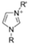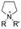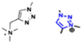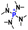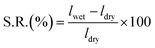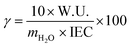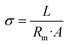Open Access Article
Open Access ArticleDurability and degradation of Anion Exchange Membranes in water electrolyzers†
Nicholas Carboni a,
Maria Assunta Navarra
a,
Maria Assunta Navarra *ab,
Stefano Passerini
*ab,
Stefano Passerini *ac and
Jürgen Garche*d
*ac and
Jürgen Garche*d
aDepartment of Chemistry, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy. E-mail: mariassunta.navarra@uniroma1.it
bHydro-Eco Research Center, Sapienza University of Rome, Via A. Scarpa 16, Rome 00161, Italy
cAustrian Institute of Technology (AIT), Center for Transportation Technologies, Giefinggasse 4, 1210 Vienna, Austria
dVisiting Professor (May/Jun 2023) at the Sapienza University of Rome, Institute for Theoretical Chemistry, Ulm University, Helmholtzstr. 16, 89081 Ulm, Germany
First published on 15th January 2026
Abstract
Anion Exchange Membrane Water Electrolyzers (AEMWEs) have emerged in recent years as an attractive alternative to Proton Exchange Membrane Water Electrolyzers (PEMWEs) and Alkaline Water Electrolyzers (AWEs) to produce hydrogen, thanks to the possibility of using cost-effective catalyst materials and less expensive and non-fluorinated Anion Exchange Membranes (AEMs). The major drawback of these systems is the limited durability of AEMs because of mechanical, thermal and chemical degradation, which are influenced by the operating parameters (temperature and pressure) of the AEMWE device. Chemical degradation is, especially, the most severe due to the highly alkaline operating environment of AEMWEs. Investigating the causes of these failures is crucial for optimizing the AEM stability. This review focuses on the degradation mechanisms involved and on possible strategies to mitigate them. An overview of the working principles of AEMWEs is provided, together with the state-of-the-art and the main functional properties of AEMs. Also, the most used cationic functional groups and polymer backbones are analysed along with their degradation pathways.
1 Introduction
Given the significant global energy consumption and growing climate concerns, hydrogen (H2) has emerged as an appealing energy carrier.1 The production and utilization of hydrogen through energy conversion technologies, such as water electrolysers powered by renewable sources and fuel cells, have received great attention. Recently, the anion exchange membrane (AEM) technology has attracted interest for both fuel cells (AEMFCs)2,3 and water electrolyzers (AEMWEs),4,5 offering several advantages over proton exchange membrane (PEM) devices operating under acidic conditions. The possible use of cost-effective materials, such as nickel and other non-noble metals as catalysts6,7 and the employment of less expensive polymeric AEMs as the electrolyte to replace perfluorinated Nafion, is the most attractive characteristic. While there have been substantial progress and achievements in AEMFC and AEMWE technologies,8,9 a challenge remains related to the performance of AEM materials, especially in the long term. To meet the operational requirements, further development of AEM materials is necessary, focusing on ionic conductivity as well as chemical, mechanical and thermal stability. Thus, a better understanding of the ion conduction and degradation mechanisms in AEMs is essential for their further development.A long service life under a wide range of operating conditions is, in fact, a prerequisite for an economical levelized cost of hydrogen (LCOH), as shown by techno-economic model calculations by Titheridge et al.10
They conclude that degradation in AEMWEs increases cell voltage and shortens stack lifetime, which raises both electricity consumption and maintenance costs, limiting scalability. It also forces conservative operation and adds plant complexity (e.g., minimization of CO2 intrusion and alkali management), all of which negatively affect system efficiency and economics.
Approaches such as the use of a stable membrane, optimized stack design and optimal operational controls (temperature, start/stop management, and CO2 exclusion) can extend lifetime and reduce performance decay.
Techno-economic assessments show that halving degradation rates or doubling stack lifetime can substantially lower the levelized cost of hydrogen by cutting both electricity and replacement expenses. Investing in more durable materials or better controls often yields a net cost benefit, since electricity and stack replacements dominate lifecycle costs.
AEMs contain fixed cations on their polymer structures alongside mobile hydroxide counter anions. The ionic conductivity of AEMs is closely linked to their intrinsic structures, particularly the fixed cations' chemistry, the composition of the polymer backbone, and the linkage between these components.11,12 A main problem of AEM materials is their chemical stability, since OH− is a strong nucleophile; therefore overcoming alkaline degradation of AEMs is not an easy task.
The AEM cations exhibit distinct electrostatic attraction with hydroxide ions, causing a profound impact on AEM conductivity and durability. The latter is mainly determined by the following degradation mechanisms: Hofmann elimination (E2), nucleophilic substitution (SN2), and rearrangements.13
Regarding AEM performance and durability, the polymer backbone plays a key role, since its degradation result in severe damage to the membranes. In fact, the AEM backbone largely determines the mechanical and thermal stability, which is important especially in AEMWEs working in differential pressure mode.
Recent advancements have seen a substantial improvement in AEMWE performance, with current densities often exceeding 1 A cm−2.14–18 However, state-of-the-art AEMWEs have a relatively short operational life (typically less than 200 hours), leaving the long-term durability challenge unsolved. Furthermore, achieving high performance often relies on platinum group metal (PGM) catalysts and/or the use of a circulating KOH solution. Using pure water with non-PGM catalysts poses challenges, primarily due to the higher cell resistance, but also because of the fast dissolution of the catalyst itself.19
In general, water electrolysis is essentially the reverse process of water formation; therefore, AEMWEs and AEMFCs are very similar.8 Consequently, the functions and challenges of AEMs are analogous in both kind of devices, particularly those regarding ionic conductivity and alkaline stability. Nevertheless, the distinct operational environments of these devices call for some variations in the AEM characteristic properties. AEMFCs require high humidification and necessitate AEMs with rapid water uptake and permeability, favouring thin but mechanically stable AEMs to enable high power densities. On the other hand, AEMWEs require an increased AEM thickness (or stiffness) to withstand differential pressure operation. This results in an elevated resistance of the AEM, which is offset by using low-concentration alkaline electrolytes instead of pure water.
Despite the significant progress achieved in AEMFCs and AEMWEs, a deeper understanding of the degradation mechanisms, to develop more stable AEMs, is necessary. For this purpose, this review provides for the first time a unique and comprehensive overview of the properties and degradation mechanisms of anion exchange membranes, focusing on the most commonly used commercial membranes, cationic functional groups, and polymer backbones. Particular emphasis is placed on the analysis of degradation mechanisms, going through mechanical, thermal, and chemical pathways in detail, while also covering performance degradation arising from the operating conditions. Furthermore, the review discusses various mitigation strategies aimed at enhancing AEM durability, one of the major challenges currently hindering the large-scale commercialization of anion exchange membrane water electrolyzers.
2 AEM water electrolyzers
The main component in the electrolyzer design is the membrane electrode assembly (MEA), which is a layered structure with the membrane sandwiched between two porous transport layers (PTLs), each coated with the anode or the cathode catalysts on the membrane side, as illustrated in Fig. 1. The PTLs provide the effective mass transport of water towards the catalyst layer and gas products away. The MEA is pressed between two nickel or stainless-steel bipolar plates to ensure correct supply of water or electrolyte solution to the electrodes and correct gas management. An electrolyte solution (usually KOH or K2CO3) is fed into the device to achieve better ionic conductivity and catalyst activity.20 Water is reduced at the cathode side and produces H2 and OH− through the hydrogen evolution reaction (HER), while for the oxygen evolution reaction (OER), OH− spontaneously diffuses across the AEM and is oxidized at the anode side. The following equations display the related reactions:21| Anode: 4OH− → O2 + 2H2O + 4e− |
| Cathode: 4H2O + 4e− → 2H2 + 4OH− |
| Overall: 2H2O → 2H2 + O2 |
 | ||
| Fig. 1 Schematic of the AEM water electrolyzer and MEA. Reprinted from Energy Chem., vol 4, Xu et al.20, Anion exchange membrane water electrolyzer: electrode design, lab-scaled testing system and performance evaluation, p. 100087, Copyright (2022) with permission from Elsevier. | ||
At 25 °C, the thermodynamic potential of the overall reaction is about 1.23 V. However, the overpotentials caused by electron transfer, mass transfer, etc., and the ohmic resistances, require greater cell voltages.20
AEMWE cell performance is strongly influenced not only by the current and applied potential but also by operating parameters such as feed type, electrolyte solution, cell temperature and pressure.22 The reaction kinetics are enhanced when the cell is operated at high temperatures, but high pressures, although helpful for the subsequent hydrogen storage, directly raise the open-circuit voltage due to impeded water diffusion within the electrode and membrane.23 The ohmic potential drop is decreased, and OH− conduction is enhanced when a basic electrolyte solution is used instead of pure water.24 The performance of AEMWE cells is also impacted by single-side (water is supplied to either the anode or the cathode) or double-sided (water supplied to both the anode and the cathode) feeding.25 In particular, the single-side feed to the anode is advantageous for dry H2 gas collection at the cathode, eliminating extra processing to separate the produced H2 from the liquid reactants.
The stability and longevity of the electrolysers should be considered when determining the operating conditions. The polymer backbone or functional groups of a membrane will deteriorate at temperatures over its thermal stability range and this degradation is amplified when a highly concentrated alkali solution is added, resulting in a decline of MEA performance.26,27 As a result, proper operation is crucial for the stability of the materials used in AEMWE cells as well as the cells' performance. To shed light on the implications for the electrolysis performance, the operating conditions of AEMWEs and their effect on the stability of the AEM will be discussed in Section 5.2.
Table 1 shows the performance and stability data of the most used commercial AEMs for electrochemical water splitting. The evaluated system performance is based on the current density achieved at specific voltages, while durability is the system's ability to maintain stability over extended operational periods. Details on the operating conditions and adopted catalysts are also given.28
| Membranes | Anode (catalyst loading: mg cm−2) | Cathode (catalyst loading: mg cm−2) | Temperature (°C) | KOH molarity (M) | Electrochemical performance | Durability | Ref. |
|---|---|---|---|---|---|---|---|
| Fumasep FAA-3-50 | Stainless steel | Pt/C (0.5) | 60 | 1 | 1.40 A cm −2 at 2.0 V | — | 29 |
| NiFe2O4 | Pt/C (0.5) | 60 | 1 | 1.5 A cm−2 at 1.8 V; 2.0 A cm−2 at 2 V | ∼3 mA h−1 @ 2 V for 120 h | 30 | |
| IrO2 | Pt/C (0.5) | 60 | 1 | 2 A cm−2 at 2.2 V | — | 30 | |
| NiO | Pt/C (0.5) | 60 | 1 | 1.68 A cm−2 at 2.2 V | — | 30 | |
| IrO2 (3) | Pt/C (0.5) | 60 | 1 | 0.6 A cm−2 at 1.8 V; 1.2 A cm−2 at 2.0 V | 1 mA cm−2 h−1 @ 1.8 V for 120 h | 31 | |
| IrO2 (3) | Pt/C (0.5) | 70 | 1 | 0.9 A cm−2 at 1.8 V; 1.7 A cm−2 at 2.0 V | — | 31 | |
| IrO2 (3) | Pt/C (0.5) | 80 | 1 | 1.05 A cm−2 at 1.8 V; 1.2 A cm−2 at 2.0 V | — | 31 | |
| IrO2 (2) | Pt/C (2) | 60 | 1 | 1.0 A cm−2 at 2.15 V | ∼3 mV h−1 @ 500 mA cm−2 for 8 h | 32 | |
| NiMn2O4 | Pt/C (0.5) | 80 | 1 | 0.53 A cm−2 at 2.0 V | 120 µV h−1 @ 400 mA cm−2 for 1000 h (@ 50 °C) | 33 | |
| Pt/C (1) | Pt/C (1) | 60 | 0.1 | 0.03 A cm−2 at 2.0 V | ∼50 µA cm−2 h−1 @ 2 V for 100 h | 34 | |
| Ni foam | Ni foam | 60 | 2 | 2 A cm−2 at 2.65 V | — | 35 | |
| A201 (Tokuyama) | IrO2 (Premion®, Alfa Aesar) | Pt/C (Pt 46.5 wt%, Tanaka K. K.) | 50 | 1 | 1.07 A cm−2 at 1.8 V | 0.02 A cm−2 per voltage cycle at 1.8 V for 1000 cycles (from 1.5 to 2.2 V at a scan rate of 20 mV s−1) | 36 |
| Aemion™ | Commercial nickel felt (75% porous, BEKAERT) | Commercial nickel felt (75% porous, BEKAERT) | 60 | 1 | 0.5 A cm−2 at 2.3 V | 2 mV h−1 @ 200 mA cm−2 for 100 h | 6 |
| IrO2 (1.5) | Pt/C (0.1) | 70 | 1 | 1.0 A cm−2 at 1.75 V | ∼5 mV h−1 @ 10 mA cm−2 for 20 h | 37 | |
| NiS2/Ni3S4 (5) | Pt/C (0.8) | 60 | 1 | 1.5 A cm−2 at 2 V | 0.12 mV h−1 @ 1000 mA cm−2 for 500 h | 38 | |
| Sustainion® X37-50 | Stainless steel | Pt/C (0.5) | 60 | 1 | 2.74 A cm−2 at 2.0 V | — | 29 |
| Commercial IrO2/CP | Ni@Ni(OH)2/Ti | 50 | 1 | 1.0 A cm−2 at 2.0 V | — | 39 | |
| NiFe | Ni | 40 | 1 | 0.3 A cm−2 at 2.0 V | 1 mV h−1 @ 400 mA cm−2 for 7 days | 40 | |
| IrO2 (2) | Pt/C (2) | 60 | 1 | 1.8 A cm−2 at 2.0 V | ∼5 mV h−1 @ 1000 mA cm−2 for 100 h | 41 | |
| NiFe2O4 (1.8) | RANEY® nickel (2.7) | 60 | 1 | 0.744 A cm−2 at 1.8 V | 0.7 µV h−1 @ 1000 mA cm−2 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 h 100 h |
42 | |
| IrO2/CP | Pt/C/CP (40 wt%, EP40) | 50 | 1 | 0.96 A cm−2 at 1.9 V | — | 43 | |
| CuCoO | NiCoO–NiCo/C | 60 | 1 | 0.504 A cm−2 at 1.8 V | 2.0 mV h−1 440 mA cm−2 for 150 h | 44 | |
| Orion TM1 | IrO2 | Pt/C | 70 | 1 | 2.75 A cm−2 at 1.9 V | 55 mV h−1@ 500 mA cm−2 for 50 h | 5 |
| XION™ composite-72-10CL | IrOx | PtNi | 60 | 0.3 | 2.48 A cm−2 at 2.0 V | 5 mV h−1 @ 1000 mA cm−2 for 50 h | 45 |
| PiperION™ (Versogen) | Ni foam (Bekaert) | Pt/C (0.5) | 60 | 1 | 0.62 A cm−2 at 2 V | — | 38 |
Currently, the large-scale application of AEMWEs is impeded by the relatively poor durability of the available AEMs. It should be noted that a Sustainion® X37-50 anion exchange membrane has demonstrated the best performance and long-term durability in electrochemical water-splitting systems. Its optimized design, based on a 1,2,4,5-tetramethylimidazole functional group, provides high hydroxide conductivity (≈115 mS cm−1 at 60 °C) and good chemical stability in alkaline media.
When paired with Pt/C catalysts, the Sustainion membrane achieved 1.8 A cm−2 at 2.0 V, outperforming Fumasep® membranes (≈1.2 A cm−2 at the same voltage), underscoring the importance of ionic group chemistry for efficient charge transport. In configurations using NiFe2O4 anodes and RANEY® Ni cathodes, Sustainion X37-50 exhibited 0.744 A cm−2 at 1.8 V for >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h, with a voltage degradation rate of only 0.7 µV h−1.
000 h, with a voltage degradation rate of only 0.7 µV h−1.
In contrast FAA-3-50 membranes showed poor durability and high degradation rates not exceeding 1000 hours of operation.
Aemion™, based on methylated polybenzimidazole, achieves high current densities (1.0–1.5 A cm−2 at ∼2.0 V) but undergoes ring-opening degradation of the imidazolium moiety under alkaline conditions. XION composite membranes exhibit 2.48 A cm−2 at 2.0 V with promising mechanical reinforcement but require further long-term validation, and PiperION™, featuring a rigid aryl backbone and piperidinium cations, demonstrated 0.62 A cm−2 at 2.0 V (1 M KOH, 60 °C), showing potential though lacking extended durability data. Overall, Sustainion® X37-50 stands out for its balance of high conductivity, alkaline resilience, and extended operational stability, but for all other reported AEMWEs, longevity no longer than 3000 h has been reported, highlighting the need of intensive research before the commercialization threshold of AEMWEs can be reached. Table 2 shows AEMWE state-of-the-art key performance indicators (KPIs) along with the expected ones for 2050.46,47
| 2020 | Target 2050 | R&D focus | |
|---|---|---|---|
| Lifetime (stack) | >5000 hours | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours 000 hours |
Membrane, electrodes |
| Stack unit size | 2.5 kW | 2 MW | MEA |
| Electrode area | <300 cm2 | 1000 cm2 | MEA |
| Cold start (to nominal load) | <20 minutes | <5 minutes | Insulation (design) |
| Capital costs (stack) minimum 1 MW | No estimation available | <USD 100/kW | MEA |
| Capital cost (system) minimum 10 MW | No estimation available | <USD 200/kW | Rectifier |
3. AEM
3.1 Membrane structure: backbone
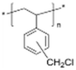
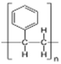
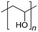
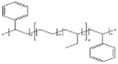
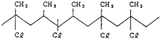
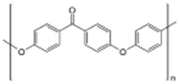
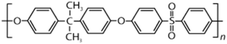
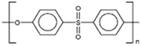
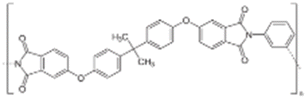
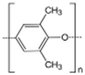
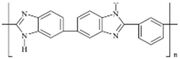
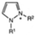
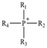
AEMs are typically composed of a polymeric backbone with grafted cationic groups to assure the ionic conductivity. Their stability depends on the chemical structure of both the cationic groups and the polymer backbone.48Numerous polymers have been explored for the application as AEMs in alkaline electrolyzers and fuel cells. For example, poly(vinyl benzyl chloride) (PVBC),49 polystyrene (PS),50,51 poly(vinyl alcohol) (PVA),52–54 styrene-(ethylene–butylene) (SEBS),55,56 chlorinated polypropylene (CPP),57,58 polyether ether ketone (PEEK),59–61 polysulfone (PSU)62–64 polyethersulfone (PES),65,66 polyetherimide (PEI),67,68 poly(p-phenylene oxide) (PPO),69,70 polybenzimidazole (PBI),71 and poly(terphenylene),72,73 among others. The most common polymer backbone structures are shown in Table 3.
The backbone structure affects the aggregation of cationic side groups and hydroxide anions by hydrophilic/hydrophobic regulation; moreover, the cation linkage (different locations of cations along the polymer backbones by graft/comb-shape) and distribution of the microphase morphology act as key factors affecting the properties of AEMs as well. A flexible and long alkyl side chain might be used to manipulate the cation linkage and degree of phase separation, improving the ionic conductivity and alkaline stability.74 Several factors need to be accounted to design an optimal polymer chain, for example cation strings/clusters tethered to the backbone increase the IEC, free space, and mobility,75 but cationic groups inserted on pendant electron-donating alkyl spacer sidechains along the backbone have been shown to largely reduce the detrimental elimination reactions, with the steric effects maximized for the alkyl chain's length of four or six carbon atoms.76 Therefore, constructing a well-connected hydroxide pathway by linkage adjustment is imperative to obtain optimal stability and ionic conductivity.
3.2 Membrane structure: cationic functional groups
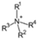
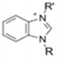
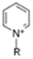
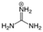
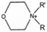
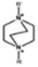
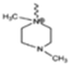
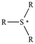
The cationic functional groups have a strong impact on the ionic conductivity and stability of AEMs.A variety of cationic groups have been synthesized and studied by experimental and computational investigations, such as quaternary ammonium,77–80 gemini quaternary ammonium,81 spirocyclic quaternary ammonium82,83 imidazolium,84–86 benzimidazolium,87,88 pyridinium,89,90 pyrrolidinium,91,92 guanidinium,93–95 pyrazolium,96 morpholinium,97,98 1,4-diazabicyclo-[2.2.2]-octane (DABCO),99,100 1,2,3-triazoles,101,102 piperazinium,103 methylated melamine,104 phosphazenium105,106 and tetrakis(dialkylamino)phosphonium,106 quaternary phosphonium,107–109 tertiary sulfonium,110 triarylsulfonium,110 and metal cations.111–113 Table 4 shows the structures of the most common functional groups.
Among the cationic functional groups shown in Table 4, quaternary ammonium (QA) cations have been mostly researched due to their maturity and low cost in synthesis.114,115
The major problem of all functional groups is their stability in alkaline environments: the synthesis of highly conductive and stable cations is imperative to develop efficient and effective AEMs and AEMWE devices.
3.3 Commercially available membranes
Anion exchange membranes have been under development for over seven decades.116 However, due to the low alkaline stability of the earlier membranes, they were predominantly used and optimized for applications in less aggressive environments, such as desalination, electrodeionization, or electrodialysis.117 While there were early reports on AEM-based fuel cells,118 research on AEMWEs started relatively late.25,119 Consequently, even though several membranes have been produced by companies, Table 5 features only a selection of those that have been studied in AEMWEs. Thus, the table does not encompass the properties of all available membranes or membrane grades. Additional membranes are accessible upon request, with variations in thickness, inclusion of a support and/or other enhancements.120 It should be noted that the comparison of hydroxide conductivity values for AEMs is often unreliable because different measurement procedures are used by manufacturers. For example, Fumatech determines hydroxide conductivity by first immersing membranes in KOH solution to ensure ion exchange, followed by rinsing in pure water to remove excess KOH. However, complete ion exchange is not always achieved, and unless the process is conducted in a closed, CO2-free environment—such as using nitrogen-purged water or within a glove box—carbonation can occur, lowering accuracy.121| Brand name | Company | Country | Product code | Thickness (µm) | IEC (meq g−1) | Ion conductivity (mS cm−1) | ASR (Ω cm2) | Dimensional stability (%) | Tensile strength (MPa) | Elongation at break (%) | Structure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a From the technical data sheet. | |||||||||||
| Fumasep® FAA3 | Fumatech | Germany | FAA-3-30 | 25–35a | 1.7–2.1 (Cl)a | 4–7 (Cl)a 40 (OH)120 | 0.3–0.5 (Cl)a | 0–2 (Br)a | 25–40a | 20–40a | Polyaromatic polymer with ether bonds in the main chain, and quaternary ammonium groups attached to the main chain |
| FAA-3-50 | 47–53a | 1.85a | As above | <2.5(Cl)a | As above | As above | As above | ||||
| FAA-3-PK-75 | 75a | 1.39 (Cl)a | >2.5 (Cl)a | <2.0 (Cl)a | 0 (Br)a | 20–45a | 30–50a | ||||
| A201 | Tokuyama | Japan | A201 | 28 (ref. 120 ) | 1.8 (ref. 120) | 42 (OH)120 | Na | 2 (MD)120 6 (TD)120 | 96 (dry, Cl)122 | 62 (dry, Cl)122 | Hydrocarbon-based membrane with quaternary ammonium groups |
| AEMION™ | Ionomr | Canada | AF1-HNN8-50-X | 50a | 2.1–2.5a | >80a | 0.13a | na | 60 (dry, I)a | 85–110 (dry, I)a |  |
| AF1-HNN8-25-X | 25a | 2.1–2.5a | >80a | 0.063a | na | 60 (dry, I)a | 85–110 (dry, I)a | ||||
| AF1-HNN5-50-X | 50a | 1.4–1.7a | 15–25a | 0.42–0.67a | na | 60 (dry, I)a | 85–110 (dry, I)a | ||||
| AF1-HNN5-25-X | 25a | 1.4–1.7a | 15–25a | 0.21–0.33a | na | 60 (dry, I)a | 85–110 (dry, I)a | ||||
| Sustainion® | Dioxide materials | USA | Sustainion X37–50 | 50 (ref. 123) | na | 80 (1 M KOH, 30 °C)123 | 0.045 (1 M KOH)123 | Cracks when dry | Cracks when dry | Cracks when dry |  |
| Orion TM1 | Orion polymer | USA | Pure material m-TPN172 | 24 (ref. 72) | 2.19(OH)a | 19 (Cl)72 54 (OH)72 >60a | na | 6 (Cl)72 10 (OH)72 | 30 (ref. 72) | 35 (ref. 72) | 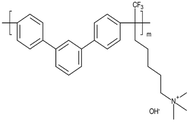 |
| XION™ | Xergy Inc | USA | XION™ composite-72-10CL | 10a | 3.4–3.6a | na | na | na | na | na | 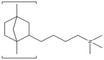 |
| PiperION® | Versogen | USA | PiperION® self-supporting | 20–80a | 2.35 a | 150 (OH, 80 °C)a | na | na | >30–50a | >20–100a | 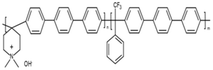 |
An alternative and more precise approach is electrochemical carbonate removal, where a voltage is applied under CO2-free conditions to purge carbonates from the membrane. Conductivity values obtained through this method are significantly higher and better represent those observed in practical AEM water electrolyzers.124
Water permeability, another key membrane property, is often omitted from tabulated data but plays a critical role in system performance. When the electrolyte solution is supplied to both electrodes, water transport through the membrane is less significant since the balance is easily maintained. However, feeding the electrolyte solution only to the anode chamber can be advantageous and it is a very common practice.36 This mode minimizes gas bubble blockage in catalyst layers, enhances performance, and reduces hydrogen gas humidity, minimizing the efforts to dry the hydrogen.
If water transport from anode to cathode is insufficient, mass transport limitations may arise due to unbalanced hydration or electro-osmotic drag, where hydroxide ions move from the cathode to the anode. Conversely, excessive water crossover can lead to cathode flooding and reduced efficiency. Proper control of membrane hydration is therefore crucial for stable electrolyzer operation.
Mechanical properties are typically reported under controlled laboratory conditions, usually in the dry halide form at room temperature. However, the more relevant wet hydroxide form at elevated temperature is rarely characterized, as it requires inert, humidity-controlled testing environments. Consequently, reported data often fail to represent real operating conditions.
For practical AEM applications, high tensile strength, high Young's modulus, and high elongation at break are desirable. A flexible membrane capable of withstanding mechanical stress without cracking is particularly valuable, as it enhances durability and operational reliability under electrochemical conditions.120
4 AEM functional properties
4.1 Introduction
Chemical homogeneity, structure, stability, and mechanical properties are in the main focus of AEM characterization techniques. Analytical techniques such as energy-dispersive X-ray (EDX), nuclear magnetic resonance (NMR), Fourier-transformed infrared (FTIR), and small-angle X-ray scattering (SAXS) are used to characterize the membrane morphology (e.g., pore structure and surface roughness) and molecular distribution (e.g., uniform distribution of head groups and formation of ion clusters).125 Ion Exchange Capacity (IEC), water uptake, swelling ratio, contact angle, conductivity, and alkaline stability measurements are commonly used to evaluate AEMs' performance and chemical stability;117,126 tensile strength, elongation at break and dimensional stability tests are used to evaluate AEMs' mechanical properties while thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) are helpful to determine AEMs' thermal stability.1274.2 Ionic exchange capacity
The ionic exchange capacity is a measure of the number of exchangeable ions per membrane dry weight (meq g−1 or mmol g−1).125 IEC determination procedures for AEMs are not as well defined as those for PEMs. IEC can be evaluated using a variety of techniques, such as titration, spectroscopy (UV-vis), and ion selective electrodes (such as pH probes) to quantify the amount of H+/OH− ions in a solution.128 The most popular techniques are titration methods, such as the Mohr method or acid/base titration.The approach for the acid/base titration consists of soaking the AEM in a strong base solution (such as 1 M NaOH) to convert it into the OH− form. Following, the AEM is soaked in a strong acid (e.g., HCl) solution with a known volume and concentration to convert it into the Cl− form. The resulting diluted HCl solution is then titrated with standardized NaOH to the phenolphthalein endpoint after the AEM is removed and rinsed with DI water.129
The Mohr approach involves soaking an AEM in a salt solution (such as 1 M NaCl) to transform it into the Cl− form. To help with the release of Cl−, the AEM is then washed and equilibrated in a 0.5 M Na2SO4 solution. The AEM/Na2SO4 solution is titrated until the K2CrO4 endpoint, which indicates that all chlorides have precipitated and Ag2CrO4 is currently forming, using an AgNO3 solution with K2CrO4 as the indicator.130
Achieving high ion-exchange capacity is important to maximize hydroxide conductivity in anion exchange membranes, as more cationic sites provide efficient ion transport pathways. However, increasing IEC typically raises water uptake and swelling, weakening mechanical strength and exposing the polymer to chemical degradation. This leads to a core trade-off: higher IEC improves conductivity but compromises alkaline stability.75,125,131
To mitigate this conflict, several strategies have been proposed, such as the use of chemically robust cations and ether-free backbones131 or controlled crosslinking and hydrophobic–hydrophilic phase separation, which can limit swelling while maintaining efficient ion channels.132
Additionally, developing AEMs with cationic sites separated from the main chain shields the backbone from hydroxide attack, balancing IEC and durability. Optimizing membrane morphology and hydration enables high conductivity at moderate IEC levels, achieving both performance and durability.133,134
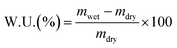
4.3 Water uptake
The water uptake (W.U.) is the change in the membrane mass in response to water exposure. It is measured as described in ref. 135, and it is defined as:Here, mwet is the weight of the membrane after water exposure at a certain temperature, and mdry is the weight of the AEM after drying.
4.4 Swelling ratio
The swelling ratio (S.R.) is a measure of the linear expansion of the membranes when exposed to water136 and is calculated as:Here, lwet is the thickness of the membrane after water exposure at a certain temperature and ldry is the thickness of the AEM after drying.
4.5 Membrane water content
The membrane water content (γ) is related to the number of water molecules per mobile ion and is determined by dividing the water uptake by the molecular weight of water and the IEC.137The W.U. is multiplied by 10 to account for the fact that the IEC is provided in mmol g−1, while the W.U. is reported in percentage:
4.6 Hydroxide conductivity
The hydroxide conductivity (σ) can be measured in a two- or four-electrode testing cell by using electrochemical impedance spectroscopy (EIS).138 An AEM is fixed in the testing cell, and a varied AC current is provided to gather impedance data after the AEM has been soaked in DI water or KOH solution for the entire night. The membrane ionic resistance (Rm) can be determined by nonlinear least squares regression analysis, and the conductivity can then be calculated using the following formula:139where L is the distance between the electrodes, and A is the cross-sectional area perpendicular to the current flow.
To measure the OH− conductivity of AEMs without the contribution of carbonate (CO32−) and bicarbonate (HCO3−) ions, which can affect the measurements, Dekel et al.124,140 proposed a decarbonation method prior to the conductivity test that consists of applying a direct current of 100 µA in situ to the AEMs until a stable conductivity value is reached.
4.7 Water contact angle
The water contact angle (θ) is related to the membrane surface's wettability. Large contact angles signify highly hydrophobic surfaces. The sessile-drop technique can be used to measure this parameter.1414.8 Alkaline stability
The alkaline stability is a measure of how the AEM's performance and properties vary under high-pH conditions over time.135 Although testing settings differ, the general approach is to soak the AEM in a high-pH solution (such as 1–10 M KOH) at a specific temperature (either room temperature or increased temperature) over extended times, periodically testing the membrane IEC and/or conductivity. To evaluate alkaline stability at various hydration levels, Dekel et al.80 suggested an alternative ex situ alkaline stability test employing NMR and a water-free hydroxide (crown ether/KOH) solution. This allowed for control over the water/OH− ratio (γ), as it has been shown that alkaline stability is influenced by the hydration level of the nucleophile (OH−).4.9 Mechanical and thermal properties
Thermal stability, tensile strength, elongation at break, and stress–strain curves are measured to evaluate AEMs' thermal and mechanical properties. Thermogravimetric analysis and differential scanning calorimetry are used to assess the thermal stability of the membrane, which is crucial given the working temperatures of AEMFCs (up to 200 °C) and AEMWEs (usually 50–70 °C).142To evaluate the membrane's thermal stability, TGA yields the temperatures at which weight changes occur, resulting from water losses, head group decomposition, and/or polymer decomposition.143 Besides crystallization and melting features, DSC can be used to assess changes in polymer crystallinity and cross-linking, glass transition temperature and the effects of thermal cycling.144 A universal testing machine can be adopted to stretch membrane samples to measure tensile strength, elongation at break, and stress–strain curves.144,145
Several, but not all, of the mentioned parameters can be found as target specification for AEMs in Table 6 based on a recent EU Horizon 2020 call for proposals.146 These should be viewed not as universally acknowledged benchmarks, but rather as guidelines, noting that a low performance in one parameter might be offset by superior performance in another. For instance, high stability is preferred over high efficiency, but low stability or low ionic conductivity could be offset by small membrane thickness.
| Parameter | EU target values |
|---|---|
| Ion conductivity | >50 mS cm−1 |
| Area-specific resistance (ASR) | ≤0.07 Ω cm2 |
| Stability | ≤0.07 Ω cm2 after 2000 h real or simulated operation in an electrolyzer |
| Tensile strength | >15 MPa |
| Elongation at break | >100% |
| Dimensional stability | ≤1% in machine direction ≤4% in transverse direction |
Notably, the targets did not include any reference to gas permeation, even though hydrogen crossover is a widely recognized key performance indicator. That is because the area-specific resistance target value in combination with the concentration value of 2% H2 in O2, which is the limit for safe operation, indirectly controls the maximum permitted permeability.120
5 AEMWE performance degradation
5.1 Overview
The main degradation mechanisms of AEMWE cells are related to the catalyst and the AEM/ionomer.Catalyst degradation (dissolution, detachment, migration, and agglomeration) occurs at both the anode and cathode side. This is related to a relatively poor interaction between the catalyst-supporting material and the catalyst itself, which can be worsened further by the low chemical stability of some transition metal (Ni, Fe and Co)-based catalysts and noble metal (Ir and Ru) catalysts,147,148 as well as by the oxidation of the support material, e.g., carbon at the anode.149
For more information about the catalyst degradation, please see Section 5.3.2.
The degradation of the AEM/ionomer has different causes – see Fig. 2.
The chemical/electrochemical degradation is related to the alkaline environment and radical attacks; thermal degradation relates to melting and glass transitions as well as hotspots; mechanical degradation occurs mainly via swelling or local imperfections in the AEM. For more information about AEM/ionomer degradation, please see Section 5.3.1.
At the anode, the electrochemical/chemical attack on the membrane and the ionomer is particularly strong due to the high electrode potential. Based on the high instability of the ionomer, the catalyst, which is dispersed with soluble anion-conducting ionomers, will degrade. In addition, there are various anions that accumulate on the catalyst surface of the anode, which leads to reduced catalyst activity.
On the cathode side, the degradation of the AEM/ionomer is less severe because of the lower electrode potential. However, a relatively high degradation of the catalyst activity is observed especially if the electrolyte-feed contains contaminants (e.g., Mg2+, Ca2+, and Ni2+), which could plate as metal on the catalyst or precipitate as hydroxide on the catalyst because the pH strongly increases at the catalyst surroundings during the hydrogen evolution reaction.150 The metal plating and/or the hydroxide deposition cover the catalyst surface leading to both the catalyst activity reduction and mechanical stress caused by the volume increase. Anion contaminants (Cl− and Br−) may also be involved in the anodic faradaic reaction because their oxidation potential is in a similar region to the oxidation potential of OH−.151
In Fig. 3, the anode and cathode degradation phenomena are illustrated.
 | ||
| Fig. 3 Degradation phenomena occurring at the anode and cathode of an AEMWE cell: (a) new cell and (b) degraded cell. Reprinted from Joule, vol 4, Lindquist et al.,151 Membrane electrolyzers for impure-water splitting, p. 13, Copyright (2020) with permission from Elsevier. | ||
It should be mentioned that, depending on the operating conditions, the dominance of the individual mechanisms discussed above may change. The supporting electrolyte may have a strong influence. Additional electrolyte feeds (e.g., KOH or K2CO3) are commonly used to externally establish a high pH environment around the catalyst, leading to high catalyst activity, while increasing the overall ionic conductivity, i.e., enabling high current densities.
5.2 Performance degradation – operating conditions
 | ||
| Fig. 4 (a) Effect of temperature on AEMWE performance at constant pressure (1 atm). (b) Effect of pressure on AEMWE performance at constant temperature (60 °C). (c) Combined effects of pressure and temperature on the applied voltage. Reprinted from Chem. Eng. Res. Des., vol 194, Vidales et al.,23 Modeling of anion exchange membrane water electrolyzers: the influence of operating parameters, p. 13, Copyright (2023) with permission from Elsevier. | ||
The other significant operational parameter influencing AEM performance is pressure, significantly impacting the thermodynamically reversible voltage, a relationship described by the Nernst equation. In Fig. 4b, the impact of varying cathode pressures on the AEMWE cell performance is illustrated. While an increase in system operating temperature reduces energy requirements (attributed to the higher temperature aiding the phase change of hydrogen and oxygen products into their gaseous forms), elevated pressure has the opposite effect, resulting in increased cell overvoltage. To explain this, one might consider that the high operating pressure directly raises the open-circuit voltage and may impede water diffusion within the electrode and membrane, thereby increasing diffusion losses.
Fig. 4c shows temperature and pressure plotted against the resulting voltage. The most favourable operational conditions emerge when high temperature is coupled with low pressure. This observed behaviour suggests the presence of two opposing effects, resulting in a convex shape. Moreover, it appears that the effect of pressure is less pronounced at high temperatures compared to low temperature, while the influence of temperature is more substantial at higher pressures than at lower pressures. This phenomenon can be elucidated by examining the resulting current density, which rises with increasing temperature due to temperature-dependent kinetics, while it decreases at high pressures.158 According to the model, the combined effects of temperature and pressure have opposite impacts on AEMWE cells' performance, with the most favourable conditions occurring at high temperatures and low pressures. Specifically, the model identifies the optimal conditions as 75 °C and 1.8 MPa, respectively. These findings align, to a very first approximation, at least as far as the temperature is concerned, with prior research,152 which suggests an optimal temperature around 60–70 °C and an optimal pressure around 0.2 MPa. However, it must be noted that, according to these previous studies,152 higher temperatures and/or pressures lead to reduced performance due to electrode degradation and gas crossover, respectively. Thus, achieving optimal electrolysis process performance requires considering the relatively high-pressure values necessary for subsequent hydrogen storage; accordingly, practical electrolyzer working conditions involve a combination of moderate temperatures (40–80 °C) and pressures (1–5 MPa). This strategy aims to both maximize electrolyzer performance and meet the hydrogen storage requirements of the system.142,159
Mayerhöfer et al.19 and Li et al.163 reported the best performing cells from a literature survey on AEMWE cell performance, employing PGM-free OER catalysts (such as CuCoOx, NiFe and other Ni based electrodes) in different electrolytes. Although the comparison considers different AEM materials, manufacturing strategies, operating temperatures, and variable preconditioning for the MEAs, the survey highlights that operation in KOH solutions (0.1 M–1 M) results in the highest reported performance to date. The current density of the KOH-fed AEMWE cells is, in fact, typically >0.5 A cm−2 at 1.6 V, while the current density of the 1 wt% K2CO3-fed AEMWE cells is always lower than 0.35 A cm−2 at 1.6 V. The pure water-fed AEMWE cells offer intermediate performance, sometimes reaching 0.5 A cm−2 at 1.6 V. Overall, the performance of PGM-free catalyst AEMWE cells follows the trend: concentrated KOH-fed >> pure water-fed > 1 wt% K2CO3-fed.
Recently, Krivina et al.164 also demonstrated that some AEMs (e.g. PiperION®) degrade more in 1 M carbonate/bicarbonate buffer than in 1 M KOH solution, pointing out that the low conductivity of the carbonate/bicarbonate forms of PiperION® might facilitate a pH gradient leading to oxidative changes in the polymer due to the local pH drop or the absence of sufficient OH− for the OER.
The durability of pure water-fed AEMWE cells is relatively low.165,166 The main degradation mechanisms in pure water-fed AEMWEs have been associated with the detachment of the ionomeric binder from the electrocatalysts at the electrodes.163 This phenomenon is particularly severe with ionomeric binders possessing high ion exchange capacity, which creates high pH environments without the need for an additional circulating liquid electrolyte. Ionomeric binders with high IEC often exhibit substantial dimensional changes under fully hydrated conditions, weakening the adhesion of the ionomer to the catalyst's surface. The lack of adhesion frequently leads to ionomer detachment from the electrode, limiting cell durability. A synthetic approach to ionomers with high IEC and low water uptake may offer a balance between high electrochemical performance and good durability.167,168 Another durability-limiting factor related to the ionomeric binder is the electrochemical phenyl oxidation.169,170 The oxidation of phenyl groups in the ionomeric binder results in a rapid voltage jump due to localized pH changes at the electrode occurring relatively quickly. This oxidative process is more detrimental in electrolyzers than fuel cells, given that the operating voltages of the AEMWE anode (1.4–2.2 V) are much higher than those of the AEMFC cathode (0.6–1.0 V). The process begins with the adsorption of phenyl groups of the ionomeric binder onto the catalyst's surface, facilitated by the favourable interaction between the aromatic π-electrons of the phenyl group and the electronic cloud around the metal atoms.171 The adsorption energy of phenyl group fragments of the ionomer backbone on the Pt surface is even higher than that of benzene.172 Once adsorbed, the phenyl group undergoes oxidation, converting into phenol. The produced phenolic protons are effectively deprotonated by the hydroxide ions, neutralizing the alkaline medium. Avoiding the presence of phenyl groups in the anode ionomers173 can solve the issue associated with the electrochemical oxidation of ionomers.
For concentrated KOH-fed AEMWEs, the primary durability-limiting factors differ from those of pure water-fed AEMWEs due to the altered operating environments created by the additional electrolyte. In this case, high IEC ionomers may not be necessary, mitigating the performance loss associated with ionomer detachment. Moreover, the electrochemical oxidation of phenyl groups in the AEM and ionomer becomes less critical because the liquid electrolyte can neutralize the phenols without significantly altering the local pH at the catalyst–electrolyte interface.163 While circulating a concentrated alkali hydroxide solution enhances AEMWE performance and performance tolerance to ionomer degradation, the corrosive nature of the liquid electrolyte accelerates the degradation of AEMs and other AEMWE components. Consequently, the chemical and electrochemical stability of AEMs becomes a major concern for concentrated KOH-fed AEMWEs.
Lastly, it should be pointed out that the operation mode can also have an impact on the durability: Niaz et al.174 showed that frequently subjecting the cell to rest times without feeding solution may deteriorate the membrane. The solution feeding should be continuous even when the cell is subjected to the rest time. The frequent rest times without solution can, in fact, cause lower humidity levels inside the cell, resulting in irreversible degradation in the membrane.
Carbonation occurs when the cell is exposed to carbon dioxide (CO2). The primary source of CO2 is mainly the ambient air. When the cell comes into contact with CO2 or is supplied with a solution containing CO2, its reaction with hydroxide anions leads to bicarbonates and carbonates, as illustrated in the following reactions:
| OH− + CO2 ⇄ HCO3− |
| HCO3− + OH− ⇄ CO32− + H2O |
However, recent experimental175–177 and theoretical178–180 studies have enabled a much more complete understanding of the effects of CO2 on AEM performance.
Briefly, the mobilities of (bi-)carbonate are lower than OH−, resulting to an increase in the ohmic resistance of the electrolyte. Parrondo et al.181 demonstrated that short-term performance losses in the system were attributed to CO2 intrusion, emphasizing the remarkable sensitivity of these systems to CO2. Its intrusion can lead to the formation of carbonate ions, contributing to increased ohmic resistance: the mobility efficiency of HCO3− (4.61 × 10−8 m2 s−1 V−1) and CO32− (7.46 × 10−8 m2 s−1 V−1) is, in fact, much lower than that of OH− (20.64 × 10−8 m2 s−1 V−1) in aqueous solutions.182 The study revealed that effective electrolyzer cell sealing, to minimize CO2 intrusion, significantly enhances the short-term stability of water electrolyzers.183
The parasitic reactions of CO2 with the alkaline electrolyte, besides decreasing conductivity and increasing cell voltages, can also cause a range of problems including (bi-)carbonate precipitation, electrolyte pH-drift and carbonation of ionomers, lowering the local pH and reducing the catalytic efficiency (Fig. 5).184,185
 | ||
| Fig. 5 Illustration of the carbonation problem during electrolysis in a CO2 electrolyzer, taken as an example. (Bi)carbonates are produced from the reaction of CO2 with hydroxides and are oxidized at the anode to release CO2. Adapted from Ramdin et al.184 with permission from American Chemical Society. | ||
In an anion exchange membrane, bicarbonate, carbonate, and hydroxide ions will be transported to the anode, yielding the following reactions:
| 4HCO3− → 4CO2 + 2H2O + O2 + 4e− |
| 2CO32− → 2CO2 + O2 + 4e− |
| 4OH−→ 2H2O + O2 + 4e− |
Martinez-Lazaro et al.186 observed AEMWEs' performance degradation at first and then performance recovery by replacing the KOH solution and/or performing linear sweep voltammetry (LSV). They attributed the performance recovery to (bi-)carbonate's decomposition during cell potential sweeping. Also, Zignani et al.187 found that carbonation phenomena especially occur during shut-down periods. Then, as a new electrolysis cycle begins, carbonate species decompose and release CO2. This provides a decarbonation method along with the electrolysis process. Since carbonates are dissolved in water, CO2 release and water circulation that prevents carbonate accumulation are the main sources of the performance recovery. Despite being reversible, carbonation is still one of the factors contributing to the decline in electrolyzer performance. It is recommended that the MEAs' ion exchange procedure is carried out in an inert gas atmosphere prior to electrolyzer installation to prevent carbonation from ambient CO2.188
5.3 Performance degradation – materials
5.3.1.1 Chemical degradation. The chemical stability of AEMs is a crucial aspect for achieving high performance and sustainability in alkaline solid polymer membranes. Unfortunately, many AEMs exhibit low chemical stability, especially in alkaline environments. Common issues of AEMs are associated with the cation functional groups. Organic cations, in general, are susceptible to various chemical reactions, such as nucleophilic substitution SN2 at the α-position, ylide formation leading to Sommelet–Hauser or Stevens rearrangements189–191 and E2 reactions when β-hydrogens are present.189 N-conjugated cations can also undergo nucleophilic addition to the C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds.76 Additionally, when oxygen-based nucleophiles react with phosphonium cations, the Cahours–Hofmann reaction may occur to form phosphine oxide.192 These reactions contribute to the degradation of AEM performance by reducing the concentration of anion-exchange groups, thereby negatively impacting ionic conductivity.13 The alkaline environment can also lead to the degradation of the polymer backbone via hydrolysis,193 dehydrofluorination125 and cross linking,194 depending on the polymer structure. Another type of chemical degradation is attributed to the radicals that form during the operation; in particular, hydroxyl (OH˙) and superoxide (OO˙) can attack both the backbone and the functional groups.195
N bonds.76 Additionally, when oxygen-based nucleophiles react with phosphonium cations, the Cahours–Hofmann reaction may occur to form phosphine oxide.192 These reactions contribute to the degradation of AEM performance by reducing the concentration of anion-exchange groups, thereby negatively impacting ionic conductivity.13 The alkaline environment can also lead to the degradation of the polymer backbone via hydrolysis,193 dehydrofluorination125 and cross linking,194 depending on the polymer structure. Another type of chemical degradation is attributed to the radicals that form during the operation; in particular, hydroxyl (OH˙) and superoxide (OO˙) can attack both the backbone and the functional groups.195
5.3.1.1.1 Alkaline degradation of functional groups. The degradation of the cationic functional groups is one of the major problems of AEMs, especially when the electrolyzer is fed with KOH solution. OH− is a strong nucleophile that can attack positively charged sites, remove protons and add to double bonds.
Fig. 6 depicts the various degradation pathways of the cationic functional groups: (a) SN2 benzyl substitution,196 (b) SN2 methyl substitution,196 (c) β-elimination substitution,197 (d) ylide-formation that can lead to Sommelet–Hauser and Stevens rearrangements,198,199 (e) nucleophilic addition and displacement of pyridinium,200 (f) nucleophilic degradation of guanidinium94,201 (g) SN2 methyl substitution of imidazolium,202 (h) heterocycle deprotonation of imidazolium,202 (i) nucleophilic addition to the double bond,8 and (j) phosphine oxidation.13
 | ||
| Fig. 6 Degradation pathways of cationic functional groups in an alkaline environment: (a) SN2 benzyl substitution,196 (b) SN2 methyl substitution,196 (c) β-elimination substitution,197 (d) ylide-formation that can lead to Sommelet–Hauser and Stevens rearrangements,198,199 (e) nucleophilic addition and displacement of pyridinium,200 (f) nucleophilic degradation of guanidinium,94,201, (g) SN2 methyl substitution of imidazolium,202 (h) heterocycle deprotonation of imidazolium,202 (i) nucleophilic addition to the double bond,8 and (j) phosphine oxidation.13 Adapted with permission from You et al.13 (Copyright (2020) American Chemical Society) and Mustain et al.194 (with permission from the Royal Society of Chemistry). | ||
In the case of cyclic ammonium groups, ring opening reactions are also possible, as reported in Fig. 7: (a) ring opening of imidazolium,202 (b) SN2 and ring opening of piperidinium, pyrrolidinium and morpholinium,203 and (c) ring opening of N-spirocyclic ammonium ions.204
 | ||
| Fig. 7 Ring opening of cyclic ammonium ions: (a) ring opening of imidazolium,202, (b) SN2 and ring opening of piperidinium, pyrrolidinium and morpholinium,203 and (c) ring opening of N-spirocyclic ammonium ions.204 Adapted from ref. 205 (with permission from Elsevier) and ref. 194 (with permission from the Royal Society of Chemistry). | ||
5.3.1.1.2 Alkaline degradation of the polymer backbone. The chemical stability of the AEM backbone is also crucial for advanced AEMWE systems, as the backbone structure and molecular weight significantly influence the mechanical toughness of the resulting membranes. Common reactions involving the polymer backbone include the cleavage of the ether bond (e.g., in polysulfone), the quaternary carbon hydrolysis and dehydrofluorination when fluorinated carbon structures are present125 (Fig. 8).
 | ||
| Fig. 8 Degradation pathways of the polymer backbone: (a) quaternary carbon hydrolysis, (b) dehydrofluorination, (c) aril–ether bond cleavage. Adapted from Hagesteijn et al.125 (with permission from Springer Nature) and Mustain et al.194 (with permission from the Royal Society of Chemistry). | ||
Aryl ether cleavage deteriorates the mechanical properties of quaternized poly(arylene ether) AEMs.206 The degradation mechanism of the aryl ether cleavage reaction is well documented in previous literature.207–210 In brief, the electron-donating aryl ether group in the polymer backbone becomes destabilized by the presence of a positively charged (electron-withdrawing) ammonium cationic group in proximity of the backbone. Hydrolysis of the ether bond in benzyl ammonium-functionalized polymers and the consequent mechanical degradation of the AEM may occur even before the degradation of the cationic group. This is because the energy barrier for aryl ether cleavage in the benzyl ammonium-functionalized polymer backbone is 85.8 kJ mol−1, which is lower than the energy barrier for α-carbons on benzyl trimethyl ammonium (90.8 kJ mol−1).211 Addressing and mitigating these chemical degradation mechanisms are essential for maintaining the long-term mechanical and chemical stability of AEMs in alkaline environments.
5.3.1.1.3 Cross-linking of AEMs under alkaline conditions. Exposure of AEMs to highly concentrated caustic solutions commonly results in significant gel formation.212,213 This gel formation arises from cross-linking reactions of quaternized polymers occurring under high pH conditions. While cross-linking reactions are intentionally introduced in AEMs to enhance their mechanical properties,214–216 uncontrolled cross-linking may lead to undesired property changes of the AEM. The reaction rate of cross-linking depends on the concentration of unreacted alkyl halide, which varies based on the AEM synthetic route. Various cross-linking mechanisms of quaternized polymers under high pH conditions have been identified, such as amine alkylation, Williamson ether synthesis and reactions involving fluorinated backbones217,218 (Fig. 9). The property changes in AEMs, due to cross-linking reactions, differ from those resulting from chemical degradation. The IEC of AEMs typically changes minimally, as the cross-linking reaction itself does not significantly consume the ammonium functional groups. However, water uptake and hydroxide conductivity may notably decrease. This reduction in hydroxide conductivity may lead to the deterioration of AEMWE performance by increasing the cell's ohmic resistance. Simultaneously, AEMs may experience decreased elongation and increased elastic modulus, rendering them brittle and potentially leading to premature cell failure. The mitigation strategy of the undesired cross-linking reaction depends on their nature. When degradation byproducts participate in cross-linking reactions, it becomes crucial to prepare alkaline-stable AEMs. Increasing quaternization yield is important to reduce the number of unreacted halide groups in the polymers that can lead to cross-linking phenomena. A high quaternization yield can be achieved through homogeneous amination reactions and potentially by using a non-aqueous reaction medium.219 Additionally, the removal of unsaturated double bonds from the polymer chain is critical in preventing undesired cross-linking.
 | ||
| Fig. 9 Cross-linking pathways of AEMs in an alkaline environment. Adapted from Li et al.163 with permission from the Royal Society of Chemistry. | ||
5.3.1.1.4 Membrane degradation via radicals. The migration of oxygen from the anode to the cathode compartment through the anion exchange membrane can trigger the two-electron oxygen reduction reaction, leading to the formation of hydrogen peroxide radicals.220 Additionally, hydroxyl (HO˙) and hydroperoxyl (HOO˙) radicals may arise from intermediates in the oxygen evolution reaction221,222 or through cation-site catalyzed reduction of dioxygen195,223 (Fig. 10). The detection of hydrogen peroxide in AEMWE cells suggests the likelihood of radical-induced hydrolysis of polymer electrolytes.224,225 For evaluating resistance to radical-induced degradation, Fenton's reagent commonly serves as an ex situ accelerated degradation test, generating oxygen-containing free radicals in solution.226
 | ||
| Fig. 10 Mechanism of superoxide and hydroxyl radical formation in AEMs through cation-site catalyzed reduction of dioxygen, as proposed by Parrondo et al.223 | ||
Ayers et al. demonstrated the decline in mechanical properties of quaternized poly(arylene ether) AEMs after exposure to Fenton's test for up to 5 hours.227 Post-Fenton's test, optical microscopy revealed surface cracking and potential dissolution of the AEMs. The predominant degradation process in polyaromatics involves the removal of OCH3 from the methoxy-substituted compound, particularly relevant to aryl ether-containing polymers such as polysulfones and polyether ketones, causing bond breakage within the C–O–C connections.228
Some possible radical degradation pathways of AEMs are reported in Fig. 11. Due to its electrophilic nature, the HO˙ radical selectively attacks the aromatic ring near the aryl ether bond, forming phenols under high pH conditions (Fig. 11a).228 Free radicals target the susceptible carbon (para position for the trimethyl ammonium hydroxide group of vinyl benzyl chloride grafts), leading to degradation of the polymer backbone in quaternized polystyrenes.229–231 Cation degradation via radical attack on benzyl triethyl ammonium is also depicted in Fig. 11d.232 In this mechanism, hydroxide ions attack the quaternary ammonium groups of the AEMs, generating ylides and water molecules through proton abstraction from the α-carbon. Subsequently, oxygen molecules in the alkaline solution capture the ylide electron, producing superoxide anion radicals and quaternary ammonium radicals, respectively. The quaternary ammonium radicals then degrade into ethylene and tertiary amine.
 | ||
| Fig. 11 Radical degradation pathways of AEMs: radicals attack both the polymer backbone and the functional groups. (a) Aryl ether polymer backbone degradation. (b) Phenyl group degradation by formation of phenolates. (c) Polymer backbone degradation of quaternized polystyrene. (d) Cationic group degradation. Adapted from Li et al.163 with permission from the Royal Society of Chemistry. | ||
Notably, an increase in IEC is associated with an increase in oxidative degradation.233 This occurrence can be ascribed to an augmented swelling ratio and water uptake, which enhance mass transport of Reactive Oxygen Species (ROS) within water channels. This improved transport, in turn, promotes the attack on vulnerable sites. Moreover, the negative impact on the stability of the AEM backbone may arise either from the introduction of a positive headgroup, or the headgroup itself may play a catalytic or mediator role in accelerating ROS attacks on susceptible sites within the AEM. In a recent study, Maxwell et al.234 used operando Raman spectroscopy to monitor non-PGM (Ni–Fe-based) AEMWEs during active operation. The goal was to track real-time chemical and structural changes in the electrodes and membrane (PiperION®) under electrolysis conditions. Raman analysis revealed that during oxygen evolution, new spectral bands appeared corresponding to oxygenated intermediates, indicative of reactive oxygen species (ROS) formation.
These ROS, including hydroxyl (˙OH) and superoxide (O2˙−) radicals, were found to attack the ionomer and polymer membrane, leading to loss of quaternary ammonium groups and reduction in ion exchange capacity. Over extended operation, the Raman spectra showed degradation products, confirming chemical oxidation of the polymer backbone.
Practically, this work demonstrates that ROS formation in non-PGM systems can be severe and stabilizing the catalyst–ionomer interface is critical. Strategies proposed include using radical-scavenging additives, ionomers with higher oxidative resistance, and catalyst coatings that suppress peroxide and radical generation.
5.3.1.2 Mechanical membrane degradation. To induce a differential pressure during the operation of AEMWEs, both the non-porous AEM and the porous transport layer must offer robust mechanical support to withstand the pressure disparity.235 The maintenance of structural integrity in fully hydrated AEMs is crucial for their mechanical properties. In PEMWEs, relatively thick (125–180 µm) perfluorosulfonic acid (PFSA) membranes are commonly employed. While thicker membranes lead to higher membrane resistance, they offer advantages such as reduced gas crossover and improved mechanical robustness.236 In contrast, AEMWEs predominantly utilize hydrocarbon AEMs due to the chemical instability of quaternized perfluorinated polymers under high pH conditions.237 Hydrocarbon AEMs, with lower gas permeability, enable the use of thinner membranes, thereby enhancing hydrogen production efficiency.
The durability of AEMWEs is closely tied to the mechanical properties of AEMs, and their degradation often results in catastrophic performance loss.238,239 This is frequently accompanied by an increase in high-frequency resistance (HFR), indicating potential interfacial failure between the AEM and the electrode. Predicting the time for this type of failure is challenging due to the involvement of various factors.
Post-mortem analysis reveals that mechanical failure in AEMs tends to occur at the edges of the MEA's active area, where mechanical stress is maximized.240 For instance, a study by Wang et al.241 demonstrated different cell failures using Low-Density Polyethylene (LDPE) and High-Density Polyethylene (HDPE) AEMs, which share similar IEC, thickness, water uptake, and conductivity, but differ significantly in mechanical properties. The HDPE-based AEM exhibits a stress at break of 35 MPa with an elongation at break of 283%, while the LDPE-based AEM has a stress at break of 23 MPa with an elongation at break of 35%. Tests conducted in AEMFCs reveal that the HDPE-based MEA has a lifetime exceeding 440 hours at 600 mA cm−2 and 70 °C under H2/CO2-free air conditions, whereas the LDPE-based MEA ceased testing at approximately 100 hours due to rapid mechanical cell degradation.
Mechanical degradation leads to premature failure, characterized by perforations, cracks, tears, or pinholes, which may result from inherent membrane defects or improper MEA fabrication processes. Local areas corresponding to the interface between lands and channels in the flow field or sealing edges in a cell, experiencing excessive or non-uniform mechanical stresses, are particularly susceptible to small perforations or tears. The constrained membrane in an assembled cell undergoes in-plane tension during shrinkage under low RH and in-plane compression during swelling under wet conditions. The migration and accumulation of catalysts into the membrane also negatively affect membrane conductivity and mechanical strength, significantly reducing ductility. A physical breach of the membrane due to local pinholes and perforations can result in the crossover of product gases into their respective reverse electrodes. The results from Huang et al.242 suggest that the mechanical failure of the membrane initiates as a random, local imperfection that propagates to catastrophic failure.
In general, mechanical degradation problems increase as the thickness of the AEM decreases. A low membrane thickness leads to a lower electrolysis voltage caused by reduced ohmic resistance.24 This was shown by Vidales and et al.23 who used AEM thicknesses of 25, 50, and 130 µm, which resulted in an increase of the total applied voltage with increasing AEM thickness. The positive effect of low ion transport resistance and the negative effect of poor mechanical properties will need to be balanced (for example, according to the EU targets in Table 6) to result in an optimal AEM thickness.24
5.3.1.3 Thermal degradation. The optimal operating temperature for AEMWEs typically falls within the range of 40–80 °C to enhance conductivity and kinetics.23 However, it introduces challenges related to durability, primarily due to the increased susceptibility to hydroxide ion (OH−) attack. Additionally, the AEM itself may exhibit instability at elevated temperatures, potentially undergoing glass transition or melting.
The occurrence of high-temperature spots becomes a concern if the membrane experiences breakage due to local pinholes and perforations. In such cases, there is a risk of H2 and O2 crossover, leading to the highly exothermic direct combustion of the oxidant and reductant on the catalyst surface, generating local hotspots. This sets off a destructive cycle wherein gas crossover and pinhole production mutually reinforce each other, inevitably accelerating the degradation of both the membrane and the entire cell.
The most important HER catalyst degradation mechanism consists of the detachment, migration, and agglomeration of carbon supported HER catalyst nanoparticles. This degradation results from weak catalyst-supporting material interactions under alkaline conditions243 and is accelerated by hydrogen bubble formation. Under low current density operation, hydrogen bubbles at the interface between the catalyst nanoparticles and the carbon support are not formed.244 As the current density of the electrolyzer increases, hydrogen bubbles overcome the critical formation size (4 nm) occurring either on top of a catalyst nanoparticle or adjacent to it. As a result, catalyst nanoparticles start to detach, migrate, and agglomerate. The detachment of catalysts is prevented by using unsupported catalysts, but the catalyst's loading and cost may increase for the sake of durability improvement.
OER catalysts, conversely, still face significant stability challenges, especially upon high current density operation. Catalyst dissolution is a well-studied degradation pathway.245 Catalysts based on the first-row transition metals, Fe, Co, and Ni, as well as their hydroxide forms, can easily dissolve into alkaline electrolytes,246–248 but catalysts based on noble metals may also incur dissolution, the extent of which decreases in the order: Ru > Ag > Au > Ir > Rh > Pt > Pd.249,250 At the OER potentials, noble metal catalysts can passivate by the formation of a stable oxide layer or dissolve in the electrolyte. The dissolution of metal oxides such as IrO2 and RuO2 is much lower than that of their metallic counterparts.251
It is noteworthy that even at Open Circuit Potential (OCP), catalyst dissolution differs drastically at different pH regimes. Mayerhöfer et al.19 studied the dissolution of CuCoOx under different conditions observing immediate dissolution for both Cu and Co in a neutral environment at OCP, indicating the thermodynamic instability of CuCoOx at this pH. Interestingly, the metal dissolution behaviour in alkaline media is more complex and severely impacted by the ionomer activation process. The catalyst is significantly more stable in an alkaline environment than under neutral conditions. While Co and Cu are expected to form stable oxides in alkaline environments and at high potentials, neutral conditions favour the dissolution of Cu2+ and Co2+ species.252,253
Catalyst dissolution may lead to thermal and mechanical problems in the membrane, affecting mechanical strength and significantly reducing ductility:254 in fact, if gas crossover happens the highly exothermic direct combustion of the oxidant and reductant occurs on the catalyst surface and consequently generates local hot-points. A destructive cycle of increasing gas crossover and pinhole production is then established, leading to inevitable degradation of membranes and their performances. Contrary to what was observed for the PEMs, performance degradation is not only due to membrane resistivity increase but also likely due to a loss of interconnection between the catalyst particles and the ionomer.157,248
To overcome the issue of catalyst degradation, various effective material design strategies have been developed such as doping255–257 or surface-structure modification258–260 of the catalyst and the formation of protective layers on the catalyst surface.261–263
6 Membrane degradation mitigation strategies
6.1 Chemical degradations
1. Synthesize β-hydrogen-absent cations: by designing cations that lack β-hydrogens, which are prone to degradation, it is possible to enhance the alkaline stability of the cationic groups.
2. Convert to coplanar arrangement and delocalize positive charges: resonance design, such as guanidinium, imidazolium, and phosphonium, involves converting cations to a coplanar arrangement and delocalizing positive charges. This strategy aims to distribute positive charges over a broader molecular structure, reducing vulnerability to alkali attack.8
3. Use cations with high steric hindrance: in recent years, cations with high steric resistance, particularly those with cyclic or spirocyclic structures such as piperidinium and 6-azonia-spiro[5.5]undecane, have been introduced to exhibit exceptional alkaline stability.8 Steric hindrance can in fact reduce the possibility of OH− attack, minimizing degradations such as ring-opening reactions, addition and SN2 substitution.
Several additional strategies have been explored to improve long-term stability against alkali attack:264
• Introduction of spacer chains: Tomoi et al.265 inhibited SN2 reactions by introducing a long spacer chain between the quaternary nitrogen atom and the benzene ring of the main chain. Alkylene spacers longer than propylene were found to make the cation less susceptible to OH− attack.
• Optimizing spacer length: Hibbs et al.266 designed poly(phenylene) AEMs with hexamethylene-trimethylammonium, exhibiting superior stability compared to benzyl-trimethylammonium-containing membranes (5% vs. 33% conductivity loss after immersion in 4 M KOH at 90 °C for 14 days). Longer spacers were observed to impede cation degradation to some extent, potentially by increasing the Hofmann elimination barrier.
Lin et al.77 reported AEM materials with different lengths of alkyl side chains between the polymer backbone and cationic groups. Increasing the length of flexible spacers (n ≥ 4) enhanced alkaline stability. The IEC and anion conductivity of membranes only decreased no more than 10% after treatment by 1 M KOH solution at 60 °C for 720 h. The influence of the alkylene spacer length between the AEM's backbone and the cation's head group is indeed an important aspect which deserves further investigation. However, controversy exists regarding the optimal spacer length to avoid Hofmann elimination. According to Marino and Kreuer,76 the spacer with chain length >4 will have a negligible effect on improving the AEMs' alkaline stability. This is because the long chain fails to provide steric strain for inhibition of the Hofmann elimination reaction. Addressing this controversy may require computational chemistry and simulation to determine the precise spacer length at which the energy for hydroxide attack is highest.
In addition to the steric strain effect,76 the influence of long spacers on enhancing microphase separation267–269 should also be considered. Microphase separation may alter the hydration state of cations, influencing their interaction with hydroxide.
Fig. 12 illustrates the alkaline stability of commonly used cationic groups, with the half-life determined by quantitatively measuring the amount of quaternary ammonium salts before and after a given time in NaOH 6 M at a temperature of 160 °C.76
 | ||
| Fig. 12 Alkaline stability of the most common cationic groups given as half-life values. Adapted from Marino and Kreuer76 with permission from John Wiley and Sons (License N° 5917551209635). | ||
Fig. 13 summarizes the alkaline stability study of You et al.,13 identifying the major degradation products. In this study the organic cations were subjected to 1 M or 2 M KOH/CD3OH solution at 80 °C for 30 days with an internal standard (3-(trimethylsilyl)-1-propanesulfonic acid sodium salt, NaDSS) in sealed NMR tubes. The decomposition processes were frequently monitored by 1H NMR analysis to assign the decomposition products and the dominating degradation pathways. The advantages of using methanol instead of water include good solvation of cations, accelerated degradation conditions in the presence of methoxide anions, the ability to avoid H/D exchange, and locked signals for 1H NMR analysis.
 | ||
| Fig. 13 Alkaline stability of the most common cationic groups and their major degradation products. Adapted with permission from You et al.,13 Copyright (2020) American Chemical Society. | ||
Summarizing their results, it can be observed that, when possible, benzyl nucleophilic substitution predominates for ammonium cations and, alternatively, SN2 and Hofmann elimination are both legitimate degradation pathways. For N-conjugated cations, SN2 and Hofmann elimination are less difficult than nucleophilic addition to the iminium carbon centres, and steric hindrance is crucial in enhancing the alkaline stability of these molecules. For these types of cations, further hydrolysis and rearrangement are also frequent. Cahours–Hofmann phosphine oxidation is a specific and rapid manner for phosphonium cations to degrade, and therefore, sterically bulky substituents close to the phosphorus atom can slow this process down considerably. When utilizing electron-rich aromatic substituents on phosphorus, ether hydrolysis will likely occur under alkaline conditions. Finally, among organic cations, tetrakisaminophosphonium is proved to be one of the most stable.
In addition to the steric hindrance effect, specific stereochemistry can play a crucial role in enhancing the stability of alkaline AEMs. For example, Bauer and Strathmann270 conducted a study on a monoquaternized 1,4-diazabicyclo[2.2.2]octane DABCO cation attached to a poly(ether sulfone) (PES) backbone. The resulting AEM exhibited remarkable resilience to OH− attacks. Despite DABCO containing β-hydrogens, the rigid cage structure of DABCO effectively hinders the antiperiplanar conformation of the N atoms with β-hydrogen, which is a prerequisite for Hofmann elimination. This structural feature contributes to the enhanced stability of the AEM under alkaline conditions, proving that considerations may, indeed, provide valuable insights for the design and optimization of AEMs with improved alkaline stability.
• Synthesize aryl ether-free AEMs to avoid aril ether bond cleavage72,271–273
• Avoid electron withdrawing functional groups in the polymer backbone to mitigate the OH− attack on the electropositive carbon.274,275 However, one should note that even without an electron-withdrawing functional group in the polymer backbone, aryl ether-containing polymers are not as robust as aryl ether-free polymers.271
• Incorporate cationic functional groups far from the polymer backbone aryl ether bond276,277
• Use less polar polymer backbones125
• Control crosslinking278–280 and the location of cationic groups to obtain optimal ion channels: to achieve an optimal location, type, and concentration of anion-conducting groups and hydrophobic side chains, through effective hydrophobic/hydrophilic region interactions, the following strategies can be pursued, depending on the polymer used: (i) linking the cationic groups to the backbone by a long aliphatic side chain,276 (ii) synthesizing polymer main chains using multiblock co-polymers containing regions of ion-conducting groups,281,282 (iii) using monomers with densely functionalized ion-conducting regions283 or separately attaching the hydrophobic side chain and ion-conducting group to the polymer backbone.284
The optimal location, type and concentration of anion-conducting groups and hydrophobic side chains can help to achieve enhanced AEM performance through effective microphase separation. Fig. 14 shows different ion channel distributions in an AEM. The scenario depicted in Fig. 14b has been demonstrated to be the most effective. In this scenario, ion channels are created to increase anion conductivity, while the hydrophobic region protects the polymer backbone, leading to improved alkaline stability.284
 | ||
| Fig. 14 Development of ion channels in AEMs. (a) Dispersed and underdeveloped ion channels, (b) interconnected ion channels conductive to the formation of “ionic highways”, and (c) segregated, overdeveloped ion channels with distinct hydrophilic/hydrophobic regions. Adapted from Hagesteijn et al.125 (with permission from Springer Nature). | ||
In summary, the diverse strategies reported above highlight the importance of tailoring both the chemical structure and morphology of AEMs to achieve the desired balance between anion conductivity and stability in alkaline environments.
6.2 Mechanical degradation
Prevention of mechanical failure of MEAs is relatively easy with several mitigating strategies:• Use edge-protect gaskets to avoid the sharp boundary between wet and dry regions of the AEM.242,285
• Prepare AEMs with minimal dimensional change between wet and dry states:218,286,287 cells employing AEMs with an elongation at break of >100% show stable performance without edge-failure.
• Prepare AEMs with ductile mechanical properties.216,288,289
• Avoid AEMs with backbone degradation: the mechanical properties of quaternized poly(arylene ether) AEMs deteriorate due to degradation reactions such as the aryl ether cleavage reaction.206
• Control crosslinking: Zhang et al.,290 for example, designed and prepared a series of crosslinked AEMs with stable sterically protected imidazolium groups. The crosslinked AEMs have a high mechanical strength of 16.7–53.2 MPa as well as controlled water uptake and dimensional stability, especially at elevated temperature.
6.3 Addition of fillers
A way to improve the properties of polymeric membranes, including AEMs, is the development of composite materials adding 1D, 2D, and 3D inorganic fillers to enhance the thermal and mechanical properties, along with chemical stability and conductivity. Composite AEMs demonstrate superior long-term stability under alkaline conditions through synergistic effects, such as physical cross-linking (e.g., acid–base interactions) or partial crystallization of the polymer near the inorganic phase.291 Among the 1D materials, the most common are titanate and carbon nanotubes, both boosting conductivity and thermal and mechanical stability of AEMs.292–294 The chemical and oxidative stability is also enhanced by fillers, due to the electrostatic interactions between the filler and the polymer matrix, which can protect the cationic groups and the backbone.292Among the 2D materials, the most common fillers used to enhance the AEM performance are Layered Double Hydroxides (LDHs)295–297 and carbon-based materials such as graphene298 and Graphene Oxide (GO).299–301
LDHs are inorganic lamellar ionic materials belonging to the group of anionic clays and their synthesis has a low cost. The structure of LDHs is based on Mg(OH)2 brucite-type blocks where the substitution of M2+ with M3+ cations generates positively charged layers. These layers are counterbalanced by mobile anions in the interlayer, allowing for reversible insertion. The lamellae are linked by van der Waals forces. LDHs exhibit satisfactory anionic conductivity and superior stability in alkaline environments, making them effective fillers for AEMs.296
A very large amount of work and effort was devoted to composite AEMs containing graphene or GO. The main features of graphene are extreme mechanical resistance, great flexibility, and thermal resistance, while GO exhibits hydrophilicity and water dispersibility due to epoxy and hydroxyl groups on its basal planes, as well as carboxyl and carbonyl groups at the layer edges. These functional groups can interact with the polymer matrix via electrostatic and hydrogen bonding, enhancing membrane stability and diminishing OH− activity against the electropositive polymer backbone and the conductive groups302 of the membranes. Moreover, the sp2 hybrid carbon structure of GO's large aromatic rings can serve as a site for scavenging oxygen free radicals generated during cell operation,303,304 and the rich oxygen containing functional groups on its two-dimensional laminated structure can provide necessary conditions for its modification through covalent or non-covalent bonds.305 For example, grafting conductive groups such as N-spirocyclic ammonium,306 imidazolium,307 and quaternary ammonium,308,309 among others, onto the surface of GO can boost the stability and conductivity of AEMs.310,311 Lastly, for what concerns the 3D fillers, the most studied are functionalized silica, Metal Organic Frameworks (MOFs) (that can also have 2D structures)312–316 and metal oxide nanoparticles such zirconia, alumina and titania, which also improve AEM resistance and conductivity.291,317–321
7 Summary
Achieving high ionic conductivity and long-term operation of AEMs in AEMWEs is not an easy task, primarily due to the degradation of AEMs in alkaline environments, temperature, pressure and applied anode potential.Currently, the large-scale application of AEMWEs is in fact impeded by the relatively poor durability of the commercially available AEMs: the best long-term AEMWE stability achieved is > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h in 1.0 M KOH at 1 A cm−2 (Motealleh et al.42) with a degradation rate of 0.7 µV h−1 using a Sustainion® anion exchange membrane. However, for all other AEMWEs reported here, longevity is no longer than 3000 h, highlighting the need for intensive research before the commercialization threshold of AEMWEs can be reached.
000 h in 1.0 M KOH at 1 A cm−2 (Motealleh et al.42) with a degradation rate of 0.7 µV h−1 using a Sustainion® anion exchange membrane. However, for all other AEMWEs reported here, longevity is no longer than 3000 h, highlighting the need for intensive research before the commercialization threshold of AEMWEs can be reached.
This review discussed the most used commercial AEMs, cationic functional groups and polymer backbones and then focused on their performance degradation. The relationship between design as well as operating parameters and performance of AEMs and AEMWEs has been analysed, enabling some mitigation strategies to be proposed. The optimal parameters identified are: (I) operating temperature in the 40–80 °C range and pressure within 1 to 5 MPa, to maximize performance and meet the hydrogen storage requirements, (II) feed electrolyzers with 0.1–1 M KOH solution instead of pure water, to boost the performances and minimize catalyst dissolution, (III) AEM thickness properly balanced to have low electrical resistance, high mechanical stability and low product crossover (usually 50 µm AEMs are used) and (IV) effective cell sealing to minimize CO2 intrusion and carbonation.
Regarding AEM stability, which is the most pressing issue in AEMWEs, some guidelines can be given to obtain stable membranes. For instance, the use of a non-polar, non-fluorinated, aryl ether-free polymer backbone with functional groups far from the main chain (spacer chain length ≥ 4) is important to optimize alkaline stability, as well as the incorporation of β-hydrogen-absent cations with coplanar structures and delocalized positive charges (such as imidazolium and phosphonium) and cyclic or spirocyclic structures (such as piperidinium and 6-azonia-spiro[5.5]undecane). Also, designing interconnected ion channels through controlled crosslinking and specific location of cationic groups is also an important strategy to optimize stability and conductivity. Controlled crosslinking can be useful to mitigate radical attacks (along with the use of a radical scavenger) and enhance mechanical stability. The latter can be further improved by using an edge-protect gasket and preparing AEMs with ductile mechanical properties and minimal dimensional change between wet and dry states. Last, the addition of a filler to obtain nanocomposite AEMs can also be a viable strategy to improve mechanical, thermal and chemical stability of the membrane (and in some cases also increase conductivity). Future research on AEMWEs should focus on overcoming key scientific and engineering barriers that currently hinder large-scale commercialization. One of the most urgent priorities is the development of standardized testing and reporting protocols for membrane conductivity and durability, which would enable meaningful cross-laboratory comparison and accelerate materials optimization. In addition, enhancing the chemical, thermal and mechanical stability of AEMs remains a fundamental challenge, as degradation of both the cationic head groups and polymer backbone continues to limit operational lifetimes. Moreover, the scalable synthesis of cost-effective and environmentally friendly ionomers and membrane materials will be crucial for achieving sustainable and economically viable hydrogen production. Collectively, addressing these challenges will be essential to develop next-generation AEMWEs for green hydrogen production.
Conflicts of interest
There are no conflicts to declare.Abbreviations
| AEMWE | Anion Exchange Membrane Water Electrolyzer |
| PEMWE | Proton Exchange Membrane Water Electrolyzer |
| AEM | Anion Exchange Membrane |
| AEMFC | Anion Exchange Membrane Fuel Cell |
| PEM | Proton Exchange Membrane |
| PGM | Platinum Group Metal |
| PTLs | Porous Transport Layers |
| HER | Hydrogen Evolution Reaction |
| OER | Oxygen Evolution Reaction |
| QA | Quaternary Ammonium |
| IEC | Ion Exchange Capacity |
| W.U. | Water Uptake |
| S.R. | Swelling Ratio |
| EIS | Electrochemical Impedance Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
| ASR | Area-Specific Resistance |
| LSV | Linear Sweep Voltammetry |
| MEA | Membrane Electrode Assembly |
| HFR | High-Frequency Resistance |
| LDPE | Low-Density Polyethylene |
| HDPE | High-Density Polyethylene |
| OCP | Open Circuit Potential |
| LDHs | Layered Double Hydroxides |
| GO | Graphene Oxide |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
Elettrochimica ed Energia A.P.S. and Mrs Sonia Cirinnà are gratefully acknowledged for supporting the manuscript preparation.References
- E. S. Hanley, J. P. Deane and B. P. Ó. Gallachóir, Renew. Sustain. Energy Rev., 2018, 82, 3027–3045 CrossRef.
- M. Hren, M. Božič, D. Fakin, K. S. Kleinschek and S. Gorgieva, Sustain. Energy Fuels, 2021, 5, 604–637 RSC.
- M. M. Hossen, M. S. Hasan, M. R. I. Sardar, J. bin Haider, Mottakin, K. Tammeveski and P. Atanassov, Appl. Catal., B, 2023, 325, 121733 CrossRef CAS.
- H. A. Miller, K. Bouzek, J. Hnat, S. Loos, C. I. Bernäcker, T. Weißgärber, L. Röntzsch and J. Meier-Haack, Sustain. Energy Fuels, 2020, 4, 2114–2133 RSC.
- S. Y. Kang, J. E. Park, G. Y. Jang, O.-H. Kim, O. J. Kwon, Y.-H. Cho and Y.-E. Sung, Int. J. Hydrogen Energy, 2022, 47, 9115–9126 Search PubMed.
- A. Khataee, A. Shirole, P. Jannasch, A. Krüger and A. Cornell, J. Mater. Chem. A, 2022, 10, 16061–16070 RSC.
- H. Wang, Y. Tong, K. Li and P. Chen, J. Colloid Interface Sci., 2022, 628, 306–314 Search PubMed.
- B. Yang and Z. Cunman, Chem. Eng. J., 2023, 457, 141094 CrossRef CAS.
- X. Chen, Y. Zhan, J. Tang, X. Yang, A. Sun, B. Lin, F. Zhu, H. Jia and X. Lei, J. Environ. Chem. Eng., 2023, 11, 110749 Search PubMed.
- L. J. Titheridge and A. T. Marshall, Int. J. Hydrogen Energy, 2024, 49, 518–532 Search PubMed.
- J. Han, W. Song, X. Cheng, Q. Cheng, Y. Zhang, C. Liu, X. Zhou, Z. Ren, M. Hu, T. Ning, L. Xiao and L. Zhuang, ChemSusChem, 2021, 14, 5021–5031 Search PubMed.
- Z. Tao, C. Wang, X. Zhao, J. Li and Q. Ren, Mater. Chem. Front., 2021, 5, 6904–6912 Search PubMed.
- W. You, K. M. Hugar, R. C. Selhorst, M. Treichel, C. R. Peltier, K. J. T. Noonan and G. W. Coates, J. Org. Chem., 2021, 86, 254–263 CrossRef CAS PubMed.
- N. Chen, S. Y. Paek, J. Y. Lee, J. H. Park, S. Y. Lee and Y. M. Lee, Energy Environ. Sci., 2021, 14, 6338–6348 Search PubMed.
- J. Xiao, A. M. Oliveira, L. Wang, Y. Zhao, T. Wang, J. Wang, B. P. Setzler and Y. Yan, ACS Catal., 2021, 11, 264–270 CrossRef CAS.
- M. S. Cha, J. E. Park, S. Kim, S.-H. Han, S.-H. Shin, S. H. Yang, T.-H. Kim, D. M. Yu, S. So, Y. T. Hong, S. J. Yoon, S.-G. Oh, S. Y. Kang, O.-H. Kim, H. S. Park, B. Bae, Y.-E. Sung, Y.-H. Cho and J. Y. Lee, Energy Environ. Sci., 2020, 13, 3633–3645 Search PubMed.
- Y. Ma, L. Li, X. You, H. Lin, G. Yi, X. Su, A. Zhu, Q. Liu and Q. Zhang, Chem. Eng. J., 2024, 480, 148225 Search PubMed.
- J. Hyun Oh, G. Ho Han, J. Kim, J. Eun Lee, H. Kim, S. Kyung Kang, H. Kim, S. Wooh, P. Soo Lee, H. Won Jang, S. Young Kim and S. Hyun Ahn, Chem. Eng. J., 2023, 460, 141727 CrossRef CAS.
- B. Mayerhöfer, F. D. Speck, M. Hegelheimer, M. Bierling, D. Abbas, D. McLaughlin, S. Cherevko, S. Thiele and R. Peach, Int. J. Hydrogen Energy, 2022, 47, 4304–4314 Search PubMed.
- Q. Xu, L. Zhang, J. Zhang, J. Wang, Y. Hu, H. Jiang and C. Li, EnergyChem, 2022, 4, 100087 CrossRef CAS.
- S. Y. Kang, J. E. Park, G. Y. Jang, O. H. Kim, O. J. Kwon, Y. H. Cho and Y. E. Sung, Int. J. Hydrogen Energy, 2022, 47, 9115–9126 Search PubMed.
- A. Lim, M. K. Cho, S. Y. Lee, H.-J. Kim, S. J. Yoo, Y.-E. Sung, J. H. Jang and H. S. Park, J. Electrochem. Sci. Technol., 2017, 8, 265–273 Search PubMed.
- A. Gomez Vidales, N. C. Millan and C. Bock, Chem. Eng. Res. Des., 2023, 194, 636–648 Search PubMed.
- L. Zeng and T. S. Zhao, Nano Energy, 2015, 11, 110–118 CrossRef CAS.
- Y. Leng, G. Chen, A. J. Mendoza, T. B. Tighe, M. A. Hickner and C.-Y. Wang, J. Am. Chem. Soc., 2012, 134, 9054–9057 CrossRef CAS PubMed.
- S. Jeong, J. Lee, S. Woo, J. Seo and B. Min, Energies, 2015, 8, 7084–7099 Search PubMed.
- M. Faraj, M. Boccia, H. Miller, F. Martini, S. Borsacchi, M. Geppi and A. Pucci, Int. J. Hydrogen Energy, 2012, 37, 14992–15002 CrossRef CAS.
- W. Ng, W. Wong, N. Rosli and K. Loh, Separations, 2023, 10, 424 Search PubMed.
- A. H. Faqeeh and M. D. Symes, Electrochim. Acta, 2023, 444, 142030 CrossRef CAS.
- A. Caprì, I. Gatto, C. Lo Vecchio, S. Trocino, A. Carbone and V. Baglio, ChemElectroChem, 2023, 10(1) DOI:10.1002/celc.202201056.
- I. Gatto, A. Caprì, C. Lo Vecchio, S. Zignani, A. Patti and V. Baglio, Int. J. Hydrogen Energy, 2023, 48, 11914–11921 CrossRef CAS.
- L. Wan, Z. Xu and B. Wang, Chem. Eng. J., 2021, 426, 131340 Search PubMed.
- A. Carbone, S. C. Zignani, I. Gatto, S. Trocino and A. S. Aricò, Int. J. Hydrogen Energy, 2020, 45, 9285–9292 Search PubMed.
- S. Patra, B. Soman, T. Vineesh, N. Shyaga and T. N. Narayanan, Mater. Chem. Front., 2020, 4, 567–573 RSC.
- D. D. Tham and D. Kim, J. Membr. Sci., 2019, 581, 139–149 Search PubMed.
- M. K. Cho, H.-Y. Park, H. J. Lee, H.-J. Kim, A. Lim, D. Henkensmeier, S. J. Yoo, J. Y. Kim, S. Y. Lee, H. S. Park and J. H. Jang, J. Power Sources, 2018, 382, 22–29 Search PubMed.
- S. Ruck, A. Körner, A. Hutzler, M. Bierling, J. Gonzalez, W. Qu, C. Bock, S. Thiele, R. Peach and C. V Pham, J. Phys.: Energy, 2022, 4, 044007 CAS.
- L. Xia, W. Jiang, H. Hartmann, J. Mayer, W. Lehnert and M. Shviro, ACS Appl. Mater. Interfaces, 2022, 14, 19397–19408 Search PubMed.
- W. Guo, J. Kim, H. Kim, S. Hong, H. Kim, S. Y. Kim and S. H. Ahn, Mater. Today Chem., 2022, 24, 100994 Search PubMed.
- E. López-Fernández, C. Gómez-Sacedón, J. Gil-Rostra, J. P. Espinós, J. J. Brey, A. R. González-Elipe, A. de Lucas-Consuegra and F. Yubero, Renew. Energy, 2022, 197, 1183–1191 CrossRef.
- N. Chen, S. Y. Paek, J. Y. Lee, J. H. Park, S. Y. Lee and Y. M. Lee, Energy Environ. Sci., 2021, 14, 6338–6348 Search PubMed.
- B. Motealleh, Z. Liu, R. I. Masel, J. P. Sculley, Z. Richard Ni and L. Meroueh, Int. J. Hydrogen Energy, 2021, 46, 3379–3386 Search PubMed.
- W. Guo, J. Kim, H. Kim and S. H. Ahn, Int. J. Hydrogen Energy, 2021, 46, 19789–19801 Search PubMed.
- Y. S. Park, J. Jeong, Y. Noh, M. J. Jang, J. Lee, K. H. Lee, D. C. Lim, M. H. Seo, W. B. Kim, J. Yang and S. M. Choi, Appl. Catal., B, 2021, 292, 120170 Search PubMed.
- N. U. Hassan, E. Motyka, J. Kweder, P. Ganesan, B. Brechin, B. Zulevi, H. R. Colón-Mercado, P. A. Kohl and W. E. Mustain, J. Power Sources, 2023, 555, 232371 Search PubMed.
- IRENA (2020), Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.50C Climate Goal, International Renewable Energy Agency, Abu Dhabi Search PubMed.
- EERA European Energy Research Alliance Joint Research Programme on Fuel Cells and Hydrogen technologies (JP FCH) Implementation Plan 2018–2030.
- H. -W. Zhang, D. -Z. Chen, Y. Xianze and S. -B. Yin, Fuel Cells, 2015, 15, 761–780 Search PubMed.
- W. Lu, Z. Yang, H. Huang, F. Wei, W. Li, Y. Yu, Y. Gao, Y. Zhou and G. Zhang, Ind. Eng. Chem. Res., 2020, 59, 21077–21087 Search PubMed.
- D. Li, E. J. Park, W. Zhu, Q. Shi, Y. Zhou, H. Tian, Y. Lin, A. Serov, B. Zulevi, E. D. Baca, C. Fujimoto, H. T. Chung and Y. S. Kim, Nat. Energy, 2020, 5, 378–385 Search PubMed.
- S. K. Tuli, A. L. Roy, R. A. Elgammal, T. A. Zawodzinski and T. Fujiwara, Polym. Int., 2018, 67, 1302–1312 Search PubMed.
- T. Zhou, J. Zhang, J. Jingfu, G. Jiang, J. Zhang and J. Qiao, Synth. Met., 2013, 167, 43–50 Search PubMed.
- T. Zhou, J. Zhang, J. Qiao, L. Liu, G. Jiang, J. Zhang and Y. Liu, J. Power Sources, 2013, 227, 291–299 Search PubMed.
- P. Y. Xu, C. H. Zhao and Q. L. Liu, J. Appl. Polym. Sci., 2013, 130, 1172–1178 Search PubMed.
- J. Zhou, J. Guo, D. Chu and R. Chen, J. Power Sources, 2012, 219, 272–279 Search PubMed.
- Q. H. Zeng, Q. L. Liu, I. Broadwell, A. M. Zhu, Y. Xiong and X. P. Tu, J. Membr. Sci., 2010, 349, 237–243 CrossRef CAS.
- J. Hong, M. Park, S. Hong and B. Kim, J. Appl. Polym. Sci., 2009, 112, 830–835 CrossRef CAS.
- J. Hong and S. Hong, J. Appl. Polym. Sci., 2010, 115, 2296–2301 Search PubMed.
- X. Yan, S. Gu, G. He, X. Wu and J. Benziger, J. Power Sources, 2014, 250, 90–97 CrossRef CAS.
- J. Han, H. Peng, J. Pan, L. Wei, G. Li, C. Chen, L. Xiao, J. Lu and L. Zhuang, ACS Appl. Mater. Interfaces, 2013, 5, 13405–13411 CrossRef CAS PubMed.
- X. Yan, G. He, S. Gu, X. Wu, L. Du and H. Zhang, J. Membr. Sci., 2011, 375, 204–211 Search PubMed.
- L. Zeng and T. S. Zhao, Electrochem. Commun., 2013, 34, 278–281 Search PubMed.
- J. Yan, H. D. Moore, M. R. Hibbs and M. A. Hickner, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 1790–1798 Search PubMed.
- C. Simari, M. H. Ur Rehman, A. Caprì, I. Gatto, V. Baglio and I. Nicotera, Mater. Today Sustain., 2023, 21, 100297 Search PubMed.
- S. S. Koilpillai and S. Dharmalingam, Int. J. Energy Res., 2015, 39, 317–325 Search PubMed.
- S. S. He and C. W. Frank, J. Mater. Chem. A, 2014, 2, 16489–16497 Search PubMed.
- G. Wang, Y. Weng, J. Zhao, R. Chen and D. Xie, J. Appl. Polym. Sci., 2009, 112, 721–727 Search PubMed.
- G. Wang, Y. Weng, J. Zhao, D. Chu, D. Xie and R. Chen, Polym. Adv. Technol., 2010, 21, 554–560 CrossRef CAS.
- K. H. Gopi, S. G. Peera, S. D. Bhat, P. Sridhar and S. Pitchumani, Int. J. Hydrogen Energy, 2014, 39, 2659–2668 Search PubMed.
- N. Li, L. Wang and M. Hickner, Chem. Commun., 2014, 50, 4092 Search PubMed.
- A. Katzfuß, S. Poynton, J. Varcoe, V. Gogel, U. Storr and J. Kerres, J. Membr. Sci., 2014, 465, 129–137 CrossRef.
- W.-H. Lee, E. J. Park, J. Han, D. W. Shin, Y. S. Kim and C. Bae, ACS Macro Lett., 2017, 6, 566–570 CrossRef CAS PubMed.
- X. Wang, C. Lin, Y. Gao and R. G. H. Lammertink, J. Membr. Sci., 2021, 635, 119525 CrossRef CAS.
- Y. Wang, D. Zhang, X. Liang, M. A. Shehzad, X. Xiao, Y. Zhu, X. Ge, J. Zhang, Z. Ge, L. Wu and T. Xu, J. Membr. Sci., 2020, 595, 117483 CrossRef CAS.
- Y. Zhang, W. Chen, T. Li, X. Yan, F. Zhang, X. Wang, X. Wu, B. Pang and G. He, J. Membr. Sci., 2020, 613, 118507 CrossRef CAS.
- M. G. Marino and K. D. Kreuer, ChemSusChem, 2015, 8, 513–523 CrossRef CAS PubMed.
- C. X. Lin, X. L. Huang, D. Guo, Q. G. Zhang, A. M. Zhu, M. L. Ye and Q. L. Liu, J. Mater. Chem. A, 2016, 4, 13938–13948 Search PubMed.
- N. J. Robertson, H. A. Kostalik, T. J. Clark, P. F. Mutolo, H. D. Abruña and G. W. Coates, J. Am. Chem. Soc., 2010, 132, 3400–3404 CrossRef CAS PubMed.
- W. Liu, L. Liu, J. Liao, L. Wang and N. Li, J. Membr. Sci., 2017, 536, 133–140 CrossRef CAS.
- D. R. Dekel, M. Amar, S. Willdorf, M. Kosa, S. Dhara and C. E. Diesendruck, Chem. Mater., 2017, 29, 4425–4431 Search PubMed.
- J. Si, S. Lu, X. Xu, S. Peng, R. Xiu and Y. Xiang, ChemSusChem, 2014, 7, 3389–3395 CrossRef CAS PubMed.
- C. Long, C. Lu, Y. Li, Z. Wang and H. Zhu, Int. J. Hydrogen Energy, 2020, 45, 19778–19790 Search PubMed.
- U. Salma and Y. Nagao, Polym. Degrad. Stab., 2020, 179, 109299 Search PubMed.
- X. Gao, F. Lu, B. Dong, A. Wu, N. Sun and L. Zheng, J. Mater. Chem. A, 2016, 4, 13316–13323 RSC.
- U. Salma, D. Zhang and Y. Nagao, ChemistrySelect, 2020, 5, 1255–1263 Search PubMed.
- D. Guo, C. X. Lin, E. N. Hu, L. Shi, F. Soyekwo, Q. G. Zhang, A. M. Zhu and Q. L. Liu, J. Membr. Sci., 2017, 541, 214–223 Search PubMed.
- X. Lin, X. Liang, S. D. Poynton, J. R. Varcoe, A. L. Ong, J. Ran, Y. Li, Q. Li and T. Xu, J. Membr. Sci., 2013, 443, 193–200 Search PubMed.
- L.-C. Jheng, C.-K. Tai, S. L.-C. Hsu, B.-Y. Lin, L. Chen, B.-C. Wang, L.-K. Chiang and W.-C. Ko, Int. J. Hydrogen Energy, 2017, 42, 5315–5326 Search PubMed.
- C. Li, S. Zhang, S. Wang, X. Xie, C. Deng and P. Pei, Int. J. Hydrogen Energy, 2014, 39, 14362–14369 CrossRef CAS.
- Y. Choi, J. Park, K. Yeon and S. Moon, J. Membr. Sci., 2005, 250, 295–304 CrossRef CAS.
- F. Gu, H. Dong, Y. Li, Z. Sun and F. Yan, Macromolecules, 2014, 47, 6740–6747 Search PubMed.
- M. Döbbelin, I. Azcune, M. Bedu, A. Ruiz de Luzuriaga, A. Genua, V. Jovanovski, G. Cabañero and I. Odriozola, Chem. Mater., 2012, 24, 1583–1590 Search PubMed.
- A. N. Mondal, Y. He, L. Wu, M. I. Khan, K. Emmanuel, Md. M. Hossain, L. Ge and T. Xu, J. Mater. Chem. A, 2017, 5, 1022–1027 RSC.
- D. S. Kim, A. Labouriau, M. D. Guiver and Y. S. Kim, Chem. Mater., 2011, 23, 3795–3797 Search PubMed.
- T. A. Sherazi, S. Zahoor, R. Raza, A. J. Shaikh, S. A. R. Naqvi, G. Abbas, Y. Khan and S. Li, Int. J. Hydrogen Energy, 2015, 40, 786–796 CrossRef CAS.
- T. Jiang, C. Wang, T. Wang, X. Wang, X. Wang, X. Li, Y. Ding and H. Wei, J. Membr. Sci., 2022, 660, 120843 CrossRef CAS.
- C. G. Morandi, R. Peach, H. M. Krieg and J. Kerres, J. Mater. Chem. A, 2015, 3, 1110–1120 RSC.
- L. Ye, L. Zhai, J. Fang, J. Liu, C. Li and R. Guan, Solid State Ionics, 2013, 240, 1–9 CrossRef CAS.
- X. Wang, C. Xu, B. T. Golding, M. Sadeghi, Y. Cao and K. Scott, Int. J. Hydrogen Energy, 2011, 36, 8550–8556 Search PubMed.
- X. Wu, W. Chen, X. Yan, G. He, J. Wang, Y. Zhang and X. Zhu, J. Mater. Chem. A, 2014, 2, 12222 Search PubMed.
- N. Li, M. D. Guiver and W. H. Binder, ChemSusChem, 2013, 6, 1376–1383 CrossRef CAS PubMed.
- L. Liu, S. He, S. Zhang, M. Zhang, M. D. Guiver and N. Li, ACS Appl. Mater. Interfaces, 2016, 8, 4651–4660 Search PubMed.
- C. G. Arges, J. Parrondo, G. Johnson, A. Nadhan and V. Ramani, J. Mater. Chem., 2012, 22, 3733 Search PubMed.
- Y.-C. Cao, X. Wang, M. Mamlouk and K. Scott, J. Mater. Chem., 2011, 21, 12910 Search PubMed.
- C. G. Arges, L. Wang and V. Ramani, RSC Adv., 2014, 4, 61869–61876 Search PubMed.
- K. J. T. Noonan, K. M. Hugar, H. A. Kostalik, E. B. Lobkovsky, H. D. Abruña and G. W. Coates, J. Am. Chem. Soc., 2012, 134, 18161–18164 Search PubMed.
- S. Gu, J. Skovgard and Y. S. Yan, ChemSusChem, 2012, 5, 843–848 Search PubMed.
- X. Yan, S. Gu, G. He, X. Wu, W. Zheng and X. Ruan, J. Membr. Sci., 2014, 466, 220–228 Search PubMed.
- S. Gu, R. Cai, T. Luo, Z. Chen, M. Sun, Y. Liu, G. He and Y. Yan, Angew. Chem., 2009, 121, 6621–6624 CrossRef.
- B. Zhang, S. Gu, J. Wang, Y. Liu, A. M. Herring and Y. Yan, RSC Adv., 2012, 2, 12683 Search PubMed.
- Y. Zha, M. L. Disabb-Miller, Z. D. Johnson, M. A. Hickner and G. N. Tew, J. Am. Chem. Soc., 2012, 134, 4493–4496 Search PubMed.
- N. Chen, H. Zhu, Y. Chu, R. Li, Y. Liu and F. Wang, Polym. Chem., 2017, 8, 1381–1392 Search PubMed.
- M. Treichel, J. C. Gaitor, C. Birch, J. L. Vinskus and K. J. T. Noonan, Polymer, 2022, 249, 124811 Search PubMed.
- D. R. Dekel, S. Willdorf, U. Ash, M. Amar, S. Pusara, S. Dhara, S. Srebnik and C. E. Diesendruck, J. Power Sources, 2018, 375, 351–360 Search PubMed.
- S. Xiong, L. Ma, L. Jiang, X. Hu, G. Fu, J. Hao, H. Gao, P. Liu, L. Tan, X. Liu, Q. Wu and D. Ouyang, Sci. Total Environ., 2023, 858, 160127 CrossRef CAS PubMed.
- T. R. E. Kressman, Nature, 1950, 165, 568 Search PubMed.
- G. Merle, M. Wessling and K. Nijmeijer, J. Membr. Sci., 2011, 377, 1–35 CrossRef CAS.
- J. Jr. Perry, Proc., Annu. Power Sources Conf., 1960, 14, 50–52 CAS.
- J. Ahn, A. Heinzel and K. Ledjeff, DECHEMA Monogr., 1990, 121, 109 Search PubMed.
- D. Henkensmeier, M. Najibah, C. Harms, J. Žitka, J. Hnát and K. Bouzek, J. Electrochem. Energy Convers. Storage, 2021, 18 DOI:10.1115/1.4047963.
- N. Ziv, W. E. Mustain and D. R. Dekel, ChemSusChem, 2018, 11, 1136–1150 CrossRef CAS PubMed.
- A. Marinkas, I. Struźyńska-Piron, Y. Lee, A. Lim, H. S. Park, J. H. Jang, H.-J. Kim, J. Kim, A. Maljusch, O. Conradi and D. Henkensmeier, Polymer, 2018, 145, 242–251 CrossRef CAS.
- Z. Liu, S. D. Sajjad, Y. Gao, H. Yang, J. J. Kaczur and R. I. Masel, Int. J. Hydrogen Energy, 2017, 42, 29661–29665 Search PubMed.
- N. Ziv and D. R. Dekel, Electrochem. Commun., 2018, 88, 109–113 CrossRef CAS.
- K. F. L. Hagesteijn, S. Jiang and B. P. Ladewig, J. Mater. Sci., 2018, 53, 11131–11150 CrossRef CAS.
- J. R. Varcoe, P. Atanassov, D. R. Dekel, A. M. Herring, M. A. Hickner, P. A. Kohl, A. R. Kucernak, W. E. Mustain, K. Nijmeijer, K. Scott, T. Xu and L. Zhuang, Energy Environ. Sci., 2014, 7, 3135–3191 RSC.
- L. Wang, Y. Liu and J. Wang, J. Appl. Polym. Sci., 2019, 136(44) DOI:10.1002/app.48169.
- F. Karas, J. Hnát, M. Paidar, J. Schauer and K. Bouzek, Int. J. Hydrogen Energy, 2014, 39, 5054–5062 Search PubMed.
- O. D. Thomas, K. J. W. Y. Soo, T. J. Peckham, M. P. Kulkarni and S. Holdcroft, J. Am. Chem. Soc., 2012, 134, 10753–10756 Search PubMed.
- M. Khan, R. Luque, S. Akhtar, A. Shaheen, A. Mehmood, S. Idress, S. Buzdar and A. ur Rehman, Materials, 2016, 9, 365 Search PubMed.
- L. Liu, H. Ma, M. Khan and B. S. Hsiao, Membranes, 2024, 14, 85 CrossRef CAS PubMed.
- J. R. Varcoe, P. Atanassov, D. R. Dekel, A. M. Herring, M. A. Hickner, P. A. Kohl, A. R. Kucernak, W. E. Mustain, K. Nijmeijer, K. Scott, T. Xu and L. Zhuang, Energy Environ. Sci., 2014, 7, 3135–3191 Search PubMed.
- D. R. Dekel, J. Power Sources, 2018, 375, 158–169 CrossRef CAS.
- S. Willdorf-Cohen, A. Zhegur-Khais, J. Ponce-González, S. Bsoul-Haj, J. R. Varcoe, C. E. Diesendruck and D. R. Dekel, ACS Appl. Energy Mater., 2023, 6, 1085–1092 Search PubMed.
- H. Strathmann, Ion-exchange membrane separation processes, in AmsterdamIon-exchange membrane separation processes, 2004 Search PubMed.
- Y. Tanaka, S.-H. Moon, V. V. Nikonenko and T. Xu, Int. J. Chem. Eng., 2012, 2012, 1–3 Search PubMed.
- X. Luo, A. Wright, T. Weissbach and S. Holdcroft, J. Power Sources, 2018, 375, 442–451 Search PubMed.
- J.-S. Park, J.-H. Choi, J.-J. Woo and S.-H. Moon, J. Colloid Interface Sci., 2006, 300, 655–662 Search PubMed.
- F. Müller, C. A. Ferreira, D. S. Azambuja, C. Alemán and E. Armelin, J. Phys. Chem. B, 2014, 118, 1102–1112 CrossRef PubMed.
- A. Zhegur-Khais, F. Kubannek, U. Krewer and D. R. Dekel, J. Membr. Sci., 2020, 612, 118461 Search PubMed.
- S. Jiang and B. P. Ladewig, ACS Appl. Mater. Interfaces, 2017, 9, 38612–38620 Search PubMed.
- I. Vincent and D. Bessarabov, Renew. Sustain. Energy Rev., 2018, 81, 1690–1704 CrossRef CAS.
- Z. Li, X. He, Z. Jiang, Y. Yin, B. Zhang, G. He, Z. Tong, H. Wu and K. Jiao, Electrochim. Acta, 2017, 240, 486–494 Search PubMed.
- R. Narducci, J.-F. Chailan, A. Fahs, L. Pasquini, M. L. Di Vona and P. Knauth, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 1180–1187 CrossRef CAS.
- Y. Wu, C. Wu, T. Xu and Y. Fu, J. Membr. Sci., 2009, 329, 236–245 Search PubMed.
- freeTextSearchKeyword=AEM;typeCodes=1;statusCodes=31094501,31094502;programCode=H2020;programDivisionCode=null;focusAreaCode=null;crossCuttingPriorityCode=null;callCode=Default;sortQuery=openingDate;orderBy=asc;onlyTenders=false, https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/opportunities/topic-details/fch-02-4-2019.
- S. Ratso, I. Kruusenberg, U. Joost, R. Saar and K. Tammeveski, Int. J. Hydrogen Energy, 2016, 41, 22510–22519 Search PubMed.
- S. Kabir, A. Zadick, P. Atanassov, L. Dubau and M. Chatenet, Electrochem. Commun., 2017, 78, 33–37 Search PubMed.
- H. A. Miller, F. Vizza, M. Marelli, A. Zadick, L. Dubau, M. Chatenet, S. Geiger, S. Cherevko, H. Doan, R. K. Pavlicek, S. Mukerjee and D. R. Dekel, Nano Energy, 2017, 33, 293–305 Search PubMed.
- W. Tong, M. Forster, F. Dionigi, S. Dresp, R. Sadeghi Erami, P. Strasser, A. J. Cowan and P. Farràs, Nat. Energy, 2020, 5, 367–377 Search PubMed.
- G. A. Lindquist, Q. Xu, S. Z. Oener and S. W. Boettcher, Joule, 2020, 4, 2549–2561 Search PubMed.
- L. An, T. S. Zhao, Z. H. Chai, P. Tan and L. Zeng, Int. J. Hydrogen Energy, 2014, 39, 19869–19876 Search PubMed.
- M. K. Cho, H.-Y. Park, S. Choe, S. J. Yoo, J. Y. Kim, H.-J. Kim, D. Henkensmeier, S. Y. Lee, Y.-E. Sung, H. S. Park and J. H. Jang, J. Power Sources, 2017, 347, 283–290 Search PubMed.
- B. Han, S. M. Steen, J. Mo and F.-Y. Zhang, Int. J. Hydrogen Energy, 2015, 40, 7006–7016 Search PubMed.
- V. Liso, G. Savoia, S. S. Araya, G. Cinti and S. K. Kær, Energies, 2018, 11, 3273 Search PubMed.
- F. Marangio, M. Santarelli and M. Cali, Int. J. Hydrogen Energy, 2009, 34, 1143–1158 CrossRef CAS.
- D. Xu, M. B. Stevens, M. R. Cosby, S. Z. Oener, A. M. Smith, L. J. Enman, K. E. Ayers, C. B. Capuano, J. N. Renner, N. Danilovic, Y. Li, H. Wang, Q. Zhang and S. W. Boettcher, ACS Catal., 2019, 9, 7–15 CrossRef CAS.
- F. Marangio, M. Santarelli and M. Cali, Int. J. Hydrogen Energy, 2009, 34, 1143–1158 CrossRef CAS.
- I. V. Pushkareva, A. S. Pushkarev, S. A. Grigoriev, P. Modisha and D. G. Bessarabov, Int. J. Hydrogen Energy, 2020, 45, 26070–26079 Search PubMed.
- M. Mandal, ChemElectroChem, 2021, 8, 36–45 CrossRef CAS.
- G. A. Lindquist, S. Z. Oener, R. Krivina, A. R. Motz, A. Keane, C. Capuano, K. E. Ayers and S. W. Boettcher, ACS Appl. Mater. Interfaces, 2021, 13, 51917–51924 Search PubMed.
- M. R. Kraglund, M. Carmo, G. Schiller, S. A. Ansar, D. Aili, E. Christensen and J. O. Jensen, Energy Environ. Sci., 2019, 12, 3313–3318 RSC.
- D. Li, A. R. Motz, C. Bae, C. Fujimoto, G. Yang, F.-Y. Zhang, K. E. Ayers and Y. S. Kim, Energy Environ. Sci., 2021, 14, 3393–3419 Search PubMed.
- R. A. Krivina, G. A. Lindquist, M. C. Yang, A. K. Cook, C. H. Hendon, A. R. Motz, C. Capuano, K. E. Ayers, J. E. Hutchison and S. W. Boettcher, ACS Appl. Mater. Interfaces, 2022, 14, 18261–18274 Search PubMed.
- X. Chu, Y. Shi, L. Liu, Y. Huang and N. Li, J. Mater. Chem. A, 2019, 7, 7717–7727 RSC.
- T. Pandiarajan, L. John Berchmans and S. Ravichandran, RSC Adv., 2015, 5, 34100–34108 RSC.
- X. Luo, D. I. Kushner, J. Li, E. J. Park, Y. S. Kim and A. Kusoglu, Adv. Funct. Mater., 2021, 31(20) DOI:10.1002/adfm.202008778.
- Y. S. Kim, ACS Appl. Polym. Mater., 2021, 3, 1250–1270 CrossRef CAS.
- D. Li, I. Matanovic, A. S. Lee, E. J. Park, C. Fujimoto, H. T. Chung and Y. S. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 9696–9701 CrossRef CAS PubMed.
- S. Maurya, A. S. Lee, D. Li, E. J. Park, D. P. Leonard, S. Noh, C. Bae and Y. S. Kim, J. Power Sources, 2019, 436, 226866 CrossRef CAS.
- D. Li, H. T. Chung, S. Maurya, I. Matanovic and Y. S. Kim, Curr. Opin. Electrochem., 2018, 12, 189–195 CrossRef CAS.
- I. Matanovic, S. Maurya, E. J. Park, J. Y. Jeon, C. Bae and Y. S. Kim, Chem. Mater., 2019, 31, 4195–4204 CrossRef CAS.
- M. S. Ide, B. Hao, M. Neurock and R. J. Davis, ACS Catal., 2012, 2, 671–683 CrossRef CAS.
- A. K. Niaz, A. Akhtar, J.-Y. Park and H.-T. Lim, J. Power Sources, 2021, 481, 229093 Search PubMed.
- W. A. Rigdon, T. J. Omasta, C. Lewis, M. A. Hickner, J. R. Varcoe, J. N. Renner, K. E. Ayers and W. E. Mustain, J. Electrochem. Energy Convers. Storage, 2017, 14(2) DOI:10.1115/1.4033411.
- M. Inaba, Y. Matsui, M. Saito, A. Tasaka, K. Fukuta, S. Watanabe and H. Yanagi, Electrochemistry, 2011, 79, 322–325 CrossRef CAS.
- Y. Zheng, T. J. Omasta, X. Peng, L. Wang, J. R. Varcoe, B. S. Pivovar and W. E. Mustain, Energy Environ. Sci., 2019, 12, 2806–2819 RSC.
- M. R. Gerhardt, L. M. Pant and A. Z. Weber, J. Electrochem. Soc., 2019, 166, F3180–F3192 CrossRef CAS.
- M. R. Gerhardt, L. M. Pant, H.-S. Shiau and A. Z. Weber, ECS Trans., 2018, 86, 15–24 CrossRef CAS.
- J. A. Wrubel, A. A. Peracchio, B. N. Cassenti, T. D. Myles, K. N. Grew and W. K. S. Chiu, J. Electrochem. Soc., 2017, 164, F1063–F1073 CrossRef CAS.
- J. Parrondo, C. G. Arges, M. Niedzwiecki, E. B. Anderson, K. E. Ayers and V. Ramani, RSC Adv., 2014, 4, 9875 RSC.
- J. Li, C. Liu, J. Ge, W. Xing and J. Zhu, Chem.–Eur. J., 2023, 29(26) DOI:10.1002/chem.202203173.
- A. collier, H. wang, X. ziyuan, J. Zhang and D. Wilkinson, Int. J. Hydrogen Energy, 2006, 31, 1838–1854 CrossRef CAS.
- M. Ramdin, O. A. Moultos, L. J. P. van den Broeke, P. Gonugunta, P. Taheri and T. J. H. Vlugt, Ind. Eng. Chem. Res., 2023, 62, 6843–6864 CrossRef CAS.
- B. Chen, P. Mardle and S. Holdcroft, J. Power Sources, 2022, 550, 232134 CrossRef CAS.
- A. Martinez-Lazaro, A. Caprì, I. Gatto, J. Ledesma-García, N. Rey-Raap, A. Arenillas, F. I. Espinosa-Lagunes, V. Baglio and L. G. Arriaga, J. Power Sources, 2023, 556, 232417 CrossRef CAS.
- S. Campagna Zignani, M. Lo Faro, A. Carbone, C. Italiano, S. Trocino, G. Monforte and A. S. Aricò, Electrochim. Acta, 2022, 413, 140078 CrossRef CAS.
- G. Huang, M. Mandal, N. U. Hassan, K. Groenhout, A. Dobbs, W. E. Mustain and P. A. Kohl, J. Electrochem. Soc., 2021, 168, 024503 CrossRef CAS.
- S. Raiguel, W. Dehaen and K. Binnemans, Green Chem., 2020, 22, 5225–5252 Search PubMed.
- C. G. Arges and V. Ramani, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 2490–2495 CrossRef CAS PubMed.
- T. S. Stevens, E. M. Creighton, A. B. Gordon and M. MacNicol, J. Chem. Soc., 1928, 0, 3193–3197 Search PubMed.
- C. T. Womble, J. Kang, K. M. Hugar, G. W. Coates, S. Bernhard and K. J. T. Noonan, Organometallics, 2017, 36, 4038–4046 CrossRef CAS.
- Z. Zakaria and S. K. Kamarudin, Int. J. Energy Res., 2021, 45, 18337–18354 CrossRef CAS.
- W. E. Mustain, M. Chatenet, M. Page and Y. S. Kim, Energy Environ. Sci., 2020, 13, 2805–2838 RSC.
- S. Wierzbicki, J. C. Douglin, A. Kostuch, D. R. Dekel and K. Kruczała, J. Phys. Chem. Lett., 2020, 11, 7630–7636 Search PubMed.
- A. D. Mohanty and C. Bae, J. Mater. Chem. A, 2014, 2, 17314–17320 RSC.
- P. G. Stevens and J. H. Richmond, J. Am. Chem. Soc., 1941, 63, 3132–3136 CrossRef CAS.
- G. Ghigo, S. Cagnina, A. Maranzana and G. Tonachini, J. Org. Chem., 2010, 75, 3608–3617 CrossRef CAS PubMed.
- S. Chempath, J. M. Boncella, L. R. Pratt, N. Henson and B. S. Pivovar, J. Phys. Chem. C, 2010, 114, 11977–11983 CrossRef CAS.
- A. Vöge, V. Deimede and J. K. Kallitsis, RSC Adv., 2014, 4, 45040–45049 RSC.
- W. Li, S. Wang, X. Zhang, W. Wang, X. Xie and P. Pei, Int. J. Hydrogen Energy, 2014, 39, 13710–13717 Search PubMed.
- B. Lee, D. Yun, J.-S. Lee, C. H. Park and T.-H. Kim, J. Phys. Chem. C, 2019, 123, 13508–13518 CrossRef CAS.
- H.-S. Dang and P. Jannasch, J. Mater. Chem. A, 2017, 5, 21965–21978 RSC.
- J. S. Olsson, T. H. Pham and P. Jannasch, Macromolecules, 2020, 53, 4722–4732 Search PubMed.
- U. Salma and N. Shalahin, Results Mater., 2023, 17, 100366 CrossRef CAS.
- R.-A. Becerra-Arciniegas, R. Narducci, G. Ercolani, S. Antonaroli, E. Sgreccia, L. Pasquini, P. Knauth and M. L. Di Vona, Polymer, 2019, 185, 121931 CrossRef CAS.
- C. Fujimoto, D.-S. Kim, M. Hibbs, D. Wrobleski and Y. S. Kim, J. Membr. Sci., 2012, 423–424, 438–449 CrossRef CAS.
- S. Miyanishi and T. Yamaguchi, Phys. Chem. Chem. Phys., 2016, 18, 12009–12023 RSC.
- A. Amel, L. Zhu, M. Hickner and Y. Ein-Eli, J. Electrochem. Soc., 2014, 161, F615–F621 CrossRef CAS.
- J. Sharma and V. Kulshrestha, in Alkaline Anion Exchange Membranes for Fuel Cells, Wiley, 2024, pp. 241–284 Search PubMed.
- Y.-K. Choe, C. Fujimoto, K.-S. Lee, L. T. Dalton, K. Ayers, N. J. Henson and Y. S. Kim, Chem. Mater., 2014, 26, 5675–5682 CrossRef CAS.
- L. Liu, X. Chu, J. Liao, Y. Huang, Y. Li, Z. Ge, M. A. Hickner and N. Li, Energy Environ. Sci., 2018, 11, 435–446 RSC.
- Q. Ge, J. Ran, J. Miao, Z. Yang and T. Xu, ACS Appl. Mater. Interfaces, 2015, 7, 28545–28553 CrossRef CAS PubMed.
- L. Zhu, T. J. Zimudzi, N. Li, J. Pan, B. Lin and M. A. Hickner, Polym. Chem., 2016, 7, 2464–2475 Search PubMed.
- Y. He, L. Wu, J. Pan, Y. Zhu, X. Ge, Z. Yang, J. Ran and T. Xu, J. Membr. Sci., 2016, 504, 47–54 CrossRef CAS.
- J. Y. Jeon, S. Park, J. Han, S. Maurya, A. D. Mohanty, D. Tian, N. Saikia, M. A. Hickner, C. Y. Ryu, M. E. Tuckerman, S. J. Paddison, Y. S. Kim and C. Bae, Macromolecules, 2019, 52, 2139–2147 CrossRef CAS.
- S. Miyanishi and T. Yamaguchi, Polym. Chem., 2020, 11, 3812–3820 RSC.
- Md. M. Hossain, L. Wu, X. Liang, Z. Yang, J. Hou and T. Xu, J. Power Sources, 2018, 390, 234–241 CrossRef CAS.
- E. J. Park, S. Maurya, M. R. Hibbs, C. H. Fujimoto, K.-D. Kreuer and Y. S. Kim, Macromolecules, 2019, 52, 5419–5428 CrossRef CAS.
- F. D. Speck, P. G. Santori, F. Jaouen and S. Cherevko, J. Phys. Chem. C, 2019, 123, 25267–25277 CrossRef CAS.
- K. Juodkazis, J. Juodkazytė, R. Vilkauskaitė and V. Jasulaitienė, J. Solid State Electrochem., 2008, 12, 1469–1479 CrossRef CAS.
- S. Loos, I. Zaharieva, P. Chernev, A. Lißner and H. Dau, ChemSusChem, 2019, 12, 1966–1976 CrossRef CAS PubMed.
- J. Parrondo, Z. Wang, M.-S. J. Jung and V. Ramani, Phys. Chem. Chem. Phys., 2016, 18, 19705–19712 RSC.
- H. Kaczmarek, L. Å. Lindén and J. F. Rabek, Polym. Degrad. Stab., 1995, 47, 175–188 Search PubMed.
- R. Espiritu, B. T. Golding, K. Scott and M. Mamlouk, J. Power Sources, 2018, 375, 373–386 Search PubMed.
- R. Borup, J. Meyers, B. Pivovar, Y. S. Kim, R. Mukundan, N. Garland, D. Myers, M. Wilson, F. Garzon, D. Wood, P. Zelenay, K. More, K. Stroh, T. Zawodzinski, J. Boncella, J. E. McGrath, M. Inaba, K. Miyatake, M. Hori, K. Ota, Z. Ogumi, S. Miyata, A. Nishikata, Z. Siroma, Y. Uchimoto, K. Yasuda, K. Kimijima and N. Iwashita, Chem. Rev., 2007, 107, 3904–3951 CrossRef CAS PubMed.
- K. E. Ayers, E. B. Anderson, C. B. Capuano, M. Niedzwiecki, M. A. Hickner, C.-Y. Wang, Y. Leng and W. Zhao, ECS Trans., 2013, 45, 121–130 CrossRef.
- G. Hübner and E. Roduner, J. Mater. Chem., 1999, 9, 409–418 Search PubMed.
- Y.-F. He, R.-M. Wang, Y.-Y. Liu, Y. Chang, Y.-P. Wang, C.-G. Xia and J.-S. Suo, J. Mol. Catal. A: Chem., 2000, 159, 109–113 CrossRef CAS.
- R. Espiritu, B. T. Golding, K. Scott and M. Mamlouk, J. Mater. Chem. A, 2017, 5, 1248–1267 RSC.
- I. Di Somma, R. Andreozzi, M. Canterino, V. Caprio and R. Sanchirico, AIChE J., 2008, 54, 1579–1584 Search PubMed.
- N. Ye, Y. Xu, D. Zhang, J. Yang and R. He, Polym. Degrad. Stab., 2018, 153, 298–306 CrossRef CAS.
- Z. Feng, G. Gupta and M. Mamlouk, RSC Adv., 2023, 13, 20235–20242 RSC.
- D. S. Maxwell, I. Kendrick and S. Mukerjee, J. Am. Chem. Soc., 2024, 146, 22431–22444 Search PubMed.
- U. Babic, M. Suermann, F. N. Büchi, L. Gubler and T. J. Schmidt, J. Electrochem. Soc., 2017, 164, F387–F399 CrossRef CAS.
- A. Albert, A. O. Barnett, M. S. Thomassen, T. J. Schmidt and L. Gubler, ACS Appl. Mater. Interfaces, 2015, 7, 22203–22212 CrossRef CAS PubMed.
- D. S. Kim, C. H. Fujimoto, M. R. Hibbs, A. Labouriau, Y.-K. Choe and Y. S. Kim, Macromolecules, 2013, 46, 7826–7833 CrossRef CAS.
- C. Fujimoto, D.-S. Kim, M. Hibbs, D. Wrobleski and Y. S. Kim, J. Membr. Sci., 2012, 423–424, 438–449 CrossRef CAS.
- J. Lei, Z. Wang, Y. Zhang, M. Ju, H. Fei, S. Wang, C. Fu, X. Yuan, Q. Fu, M. U. Farid, H. Kong, A. K. An, R. Deng, F. Liu and J. Wang, Carbon Neutrality, 2024, 3, 25 CrossRef CAS.
- K. H. Lee, D. H. Cho, Y. M. Kim, S. J. Moon, J. G. Seong, D. W. Shin, J.-Y. Sohn, J. F. Kim and Y. M. Lee, Energy Environ. Sci., 2017, 10, 275–285 RSC.
- L. Wang, X. Peng, W. E. Mustain and J. R. Varcoe, Energy Environ. Sci., 2019, 12, 1575–1579 RSC.
- X. Huang, R. Solasi, Y. Zou, M. Feshler, K. Reifsnider, D. Condit, S. Burlatsky and T. Madden, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 2346–2357 Search PubMed.
- Q. Wang, Y. Gao, Z. Ma, Y. Zhang, W. Ni, H. A. Younus, C. Zhang, Z. Chen and S. Zhang, J. Energy Chem., 2021, 54, 342–351 Search PubMed.
- P. Paciok, M. Schalenbach, M. Carmo and D. Stolten, J. Power Sources, 2017, 365, 53–60 CrossRef CAS.
- H. Jin, B. Ruqia, Y. Park, H. J. Kim, H. Oh, S. Choi and K. Lee, Adv. Energy Mater., 2020, 11(4) DOI:10.1002/aenm.202003188.
- J. Staszak-Jirkovský, C. D. Malliakas, P. P. Lopes, N. Danilovic, S. S. Kota, K.-C. Chang, B. Genorio, D. Strmcnik, V. R. Stamenkovic, M. G. Kanatzidis and N. M. Markovic, Nat. Mater., 2016, 15, 197–203 CrossRef PubMed.
- Y. Zhang, L. Gao, E. J. M. Hensen and J. P. Hofmann, ACS Energy Lett., 2018, 3, 1360–1365 CrossRef CAS PubMed.
- L. Xie and D. W. Kirk, Electrochim. Acta, 2020, 364, 137091 CrossRef CAS.
- S. Cherevko, A. R. Zeradjanin, G. P. Keeley and K. J. J. Mayrhofer, J. Electrochem. Soc., 2014, 161, H822–H830 Search PubMed.
- M. Schalenbach, O. Kasian, M. Ledendecker, F. D. Speck, A. M. Mingers, K. J. J. Mayrhofer and S. Cherevko, Electrocatalysis, 2018, 9, 153–161 Search PubMed.
- S. Cherevko, S. Geiger, O. Kasian, N. Kulyk, J.-P. Grote, A. Savan, B. R. Shrestha, S. Merzlikin, B. Breitbach, A. Ludwig and K. J. J. Mayrhofer, Catal. Today, 2016, 262, 170–180 Search PubMed.
- F. D. Speck and S. Cherevko, Electrochem. Commun., 2020, 115, 106739 CrossRef CAS.
- C. Stumm, M. Bertram, M. Kastenmeier, F. D. Speck, Z. Sun, J. Rodríguez-Fernández, J. V. Lauritsen, K. J. J. Mayrhofer, S. Cherevko, O. Brummel and J. Libuda, Adv. Funct. Mater., 2021, 31(13) DOI:10.1002/adfm.202009923.
- J. Wu, X. Z. Yuan, J. J. Martin, H. Wang, J. Zhang, J. Shen, S. Wu and W. Merida, J. Power Sources, 2008, 184, 104–119 Search PubMed.
- J. W. D. Ng, M. García-Melchor, M. Bajdich, P. Chakthranont, C. Kirk, A. Vojvodic and T. F. Jaramillo, Nat. Energy, 2016, 1, 16053 CrossRef CAS.
- C. Guan, W. Xiao, H. Wu, X. Liu, W. Zang, H. Zhang, J. Ding, Y. P. Feng, S. J. Pennycook and J. Wang, Nano Energy, 2018, 48, 73–80 Search PubMed.
- C. Tang, R. Zhang, W. Lu, L. He, X. Jiang, A. M. Asiri and X. Sun, Adv. Mater., 2017, 29(2) DOI:10.1002/adma.201602441.
- F. Yu, H. Zhou, Z. Zhu, J. Sun, R. He, J. Bao, S. Chen and Z. Ren, ACS Catal., 2017, 7, 2052–2057 Search PubMed.
- K. Liu, C. Zhang, Y. Sun, G. Zhang, X. Shen, F. Zou, H. Zhang, Z. Wu, E. C. Wegener, C. J. Taubert, J. T. Miller, Z. Peng and Y. Zhu, ACS Nano, 2018, 12, 158–167 CrossRef CAS PubMed.
- P. Chen, K. Xu, Z. Fang, Y. Tong, J. Wu, X. Lu, X. Peng, H. Ding, C. Wu and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 14710–14714 CrossRef CAS PubMed.
- J. Mahmood, M. A. R. Anjum, S. Shin, I. Ahmad, H. Noh, S. Kim, H. Y. Jeong, J. S. Lee and J. Baek, Adv. Mater., 2018, 30(52) DOI:10.1002/adma.201805606.
- L. Ai, T. Tian and J. Jiang, ACS Sustain. Chem. Eng., 2017, 5, 4771–4777 Search PubMed.
- L. Du, L. Luo, Z. Feng, M. Engelhard, X. Xie, B. Han, J. Sun, J. Zhang, G. Yin, C. Wang, Y. Wang and Y. Shao, Nano Energy, 2017, 39, 245–252 Search PubMed.
- J. Pan, H. Zhu, H. Cao, B. Wang, J. Zhao, Z. Sun and F. Yan, J. Membr. Sci., 2021, 620, 118794 CrossRef CAS.
- M. Tomoi, K. Yamaguchi, R. Ando, Y. Kantake, Y. Aosaki and H. Kubota, J. Appl. Polym. Sci., 1997, 64, 1161–1167 CrossRef CAS.
- M. R. Hibbs, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 1736–1742 CrossRef CAS.
- N. Li, Y. Leng, M. A. Hickner and C.-Y. Wang, J. Am. Chem. Soc., 2013, 135, 10124–10133 CrossRef CAS PubMed.
- N. Li, T. Yan, Z. Li, T. Thurn-Albrecht and W. H. Binder, Energy Environ. Sci., 2012, 5, 7888 RSC.
- J. Ran, L. Wu, B. Wei, Y. Chen and T. Xu, Sci. Rep., 2014, 4, 6486 CrossRef PubMed.
- B. Bauer, H. Strathmann and F. Effenberger, Desalination, 1990, 79, 125–144 CrossRef CAS.
- E. J. Park and Y. S. Kim, J. Mater. Chem. A, 2018, 6, 15456–15477 RSC.
- T. H. Pham, J. S. Olsson and P. Jannasch, J. Mater. Chem. A, 2019, 7, 15895–15906 Search PubMed.
- X. Wang, W. Sheng, Y. Shen, L. Liu, S. Dai and N. Li, J. Membr. Sci., 2019, 587, 117135 CrossRef CAS.
- A. Amel, S. B. Smedley, D. R. Dekel, M. A. Hickner and Y. Ein-Eli, J. Electrochem. Soc., 2015, 162, F1047–F1055 CrossRef CAS.
- C. G. Arges, L. Wang, M. Jung and V. Ramani, J. Electrochem. Soc., 2015, 162, F686–F693 CrossRef CAS.
- J. Han, Q. Liu, X. Li, J. Pan, L. Wei, Y. Wu, H. Peng, Y. Wang, G. Li, C. Chen, L. Xiao, J. Lu and L. Zhuang, ACS Appl. Mater. Interfaces, 2015, 7, 2809–2816 CrossRef CAS PubMed.
- J. Pan, J. Han, L. Zhu and M. A. Hickner, Chem. Mater., 2017, 29, 5321–5330 CrossRef CAS.
- W. You, K. M. Hugar and G. W. Coates, Macromolecules, 2018, 51, 3212–3218 CrossRef CAS.
- S.-B. Lee, C.-M. Min, J. Jang and J.-S. Lee, Polymer, 2020, 192, 122331 CrossRef.
- Q. Yang, X. L. Gao, H. Y. Wu, Y. Y. Cai, Q. G. Zhang, A. M. Zhu and Q. L. Liu, J. Power Sources, 2019, 436, 226856 Search PubMed.
- E. A. Weiber, D. Meis and P. Jannasch, Polym. Chem., 2015, 6, 1986–1996 Search PubMed.
- X. Ren, S. C. Price, A. C. Jackson, N. Pomerantz and F. L. Beyer, ACS Appl. Mater. Interfaces, 2014, 6, 13330–13333 CrossRef CAS PubMed.
- D. Chen and M. A. Hickner, Macromolecules, 2013, 46, 9270–9278 CrossRef CAS.
- J. Pan, C. Chen, Y. Li, L. Wang, L. Tan, G. Li, X. Tang, L. Xiao, J. Lu and L. Zhuang, Energy Environ. Sci., 2014, 7, 354–360 RSC.
- H. Ishikawa, T. Teramoto, Y. Ueyama, Y. Sugawara, Y. Sakiyama, M. Kusakabe, K. Miyatake and M. Uchida, J. Power Sources, 2016, 325, 35–41 CrossRef CAS.
- L. Zhu, T. J. Zimudzi, N. Li, J. Pan, B. Lin and M. A. Hickner, Polym. Chem., 2016, 7, 2589 RSC.
- X. Q. Wang, C. X. Lin, F. H. Liu, L. Li, Q. Yang, Q. G. Zhang, A. M. Zhu and Q. L. Liu, J. Mater. Chem. A, 2018, 6, 12455–12465 RSC.
- C. Xiao Lin, X. Qin Wang, E. Ning Hu, Q. Yang, Q. Gen Zhang, A. Mei Zhu and Q. Lin Liu, J. Membr. Sci., 2017, 541, 358–366 CrossRef.
- P. Dai, Z.-H. Mo, R.-W. Xu, S. Zhang and Y.-X. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 20329–20341 CrossRef CAS PubMed.
- X. Zhang, Y. Cao, M. Zhang, Y. Huang, Y. Wang, L. tableLiu and N. Li, J. Membr. Sci., 2020, 596, 117700 CrossRef CAS.
- R. Narducci, E. Sgreccia, P. Knauth and M. L. Di Vona, Polymers, 2021, 13, 3887 CrossRef CAS PubMed.
- T. Zhou, M. Wang, X. He and J. Qiao, J. Materiomics, 2019, 5, 286–295 CrossRef.
- V. Elumalai and D. Sangeetha, J. Power Sources, 2019, 412, 586–596 CrossRef CAS.
- Y. Lu, Z. Hu, Y. Liu, I. Buregeya, X. Pan, N. Li and S. Chen, Fuel Cells, 2019, 19, 663–674 CrossRef CAS.
- C. Gong, S. Zhao, W.-C. Tsen, F. Hu, F. Zhong, B. Zhang, H. Liu, G. Zheng, C. Qin and S. Wen, J. Power Sources, 2019, 441, 227176 CrossRef CAS.
- M. L. Di Vona, M. Casciola, A. Donnadio, M. Nocchetti, L. Pasquini, R. Narducci and P. Knauth, Int. J. Hydrogen Energy, 2017, 42, 3197–3205 CrossRef CAS.
- N. Chen, C. Long, Y. Li, D. Wang, C. Lu, H. Zhu and J. Yu, ACS Appl. Mater. Interfaces, 2018, 10, 18246–18256 Search PubMed.
- D. Ion-Ebrasu, B. G. Pollet, S. Caprarescu, A. Chitu, R. Trusca, V. Niculescu, R. Gabor, E. Carcadea, M. Varlam and B. S. Vasile, Int. J. Hydrogen Energy, 2020, 45, 17057–17066 Search PubMed.
- N. Carboni, L. Mazzapioda, A. Caprì, I. Gatto, A. Carbone, V. Baglio and M. A. Navarra, Electrochim. Acta, 2024, 486, 144090 Search PubMed.
- G. Das, B. J. Park, J. Kim, D. Kang and H. H. Yoon, Sci. Rep., 2019, 9, 9572 CrossRef PubMed.
- I. Arunkumar, A. R. Kim, S. H. Lee and D. J. Yoo, Int. J. Hydrogen Energy, 2024, 52, 139–153 CrossRef CAS.
- O. Movil, L. Frank and J. A. Staser, J. Electrochem. Soc., 2015, 162, F419–F426 Search PubMed.
- T. Zhu, Y. Sha, H. A. Firouzjaie, X. Peng, Y. Cha, D. M. M. M. Dissanayake, M. D. Smith, A. K. Vannucci, W. E. Mustain and C. Tang, J. Am. Chem. Soc., 2020, 142, 1083–1089 CrossRef CAS PubMed.
- Z. Wang, G. Li, W. Hou, H. Guo, L. Wang and M. Wu, ACS Nano, 2023, 17, 8671–8679 Search PubMed.
- S. Yu, P. Zhou, J. Hao and Y. Zhou, Polymer, 2023, 283, 126256 Search PubMed.
- C. Long, C. Lu, Y. Li, Z. Wang and H. Zhu, Int. J. Hydrogen Energy, 2020, 45, 19778–19790 Search PubMed.
- Q. Yang, C. X. Lin, F. H. Liu, L. Li, Q. G. Zhang, A. M. Zhu and Q. L. Liu, J. Membr. Sci., 2018, 552, 367–376 Search PubMed.
- D. Zhang, N. Ye, S. Chen, R. Wan, Y. Yang and R. He, Renew. Energy, 2020, 160, 250–260 Search PubMed.
- H. Zarrin, J. Fu, G. Jiang, S. Yoo, J. Lenos, M. Fowler and Z. Chen, ACS Nano, 2015, 9, 2028–2037 Search PubMed.
- J. Y. Chu, K. H. Lee, A. R. Kim and D. J. Yoo, Compos. B Eng., 2019, 164, 324–332 CrossRef CAS.
- D. Zhang, S. Xu, R. Wan, Y. Yang and R. He, J. Power Sources, 2022, 517, 230720 Search PubMed.
- D. Shen, Y. Liu, C. Chen, Z. Yang, H. Li, S. Xiao, W. Wu and J. Wang, Adv. Funct. Mater., 2025 DOI:10.1002/adfm.202515358.
- Y. Zheng, Y. Liu, H. Li, Z. Yang, W. Wu, J. Zhang, J. Wu and J. Wang, Adv. Funct. Mater., 2025, 35(37) DOI:10.1002/adfm.202500151.
- C. Wang, Z. Yang, W. Wu, C. Chen, Y. Li, R. Zhang, W. Li, K. Dai and J. Wang, Chem. Mater., 2024, 36, 8255–8263 CrossRef CAS.
- J. Ren, J. Xu, M. Ju, X. Chen, P. Zhao, L. Meng, J. Lei and Z. Wang, Adv. Powder Mater., 2022, 1, 100017 Search PubMed.
- Y. Lin, H. Gao, Q. Zhou, Q. Huang, M. Zhao and S. Tang, Int. J. Hydrogen Energy, 2025, 145, 75–83 Search PubMed.
- X. Liao, L. Ren, D. Chen, X. Liu and H. Zhang, J. Power Sources, 2015, 286, 258–263 Search PubMed.
- M. T. Pérez-Prior, T. García-García, A. Várez and B. Levenfeld, J. Mater. Sci., 2015, 50, 5893–5903 Search PubMed.
- S. Xu, R. Jiang, S. Jiang and Y. Gao, J. Electrochem. Soc., 2016, 163, F688–F690 CrossRef CAS.
- Y. Chen, Z. Li, N. Chen, R. Li, Y. Zhang, K. Li, F. Wang and H. Zhu, Electrochim. Acta, 2017, 255, 335–346 CrossRef CAS.
- A. R. Ferrari, D. Stucchi, T. Caielli, R. Akbari, I. C. Pellini, C. Antonini and P. Mustarelli, Solid State Ionics, 2025, 430, 116996 Search PubMed.
Footnote |
| † In memory of Prof. Bruno Scrosati (August 1937 – November 2024). |
| This journal is © The Royal Society of Chemistry 2026 |