared as described in ref. 26.
Tetrapropylpyrogallol[4]arene, 2, was prepared as described in ref. 27.
Tetrahexylpyrogallol[4]arene, 3, was prepared as described in ref. 28.
Tetranonylpyrogallol[4]arene, 4, was prepared as described in ref. 29.
Tetradodecylpyrogallol[4]arene, 5, was prepared as described in ref. 30.
Tetra-3-pentylpyrogallol[4]arene, 6, was prepared as described in ref. 31.
Tetra-4-heptylpyrogallol[4]arene, 7, was prepared as described in ref. 31.
Determination of MIC
An initial inoculum of E. coli was dissolved in 2 mL of LB Media and incubated in an incubator/shaker at 37 °C at approximately 200 rpm for 24 h. Stock solutions of the various compounds to be tested were made at 1 mM concentration. The amount by volume of each stock solution to be added to each test tube was calculated in order to determine the amount of media needed to bring the volume of solution to a total of 2 mL. Initially, the required amount of media was added to each test tube. The desired amount of each of the Pgs and antibiotic solutions was added to the test tube. Each tube was vortexed before and after adding E. coli to ensure uniform dispersion throughout the solution. An aliquot (20 μL) of the E. coli (O.D. = 0.6 (λ = 600 nm)) solution was added to each test tube and the mixture was subsequently vortexed. The test tubes were then placed in an incubator/shaker at 37 °C at approximately 200 rpm for 24 h.Determination of MIC in the presence of DMSO
In the studies involving DMSO alone or in combination with an antibiotic, the experimental procedure was as described above except that DMSO replaced pyrogallolarene.SEM procedure
E. coli bacteria were grown to mid-exponential growth phase in LB medium at 37 °C and 200 rpm, to the optical density (λ = 600 nm) of O.D. = 0.55. The bacteria were treated with 0.5%, 5%, and 16.5% by volume of DMSO for 10 minutes at 37 °C and 200 rpm. No DMSO was added to the E. coli for the untreated control. Samples (20 μL) of either treated or untreated bacteria were added on to 0.45 μm nylon membrane (Sigma-Aldrich). The bacteria were fixed with 2.5% glutaraldehyde for 60 min and washed in phosphate buffered saline (PBS) for 15 min. The samples were post-fixed in 1% OsO4 for 60 min and dehydrated with a graded ethanol series (0–100%). The sample was dried using a critical point dryer and sputter coated for 1 min with gold. Microscopy was performed by using a JEOL 6320F and images were acquired at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× and 50
000× and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification.
000× magnification.
Acknowledgements
We thank the NSF for a grant, CHE-1307324 that supported this work.References
- W. P. Weber and G. W. Gokel, Phase Transfer Catalysis in Organic Synthesis, Springer-Verlag, p.280, Berlin, 1977 Search PubMed; C. M. Starks, C. L. Liotta and M. Halpern Phase Transfer Catalysis, Chapman and Hall, New York, 1994, p.668 Search PubMed.
- C. J. Li and P. T. Anastas, Chem. Soc. Rev., 2012, 41, 1413 RSC; M. O. Simon and C. J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC.
- Drug Delivery: Principles and Applications, ed. B. Wang, T. Siahaan and R. A. Soltero, Wiley Interscience, Hoboken, NJ, 2005, p.448 Search PubMed.
- L. Isaacs, Adv. Drug Delivery Rev., 2012, 64, 763 CrossRef CAS PubMed.
- S. Kubik, R. Goddard, R. Kirchner, D. Nolting and J. Seidel, Angew. Chem., 2001, 40, 2648 CrossRef CAS.
- S. Kubik, C. Reyheller and S. Stuewe, J. Inclusion Phenom. Macrocyclic Chem., 2005, 52, 137 CrossRef CAS PubMed; S. Kubik, Chem. Soc. Rev., 2009, 38, 585–605 RSC.
- C. D. Gutsche, Calixarenes, Royal Society of Chemistry, Cambridge, 1989, vol. 1, pp. 223 Search PubMed; Calixarenes, a Versatile Class of Macrocyclic Compounds (Topics in Inclusion Science), ed. J. Vicens and V. Böhmer, Springer Verlag, Berlin, 1990, p. 280 Search PubMed; C. D. Gutsche, Calixarenes Revisited, Royal Society of Chemistry, Cambridge, 1998, vol. 6, p. 233 Search PubMed; Calixarenes in Action, ed. L. Mandolini and R. Ungaro, World Scientific Publishing Company, Hackensack, NJ, 2000, p. 271 Search PubMed; Calixarenes 2001, ed. M. -Z. Asfari, V. Böhmer, J. Harrowfield and J. Vicens, Springer Verlag, Weinheim, 2001, p. 700 Search PubMed; C. D. Gutsche, Calixarenes: An Introduction (Monographs in Supramol. Chem.), Royal Society of Chemistry, Cambridge, UK, 2nd edn, 2008, p. 248 Search PubMed; W. Sliwa and C. Kozlowski, Calixarenes and Resorcinarenes: Synthesis, Properties, and Applications; Wiley-VCH Verlag GmbH & Co., Weinheim, 2009, p. 316 Search PubMed.
- M. Mourer, R. E. Duval, C. Finance and J. B. Regnouf-de-Vains, Bioorg. Med. Chem. Lett., 2006, 16, 2960–2963 CrossRef CAS PubMed; M. Grare, M. Mourer, S. Fontanay, J. B. Regnouf-de-Vains, C. Finance and R. E. Duval, J. Antimicrob. Chemother., 2007, 60, 575 CrossRef PubMed; B. Korchowiec, M. Orlof, G. Sautrey, A. Ben Salem, J. Korchowiec, M. Mourer, H. Massimba Dibama, P. Constant, M. Daffe and J. B. Regnouf-de-Vains, Bioorg. Med. Chem., 2012, 20, 2035 CrossRef PubMed.
- G. W. Gokel, Chem. Commun., 2000, 1 RSC.
- W. M. Leevy, M. R. Gokel, G. Hughes-Strange, P. H. Schlesinger and G. W. Gokel, New J. Chem., 2005, 29, 205 RSC.
- J. L. Atkins, M. B. Patel, Z. Cusumano and G. W. Gokel, Chem. Commun., 2010, 46, 8166 RSC.
- M. B. Patel, A. Stavri, N. S. Curvey and G. W. Gokel, Chem. Commun., 2014, 50, 11562 RSC.
- M. B. Patel, N. R. Modi, J. P. Raval and S. K. Menon, Org. Biomol. Chem., 2012, 10, 1785 CAS.
- Z. W. Yu and P. J. Quinn, Biophys. Chem., 1998, 70, 35 CrossRef CAS.
- Z. W. Yu and P. J. Quinn, Biochim. Biophys. Acta, 2000, 1509, 440 CrossRef CAS.
- A. C. Williams and B. W. Barry, Adv. Drug Delivery Rev., 2004, 56, 603 CrossRef CAS PubMed.
- R. Notman, M. Noro, B. O'Malley and J. Anwar, J. Am. Chem. Soc., 2006, 128, 13982 CrossRef CAS PubMed.
- L. R. Yarbrough, F. Y. Wu and C. W. Wu, Biochemistry, 1976, 15, 2669–2676 CrossRef CAS.
- A. Gourevitch, J. M. Tynda, T. A. Puglisi and J. Lein, Antibiot. Annu., 1958, 6, 784–789 Search PubMed.
- O. A. Gomazkov, Fed. Proc. Transl. Suppl., 1964, 23, 876–878 CAS.
- Clinical and Laboratory Standards Institute, “M07–A9, “Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard,” Ninth Edition, January 2012, ISBN 1-56238-784-7, http://www.clsi.org.
- N. Ni, G. Choudhary, M. Li and B. Wang, Bioorg. Med. Chem. Lett., 2008, 18, 1567 CrossRef CAS PubMed; W. H. Park, M. N. Park, Y. H. Han and S. W. Kim, Int. J. Mol. Med., 2008, 22, 263 Search PubMed; Y. H. Han, S. H. Kim, S. Z. Kim and W. H. Park, J. Biochem. Mol. Toxicol., 2009, 23, 36 CrossRef PubMed; Y. H. Han, S. Z. Kim, S. H. Kim and W. H. Park, Chem.–Biol. Interact., 2009, 177, 107 CrossRef PubMed.
- J. Flesar, J. Havlik, P. Kloucek, V. Rada, D. Titera, M. Bednar, M. Stropnicky and L. Kokoska, Vet. Microbiol., 2010, 145, 129 CrossRef CAS PubMed.
- S. Budavari, The Merck Index, 12th Edition, p. 8184 Search PubMed.
- S. Goswami, M. D. Adhikari, C. Kar, D. Thiyagarajan, G. Das and A. Ramesh, J. Mater. Chem. B, 2013, 1, 2612 RSC.
- Q.-F. Zhang, R. D. Adams and D. Fenske, J. Inclusion Phenom. Macrocyclic Chem., 2005, 53, 275 CrossRef CAS.
- J. L. Atwood, L. J. Barbour and A. Jerga, Chem. Commun., 2001, 2376–2377 RSC.
- S. J. Dalgarno, S. A. Tucker, D. B. Bassil and J. L. Atwood, Science, 2005, 309, 2037 CrossRef CAS PubMed.
- G. W. V. Cave, S. J. Dalgarno, J. Antesberger, M. C. Farrarelli, R. M. McKinlay and J. L. Atwood, Supramol. Chem., 2008, 20, 157 CrossRef CAS.
- O. V. Kulikov, N. P. Rath, D. Zhou, I. A. Carasel and G. W. Gokel, New J. Chem., 2009, 33, 1563 RSC.
- O. V. Kulikov, M. M. Daschbach, C. R. Yamnitz, N. Rath and G. W. Gokel, Chem. Commun., 2009, 7497 RSC.
| This journal is © The Royal Society of Chemistry 2015 |