Polycyclic aromatic hydrocarbon removal from stormwater runoff by bioretention cells: a review
Yichi
Zhang
,
Jiaqing
Xiong
 *,
Jiajia
Zhou
,
Yanzheng
Liu
and
Qionghua
Zhang
*,
Jiajia
Zhou
,
Yanzheng
Liu
and
Qionghua
Zhang
Xi'an University of Architecture and Technology, China. E-mail: xiongjiaqing@xauat.edu.cn
First published on 27th October 2025
Abstract
Polycyclic aromatic hydrocarbons (PAHs) are detrimental to human health and the environment as a hazardous persistent organic pollutant of environmental concern. Research is emerging on the occurrence, form, migration characteristics and removal of PAHs from runoff stormwater by bioretention cells. This review analyses the sources of PAHs, the characteristics of their concentration distribution and their migration pattern in stormwater runoff. The mechanism of PAH removal by bioretention cells, the purification effects of different fillers and their influencing factors, and the accumulation characteristics of PAHs in bioretention cells are analysed, and the influence mechanism of PAH accumulation on the performance of bioretention cells is summarised. It is noteworthy that the typical concentration range of polycyclic aromatic hydrocarbons (PAHs) in urban stormwater runoff is 0.65–13.4 µg L−1. The average PAH concentrations in surface runoff vary across different functional zones, with levels in industrial and commercial areas generally being significantly higher than those in residential areas, green spaces, and other functional zones. Studies have shown that the overall removal efficiency of PAHs by bioretention cells can consistently exceed 80%, demonstrating their significant potential for pollution control. Based on existing research progress, this review further proposes that future efforts should focus on the following research directions: (1) induction of the decomposition of PAHs accumulated in bioretention cells into degradable products; (2) search for more effective fillers to improve their removal efficiency; (3) effects of PAH contamination on microbial functions in the filler of bioretention cells; and (4) synergistic effects of PAHs with other pollutants on bioretention cells. This review evaluates the actual PAH removal performance of bioretention facilities, which holds significant scientific and practical value for optimizing the design of low-impact development facilities and ensuring the safety of the urban water environment.
Environmental significanceAs a core technology of green infrastructure, bioretention basins play an important role in alleviating urban non-point source pollution, especially in the removal of persistent toxic pollutants such as polycyclic aromatic hydrocarbons (PAHs), which requires systematic evaluation. This paper reviews their removal mechanisms (such as adsorption, degradation, and plant absorption), key influencing factors (PAH hydrophobicity, media type, microbial communities, etc.), and optimisation strategies, providing a theoretical basis for overcoming the limitations of traditional treatment technologies. It holds significant scientific value and practical significance for ensuring water ecological safety and achieving sustainable stormwater management under the ‘dual carbon’ goals. This review fills the knowledge gap in understanding the migration and transformation patterns of PAHs in rainwater runoff, providing critical technical support for urban resilience-oriented environmental construction. |
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are compounds consisting of multiple condensed aromatic rings, and usually appear as colourless, white or light-yellow solids.1 These compounds are usually insoluble in water and non-volatile and have moderately elevated melting and boiling points.1,2 The high stability of their chemical structure makes PAHs resistant to degradation in the environment. This quality is the reason for which they are classified as persistent organic pollutants.1,3 The classification of PAHs is based on the number of rings and there are two categories: low molecular weight (LMW) and high molecular weight (HMW).4 It is evident that the physicochemical properties of PAHs are closely related to their molecular weights. With increasing molecular weight, there is an increase in the antioxidant, reduction and vapourisation resistance as well as lipophilicity of PAHs, while the water solubility decreases.5,6 In the environment, LMW PAHs are primarily present in gaseous form, whereas HMW PAHs have a propensity to adsorb to particulate matter.7 Of the more than 100 known polycyclic aromatic hydrocarbons (PAHs), the U.S. Environmental Protection Agency (USEPA) has specifically designated 16 as priority control targets based on environmental and health risk considerations (Table S1 lists the 16 PAHs and their common physicochemical properties).The contamination process of PAHs involves surface accumulation and subsequent rainfall washout.8 The main origins of PAHs accumulating on the surface are traffic emissions, industrial activities, residential heating and atmospheric deposition.9,10 Accumulated PAHs can enter surface water bodies directly with runoff and can also be carried into urban infiltration areas, such as green spaces, road greenbelts and agricultural lands, and enter the groundwater system through soil infiltration. Studies have demonstrated that rainwater runoff carries polycyclic aromatic hydrocarbons (PAHs) at levels varying from 0.65 to 13.4 µg L−1,11 constituting a significant input pathway for PAH contamination of surface waters, contributing up to 14 to 36% of the overall PAH load to aquatic ecosystems.12
PAHs cause both environmental and health risks owing to their chemical nature, bioaccumulation, persistence and toxicological effects.13 PAHs are highly bioconcentrated and can easily accumulate in the food chain to induce negative biological effects including carcinogenicity, mutagenicity and toxicity.2,14 For example, benzo(a)pyrene is recognised as a representative substance with the highest carcinogenicity among PAHs;2 anthracene and benzo(a)pyrene are responsible for allergic skin reactions in animals and humans.15,16 It is imperative to acknowledge that exposure to PAHs is inevitable at this juncture. Short-term exposure to PAHs causes symptoms such as skin irritation, inflammation, nausea and vomiting, and long-term exposure increases the incidence of genetic mutations, cardiorespiratory death and cancer.17 Thus, how to reduce the pollution load of PAHs is beginning to receive widespread attention. To remove a variety of pollutants from stormwater runoff, several countries have added facilities for stormwater management practices, which include green roofs, cisterns, and bioretention cells, to urban construction.18 As a Nature-based Solution (NbS), stormwater management facilities can provide multiple ecosystem services. They not only reduce runoff volume and remove pollutants but also create habitats for organisms and replenish groundwater, effectively mitigating urban flooding and water pollution issues.19,20 Among these, bioretention cells, serving as a typical implementation of NbS, play a crucial role in removing PAHs from stormwater runoff, owing to their unique structure and operational principles. Bioretention cells are able to achieve the dual goals of runoff water quantity control and water quality purification through the synergistic action of plants, fillers and microorganisms.21 Research has shown that bioretention cells remove PAHs mainly through adsorption, biodegradation, and plant absorption.22 Of these, particle adsorption is one of the most significant forms of removing runoff contaminants in bioretention cells.23 Therefore, the selection of the correct bioretention cell filler is critical in influencing its removal efficiency. The filler supplies both the essential support and nutrients for plant growth and contributes significantly to the water infiltration efficiency of the bioretention cells.24 However, the high hydrophobicity of PAHs may contribute to their long-term accretion within the filler, which in turn may affect the continued operational efficacy of the bioretention cells.22 Therefore, it is of profound significance to clarify the removal mechanism, degradation pathway and key control factors of PAHs in bioretention cells to enhance their removal efficiency and ensure ecological safety.
In this review, the literature related to purification of stormwater PAHs by bioretention cells was reviewed through the Web of Science database, and the search was completed on May 20, 2025. ‘PAHs’, ‘bioretention cells’, ‘stormwater runoff’, ‘soil’, and ‘microorganisms’ were used as keywords, and a large amount of literature was searched. Based on a retrieval strategy utilizing keyword combinations, this study ultimately incorporated 98 highly relevant publications from the past 15 years as the foundation for analysis. This review analyses the origins and migration of PAHs in runoff, reviews the research progress of bioretention cells regarding the removal effect and accumulation degree of PAHs, as well as discusses the effect of PAHs on the properties of bioretention cells. Finally, the challenges and future research directions for PAH removal in bioretention systems are summarized. Compared to existing studies, the innovation of this review lies in systematically revealing the “removal, accumulation, and transformation” of PAHs in bioretention systems, with particular emphasis on the impact of PAH accumulation on the functional performance of bioretention facilities. This comprehensive discussion aims to shift the research focus in this field from short-term removal efficiency to long-term system sustainability and environmental risks. It holds significant theoretical and practical implications for guiding the optimized design and risk management of future bioretention cells.
2. Migration characteristics of PAHs in stormwater runoff
2.1 Sources of PAHs in stormwater runoff
Table 1 lists the characteristic ratios that are currently in common use. The primary contributors of PAHs in stormwater runoff are traffic emissions, industrial activities, residential heating, and atmospheric deposition.9,10 The eigen-ratio method is a commonly used method for analysing the sources of these PAHs with intra-source variability and inter-source similarity, which allows effective identification of characteristic sources in a specific region, and is based on the principle of determining the main sources based on the ratio of the PAH concentrations of the individual isomers.31Fig. 1 illustrates the sources and trends of PAHs.| PAH rate of comparison | Limitations | Source | References |
|---|---|---|---|
| a Ant – anthracene, BaA – benzo(a)anthracene, BaP – benzo(a)pyrene, Bghip – benzo[g,h,i]perylene, Chr – chrysene, Flu – fluorene, IPy – indeno[1,2,3-cd]pyrene, Phe – phenanthrene, Pyr – pyrene, three + four ring PAHs – three and four ring PAHs, ∑COMB – e (FLA, PYR, BaA, CHR, BkF, BbF, BaP, IcdP and BghiP), ∑HMW – sum of four and five-ring PAHs, ∑LMW – sum of two and three-ring PAHs, ∑PAHs – sum of total non-alkylated PAHs. | |||
| Ant/(Ant + Phe) | <0.1 | Petroleum source | 25 |
| >0.1 | Pyrolysis source | ||
| BaA/(BaA + Chr) | <0.2 | Petroleum source | 26 |
| 0.2–0.5 | Petrol, diesel and crude fuel combustion | ||
| >0.5 | Grass, wood, and coal combustion | ||
| ∑COMB/∑PAHs | ∼1 | Combustion source | 27 |
| Flu/(Flu + Pyr) | <0.4 | Petroleum source | 28 |
| 0.4–0.5 | Fossil fuel combustion | ||
| >0.5 | Grass, wood, and coal combustion | ||
| IPy/(IPy + Bghip) | <0.2 | Petroleum source | 26 |
| 0.2–0.5 | Fossil fuel combustion | ||
| >0.5 | Grass, wood, and coal combustion | ||
| ∑LMW/∑HMW | <1 | Pyrolysis source | 29 |
| >1 | Petroleum source | ||
| Three + four ring PAHs/∑PAHs | > 0.9 | Wood or biomass combustion | 30 |
| ≈0.7 | Coal combustion | ||
| <0.5 | Petroleum sources or traffic emissions | ||
Transport emissions are the most representative source of PAHs. During vehicle operation, oil leaks, tailpipe emissions, tyre wear, and so on, deliver PAHs to the environment.32 B. K. Lee et al.,33 found that ∑COMB/∑PAHs of road dust in Ulsan, Korea, ranged from 0.73–0.93 and FL/Pyr ranged from 0.25–1.20, indicating that traffic emissions are one of the major sources of PAHs. Another potential source of PAHs in stormwater runoff is asphalt materials. The product asphalt is typically petroleum-based and is considered an oil-producing source. P. R. N. Fernandes et al.,34 conducted a quantitative analysis of PAH composition in asphalt binders, revealing that the contamination level of LMW PAHs was relatively low (<10 mg kg−1), while the average concentration of HMW PAHs ranged from 10.2 to 27.2 mg kg−1. The PAH concentrations decreased with decreasing road asphalt content. J. Su et al.,35 revealed a significant decrease in benzo[a]pyrene concentration (from 9.13 to 0.10 mg kg−1) and a similar decreasing trend in phenanthrene concentration (from 9.85 to 1.95 mg kg−1) as the asphalt content was reduced from 100% to 1%. This pattern was also observed in the other PAH components detected.
Industrial activities and residential heating also generate PAH pollution. It has been shown that industrial emissions contribute up to 10% of total PAH emissions.36 Industrial activities such as petroleum processing, coking and steelmaking are all significant sources of PAHs in stormwater runoff, and PAHs released during production from these industrial activities not only enter the atmosphere directly, but can also enter water bodies through stormwater runoff. Residential heating relies heavily on coal combustion, which triggers peak PAH pollution mainly during autumn and winter, when the combustion of coal, coal tar and hydrocarbons increases the amount of total PAHs in stormwater runoff.8 G. Vuković et al.,30 found that three + four ring PAHs/∑PAHs averaged 0.61 and BbF/BkF averaged 2.14 in the urban area of Belgrade in winter, which indicates that coal combustion is one of the primary contributors of PAHs. Atmospheric deposition is also a source of PAHs in urban stormwater runoff. PAHs can stay on the ground through dry deposition and can also directly contaminate stormwater through wet deposition (rain and snow).12 In this regard, wet deposition is the main source of LMW PAHs in stormwater runoff.37
2.2 Concentration distribution of PAHs in stormwater runoff
Table 2 summarises the distribution of average PAH concentrations in different functional areas for multiple locations around the world. PAH content in rainwater runoff is mostly at ng and µg levels, and the content is influenced by factors such as particle size distribution, land utilisation type, traffic volume, the number of dry days prior to rainfall, the season and the frequency of human activities.2,8,13 PAHs in urban stormwater runoff are mainly associated with particulate matter, and the small-size particulate matter has a larger specific surface area, and its average concentration increases with decreasing particle diameter of the particulate matter.12,38 L. Herngren et al.,39 investigated the particle size distribution of PAHs in rainwater, and fine particulate matter (0.45–75 µm) dominated the PAH fugacity, with significantly higher levels of PAHs than particulate matter in other particle size intervals. C. H. Shi et al.,13 analysed the concentration of PAHs fractionated by particle size in different land use types and found that PAHs smaller than 75 µm were the highest, accounting for 37% of the total average PAH concentration, followed by 75–150 µm at 26%. Urban average PAH concentrations are higher than those of suburb and rural areas. C. Wang et al.,40 compared soil PAH concentrations in urban and rural Nanjing and found that the average urban PAH concentrations were about 1.98- and 3.14-fold higher than those of suburb and rural areas, etc. In urban areas, urban centres have the highest average PAH levels among the different functional zones, and average PAH levels are usually higher in industrial and commercial areas than in other functional areas, such as residences, as a result of high traffic volumes and industrial activities at these locations. For example, K. Ciarkowska et al.,41 analysed soil PAH levels in Krakow and Zakopane, Poland, and revealed that average PAH levels were higher in the city centre than in residential and housing areas, and T. Mihankhah et al.,42 compared dust samples from diverse land use categories in Tehran, Iran, and found that the average PAH concentrations were higher in the industrial area (778 µg kg−1) and in the commercial area (813 µg kg−1) than in the residential area (359 µg kg−1). T. N. T. Nguyen et al.,12 analysed runoff samples from different land use areas (residential and industrial) in Ulsan, Korea, and found that the average PAH concentration was higher at industrial locations (272 ng L−1). In addition, the effect of the first flush during the initial stages of the rainfall has a robust influence on the change in PAH levels, with the highest PAH concentration occurring at the first flush being positively correlated with the number of days of dryness prior to the rainfall.12 T. N. T. Nguyen et al.,12 found that the concentration of PAHs in stormwater runoff decreases with increasing rainfall. Z. Zhang et al.,8 took soil samples from eight typical roads in Fengxi New City, Xi'an, in different months and found that the PAH concentrations were remarkably less in the flood season (average 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 767 µg kg−1) compared to the non-flood season (average 17
767 µg kg−1) compared to the non-flood season (average 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291 µg kg−1). The aforementioned data show that increased rainfall and stronger hydrological activities during the flood season can decrease the levels of PAHs in the environment.
291 µg kg−1). The aforementioned data show that increased rainfall and stronger hydrological activities during the flood season can decrease the levels of PAHs in the environment.
| No. | Sites | Functional partition | Average PAH concentration | References |
|---|---|---|---|---|
| 1 | Ulsan, Korea | Residential/industrial | 131/272 ng L−1 | 12 |
| 2 | Madison, USA | Commercial | 330 ng L−1 | 5 |
| 3 | Taiyuan, China | Residential/industrial/cultivation | 2109/2376/719.1 ng g−1 | 43 |
| 4 | Nanjing, China | Urban/suburb/rural | 3330/1680/1060 ng g−1 | 40 |
| 5 | Lanzhou, China | Residential/commercial/industrial | 1040/1870/2490 µg kg−1 | 44 |
| 6 | Tehran, Iran | Residential/commercial/industrial | 359/813/778 µg kg−1 | 42 |
| 7 | Krakow, Poland | Centre/residential/greenfield | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220/768/450 µg kg−1 220/768/450 µg kg−1 |
41 |
| 8 | Zakopane, Poland | Centre/residential/greenfield | 1891/1331/281 µg kg−1 | 41 |
| 9 | Lagos, Nigeria | Residential/commercial/industrial | 1344/5925/1510 µg kg−1 | 45 |
2.3 Transport and transformation of PAHs in stormwater runoff
Polycyclic aromatic hydrocarbons (PAHs) tend to bind to colloids, dissolved organic matter, or suspended particles in stormwater runoff and are finally deposited in sediments.46 The transport behaviour of PAHs at the sediment–water interface is usually described using the sediment–water fugacity fraction (ffsw).47 The state of PAHs present in stormwater runoff can be categorized as net deposition, equilibrium, and net diffusion.47 During the initial stages of stormwater runoff, net diffusion may dominate, with PAHs in sediments being released into the water body. Net diffusion is usually driven by a concentration gradient that represents the transport of PAHs between the sediment and the water body. For example, sediment erosion can be enhanced in areas of stormwater runoff where the shear stress of the water flow is greater, leading to resuspension of sediments or direct release of PAHs.47 In contrast, resuspended sediments release more contaminants than bottom settling particles.48 Net deposition generally dominates the periods of peak runoff, when suspended particulate matter settles carrying PAHs. Net deposition describes the transport of PAHs from the water column to the sediment, usually through settling and adsorption of particulate matter. The net deposition process is more significant during the terminal stages of stormwater runoff or when flow velocities are reduced. The equilibrium state means that the net deposition and net diffusion rates are equal. During the later stages of runoff, PAHs may tend to be in a dynamic equilibrium between the sediment and the water body.3. Mechanism of PAH removal in bioretention cells
Upon entering bioretention systems, PAHs are initially intercepted through rapid sorption to the filter media, followed by gradual biodegradation mediated by plant–microorganism interactions. In laboratory-scale bioretention cells, PAH removal occurs through multiple pathways: sorption (56–73% of added NAP), biodegradation (12–18%), plant uptake (2–23%), and volatilization (<1%).23 The sorption efficiency is predominantly governed by PAH hydrophobicity, with stronger hydrophobicity leading to greater retention through hydrophobic interactions with the media.38 Biodegradation efficiency shows a positive correlation with PAH bioavailability, where reduced bioavailability corresponds to slower degradation rates. Furthermore, plant uptake capacity varies significantly across species, with deep-rooted vegetation demonstrating dual advantages: enhanced direct PAH absorption and provision of extensive habitats for microbial colonization and growth.3.1 Filler adsorption
Filler adsorption is one of the crucial ways to remove PAHs in bioretention cells.49 When a rainfall incident occurs, stormwater runoff collects in bioretention cells. PAHs are quickly captured by the filler and accumulate. After stormwater runoff carries PAHs into the bioretention basin, PAHs accumulate mainly in its upper filler layer.50 The three main mechanisms associated with the adsorption of polycyclic aromatic hydrocarbons (PAHs) are electron donor–acceptor interactions, hydrogen bond formation, and π–π interactions.51 N. Esfandiar et al.,51 found that solution pH did not have a significant effect on the amount of PAHs adsorbed by all the adsorbents, while adsorption coefficients (Kd) and hydrophobicity of the adsorbents, and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd and log
Kd and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow for PAHs showed highly positive correlations. These results suggest that π–π interactions may be the main mechanism for the adsorption of PAHs onto the studied adsorbents. The octanol–water partition coefficient (Kow) is an indicative parameter for determining the adsorption capacity of PAHs, and the higher this coefficient is, the more readily PAHs are adsorbed.52 However, adsorption is a reversible process, which is usually accompanied by desorption. N. Esfandiar et al.,52 selected three PAHs (anthracene, fluorene, and pyrene) for desorption experiments and found that PAHs were tightly bound to the organic matter, which could lead to low bioavailability. Therefore, it is necessary to investigate how to weaken the binding strength of PAHs to organic matter to promote their controlled desorption, and at the same time improve the bioavailability of PAHs in the aqueous phase.
Kow for PAHs showed highly positive correlations. These results suggest that π–π interactions may be the main mechanism for the adsorption of PAHs onto the studied adsorbents. The octanol–water partition coefficient (Kow) is an indicative parameter for determining the adsorption capacity of PAHs, and the higher this coefficient is, the more readily PAHs are adsorbed.52 However, adsorption is a reversible process, which is usually accompanied by desorption. N. Esfandiar et al.,52 selected three PAHs (anthracene, fluorene, and pyrene) for desorption experiments and found that PAHs were tightly bound to the organic matter, which could lead to low bioavailability. Therefore, it is necessary to investigate how to weaken the binding strength of PAHs to organic matter to promote their controlled desorption, and at the same time improve the bioavailability of PAHs in the aqueous phase.
3.2 Plant absorption
Plants in bioretention cells primarily use their root systems to absorb PAHs from the filler. Mechanisms by which PAHs enter the plant root system include ectoplasmic, commensal and transcellular transport.53,54 Ectoplasmic transport takes place outside the plasma membrane and usually occurs only in young tissues that have not formed a structural barrier.53 Solute transport in the coplasma pathway is mediated by intercellular junctions, which form a cytoplasmic continuum by bridging the cell walls of neighbouring cells. Transcellular transport is mainly used for water absorption and requires penetration of both plasma membranes of each cell layer. Among them, coplasma transport is the main pathway for PAH absorption in plant roots.55 X. Zhan et al.,54 found that more than 55% of the phenanthrene was taken up via commensal transport. In addition, in the case of complex contamination with PAHs and other pollutants, the other pollutants may modulate or alter the transport and transformation pathways of PAHs within the plant.56 The co-existence of heavy metals and PAHs has been shown to disrupt the integrity of crop root cells, thereby modulating the internal transport and transformation pathways of PAHs. For example, some studies have shown that coexisting Cu2+ ions can produce a synergistic effect through cation–π interactions when the Cu2+ concentration is ≤100 µmol L−1, which in turn promotes the absorption and accumulation of PAHs in spinach. And Cu2+ ions also caused cell membrane lipid peroxidation and reduced the absorption of PAHs in pumpkin.573.3 Biodegradation
The microorganisms that degrade PAHs in bioretention cells mainly include bacteria and fungi, and the biodegradation is carried out by the biological enzymes produced by these microorganisms.11 PAHs can be biodegraded in the bioretention cells by aerobic and anaerobic pathways,37 and the degradation pathways are shown in Fig. 2. Under aerobic conditions, PAHs are degraded mainly by the dioxygenase pathway and the cytochrome P450 monooxygenase pathway. The dioxygenase pathway mainly involves the decomposition of PAHs into cis-dihydrodiol by bacteria under the action of dioxygenase, and then the conversion of the coenzyme NAD+ into NADH + H+ under the action of dehydrogenase, and at the same time the generation of intermediate products catechols, which then undergo ring-opening in the neighbouring or interstitial position to achieve the degradation of PAHs.58 The cytochrome P450 monooxygenase pathway is used by bacteria and fungi to generate aryl hydrocarbon oxides; phenols are formed by non-enzymatic rearrangement of aryl hydrocarbon oxides, which then combined with glucose and glucuronic acid to form glycosides; additionally, aryl hydrocarbon oxides can be generated by epoxide hydrolases to produce trans-dihydrodiols.58 The terminal electron acceptors (TEAs) under anaerobic conditions include: sulphate, nitrate, CO2 and metal ions (e.g. Fe3+ and Mn4+). Sulfate-reducing bacteria use SO32− as an electron acceptor to gradually produce S2−; denitrifying bacteria achieve nitrogen removal via nitrate reduction (NO3− → NO2− → N2); and methanogenic bacteria use CO2 as an electron acceptor to produce CH4. Metal ion-reducing bacteria (e.g., Fe3+ → Fe2+, Mn4+ → Mn3+) drive the oxidation of organic matter by reducing high-valent metals.59 In bioretention cells, the degradation of PAHs occurs predominantly under aerobic conditions. In contrast, anaerobic conditions typically result in significantly reduced degradation efficiency.37 Consequently, within deeper filter layers where oxygen availability is limited, PAHs often resist effective degradation, leading to their accumulation and higher residual concentrations in these zones.37 Furthermore, the increasing depth exacerbates the scarcity of electron acceptors, which additionally constrains anaerobic biodegradation processes.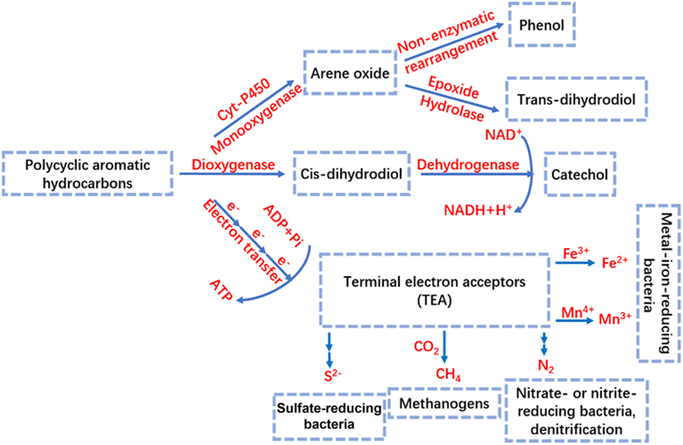 | ||
| Fig. 2 Aerobic and anaerobic degradation pathways of PAHs.58,59 | ||
4. Factors affecting PAH removal in bioretention cells
4.1 Hydrophobic properties of PAHs
K ow can be used to illustrate the partitioning of PAHs between water and organic matter in the filler, and its value increases as the molecular weight of PAHs increases.60 In bioretention cells, hydrophobic PAHs are more likely to be adsorbed by the filler through hydrophobic interaction due to their higher Kow.22 Higher Kow values indicate that PAHs are more inclined to migrate from the aqueous phase to the solid particulate matter, thus enhancing their tendency to adsorb on the filler surface.22,52 However, hydrophobic PAHs are generally more difficult to degrade.20 M. C. Leroy et al.,61 analysed the residues of PAHs in bioretention cell fillers contaminated with three types of PAHs (phenanthrene, pyrene and benzo(a)pyrene) and found that phenanthrene was the most rapidly degraded, taking about 150 days to degrade completely. In contrast, pyrene took about 350 days to degrade, while benzo(a)pyrene was not completely degraded, with 9 to 20% of benzo(a)pyrene still remaining in the filler after one year. Thus, hydrophobic PAHs, which are difficult to degrade biologically, are more likely to continue to accumulate in bioretention cells and reach dangerous levels. These substances not only persist for a long time, but also inhibit the activity of functional microorganisms, which in turn affects the degradation process of other organic compounds and nutrients.62 It is evident that the cumulative effect of highly hydrophobic PAHs and their potential ecological risks need to be given high priority.4.2 Types of plants
As an important part of the bioretention cells, plants can absorb PAHs directly from the filler. G. H. LeFevre et al.,23 compared the impact of bioretention cells with and without plants on naphthalene removal and found that naphthalene removal in the planted group was 93%, which was 15% higher than that in the unplanted group. It has been shown that the biodegradation of PAHs is enhanced in fillers with the existence of plants in comparison to unplanted fillers.63 Contaminants that enter plants are concentrated in plant roots or migrate elsewhere in the plant tissue. However, PAHs are significantly more abundant in the root system than in the leaves due to their hydrophobicity, and they are strongly trapped on the root surface, making them hard to be transported by the plant.49 Therefore, the removal efficiency of PAHs by plants is mainly related to the root system, and the key to successful inter-root remediation depends on the appropriate partnership of plants and microorganisms with degradation capacity.64,65 Plants with well-developed root systems take up higher levels of PAHs and provide more area for microbial growth. For example, W. Aprill et al.,66 pointed out that grasses in native prairies with deep roots had significantly higher rates of biodegradation of four PAHs in the soil than unvegetated conditions. In addition, plants can help soil aeration by increasing permeability and breaking up soil clods, favouring aerobic biodegradation of PAHs.67The plant type is an influential factor in the efficiency of pollutant removal. M. C. Leroy et al.,61 investigated the remediation effect of macrophytes (including cordgrass, yellow calamus, and greengrass) versus mixed herbaceous plants on soil contaminated with three polycyclic aromatic hydrocarbons (PAHs), namely, phenanthrene, pyrene, and benzo(a)pyrene, by comparing the remediation effect of these two plants, and found that yellow calamus showed a better remediation performance. The experimental data showed that the degradation kinetics of the three PAHs were most significant in the yellow calamus planting system: the half-life of phenanthrene was 77 days, pyrene was 79 days, and benzo(a)pyrene was 90 days, which were all faster than those of the other plant treatment groups. 14C tracer experiments reported in ref. 23 showed that the 14C concentration in the biomass of bluejoint sedge was about 3 times that of triticale, which indirectly reflected the higher naphthalene absorption capacity of bluejoint sedge than that of triticale.
These differences imply that the optimisation of phytoremediation in bioretention cells needs to take into account the physiological characteristics of the species, the function of the root system and the synergistic effect with microorganisms, and target the selection of highly absorptive or highly degrading plants in order to achieve a more efficient pollution treatment. The design should incorporate both deep-rooted and shallow-rooted plants to establish a vertical root architecture, enabling multi-layered interception and degradation of pollutants while enhancing the long-term operational efficiency and ecological sustainability of bioretention cells.
4.3 Microbiological functions
Microbes, as the core life components of the bioretention cell filler, drive vital ecological processes such as organic matter mineralisation, climate regulation and nutrient cycling, and achieve efficient degradation by metabolising PAHs as carbon and energy sources.68 Microbial community diversity is usually positively correlated with its degradation potential, and complex community structures not only synergistically metabolise a wide range of PAHs, but also enhance the stability of the system with the degradation rate.4.4 Filler parameters and selection
Filler parameters include filler particle size, filler water content, organic matter content, temperature and salinity. While adsorption is a superficial phenomenon, the adsorption capacity is correlated with the size of the filler.69,70 The smaller the size of the filler, the larger the specific surface area, the more adsorption active sites are available, and the larger the adsorption capacity.71 Comparative experiments reported in ref. 72 showed that the adsorption of naphthalene was higher for clay (102 mg) than for sand (91 mg) owing to the fact that clay has a larger specific surface area than sand. Y. Xu et al.,38 discovered that the average removal of naphthalene diminished with increasing particle size of the particles added to the bioretention cells. The water content of the filler is related to the dry period and rainfall intensity. The experimental data of ref. 38 showed that prolonging the dry period extended the removal efficiency of naphthalene from the filler. When the dry period increased from 2 to 8 days, the removal of naphthalene from the top filler increased from 81% to 87%, which indicated that moderate dry conditions could enhance the adsorption capacity of naphthalene on the filler. The filler organic matter is the main site for PAH adsorption. In general, fillers with high organic matter content not only have strong adsorption capacity for PAHs, but also can effectively reduce the migration and leaching of PAHs. A. Parajulee et al.,73 found that PAHs can be intensely adsorbed onto filler organic matter, which results in lower levels of particulate PAHs in runoff. In addition, changes in temperature and salinity can directly affect the solubility of PAHs, which in turn affects the removal efficiency of PAHs.Table 3 summarises the research results in recent years on the selection of different fillers for PAH removal in bioretention cells. The selection of filler types is crucial for the effective removal of PAHs in bioretention cells. It has been shown that a combination of sandy filler and organic matter can effectively remove PAHs from runoff. J. K. McIntyre et al.,74 achieved 95% removal of PAHs using 60% sand, 30% organic matter, and 10% water treatment residuals. C. J. Mitchell et al.,19 collected road runoff as a runoff source and achieved 97% removal of PAHs using 60% sand and 40% compost. J. K. McIntyre et al.,75 showed that the runoff PAH content was high in the near term (1311 µg L−1) after coal tar sealant was applied to asphalt pavements, but gradually decreased with the aging process (145 µg L−1). About 90% average removal of PAHs from these runoff sources was achieved with a composition of 60% sand and 40% organic matter, regardless of the influent ΣPAH concentration. BSM, a typical combination of sandy fill and organic matter, is currently receiving considerable attention for its ability to adsorb different pollutants. BSM is usually made from a mixture of loamy or sandy loam with organic matter such as mulch, compost, or wood chips.76 K. Zhang et al.,77 found that the removal of PAHs was maintained at a high level by using different ratios of BSM. It has been shown that the removal of PAHs can be significantly enhanced by adding modifiers to BSM. For example, N. Esfandiar et al.,76 demonstrated that incorporating coconut fibre and shredded waste tyre rubber into bioretention cells enhances adsorption capacity for naphthalene, anthracene, phenanthrene and pyrene. Notably, bioretention cells achieve higher removal rates for pyrene and phenanthrene, primarily due to their lower water solubility and bioavailability, enabling robust adsorption by the medium and reducing leaching by rainwater. X. Duan et al.,78 showed that BSM exhibited higher polycyclic aromatic hydrocarbon removal efficiency and stability when combined with water treatment residues, outperforming either BSM alone or pure soil. These studies provide new insights for realising PAH removal by BSM.
| Sites | Bioretention cell filler | Contaminant | Initial concentration | Removal rate (%) | References |
|---|---|---|---|---|---|
| Labs | Sand: 60% | Rainwater PAHs | 1311 µg L−1 | 90 | 75 |
| Compost: 40% | |||||
| Labs | Sand: 60% | Rainwater PAHs | 1.6 µg L−1 | 95 | 74 |
| Compost: 15% | |||||
| Bark: 15% | |||||
| Water treatment residues: 10% | |||||
| Labs | Soil: 100% | Naphthalene/fluorene/pyrene | 1400/300/300 µg L−1 | 80.7–82.1/81.3–83.7/83.3–85.5 | 78 |
| Soil: 63% | 80.5–89.9/87.6–96.8/92.4–93.8 | ||||
| Sand: 32% | 85.1–87.5/92.0–94.6/90.5–97.1 | ||||
| Sawdust: 5% | |||||
| Soil: 57% | |||||
| Sand: 28% | |||||
| Sawdust: 5% | |||||
| Water treatment residues: 10% | |||||
| Labs | Sand: 80% | Naphthalene/pyrene/acenaphthene/phenanthrene | 25/35/20/20 µg L−1 | >70/>99/>85/>99 | 76 |
| Silt: 10% | >85/>99/>95/>99 | ||||
| Clay: 6% | >95/>99/>95/>99 | ||||
| Compost: 4% | >70/>99/>80/>99 | ||||
| Sand: 76% | |||||
| Silt: 10% | |||||
| Clay: 5% | |||||
| Compost: 4% | |||||
| Waste tyre crumb rubber: 5% | |||||
| Sand: 76% | |||||
| Silt: 10% | |||||
| Clay: 5% | |||||
| Compost: 4% | |||||
| Coconut fibre: 5% | |||||
| Sand: 76% | |||||
| Silt: 10% | |||||
| Clay: 5% | |||||
| Compost: 4% | |||||
| Blast furnace slag: 5% | |||||
| Outdoor | Sand: 84.2% | Naphthalene/pyrene | 140/100 µg L−1 | 89.3/93.3 | 77 |
| Silt: 3.0% | 87.1/93.9 | ||||
| Clay: 12.8% | |||||
| Organic matter: 4.6% | |||||
| Sand: 96.0% | |||||
| Chalk: 0.8% | |||||
| Clay: 3.2% | |||||
| Organic matter: 0.4% | |||||
| Labs | Sand: 89% | Naphthalene/acenaphthene/phenanthrene/pyrene | 100 µg L−1 | 68–75/70–82/92–99/94–98 | 52 |
| Silt: 8% | |||||
| Clay: 3% | |||||
| Compost: 4% | |||||
| Labs | Sand: 57.1% | Naphthalene | 10 mg L−1 | 78–93 | 23 |
| Compost: 28.6% | |||||
| Soil: 14.3% | |||||
| Outdoor | Sand: 100% | Rainwater PAHs | 1.88/0.37 mg L−1 | 91.5/62.2 | 84 |
| Labs | Sand: 85.2% | Pyrene/phenanthrene/acenaphthene | 0.03 mg L−1 | >99/>99/>62.5 | 85 |
| Silt: 4.5% | |||||
| Activited carbon: 0.3% | |||||
| Zeolite: 10% | |||||
| Labs | Sand: 60% | Rainwater PAHs | 0.09–5.08 µg L−1 | >97 | 19 |
| Compost: 40% | >97 | ||||
| Sand: 60% | |||||
| Compost: 20% | |||||
| Biochar: 20% |
Biochar is a more environmentally friendly and cheaper material than activated carbon, with the cost per tonne ranging from $51 to $386, whereas activated carbon costs approximately $2200 per tonne.79 Biochar is typically produced from waste biomass as a raw material. In contrast, although activated carbon uses lignocellulosic materials as feedstock, it generally relies on primary resources such as directly harvested timber and coal, leading to significantly higher consumption of virgin natural resources in its production.80 It is now extensively utilised for bioretention cell filler improvement due to its high adsorption, porous structure and promotion of microbial growth.71 PAH molecules can diffuse into the pores of the uncarbonised portion of the biochar, where they can directly interact with PAHs and cause adsorption.81 Furthermore, the adsorption capacity of biochar for certain hydrophobic organic compounds is significantly higher than that of filler organic matter. However, filler organic matter may compete with PAH molecules for the available active sites of biochar. L. Kong et al.,82 produced wheat straw-derived biochar for remediation of PAH-contaminated soils at a pyrolysis temperature of 500 °C. The results showed that there was no significant adsorption in soils with 4.69% and 6.98% organic matter content, while higher adsorption potential was observed in soils with 1.82% organic matter content. When selecting a composite filler containing organic matter components and biochar for PAH removal, the organic matter content of the filler can be appropriately reduced. According to Section 4.1, hydrophobic PAHs tend to accumulate in the bioretention cells and thus are prone to ecological risks, and recent studies have reported that the addition of biochar will promote the biodegradation of hydrophobic PAHs in the filler by providing a better habitat for the filler microorganisms.22 Therefore, biochar has more significant advantages as a filler for bioretention cells. The properties of biochar can be modulated by the type of feedstock and reaction conditions (i.e. temperature, heating rate, residence time and reactor type). Among them, the feedstock type and the pyrolysis temperature of the reaction conditions have the greatest influence on the removal of PAHs.80 Due to different feedstock types and pyrolysis temperatures during preparation, biochar produces different amounts of PAHs, and these inherent PAHs can be emitted with the leaching of hydrophobic organics and metals, which may affect the removal of PAHs from bioretention cells.83 X. Chen et al.,83 found that the content of PAHs in biochar from sewage sludge increased and then fell with increasing pyrolysis temperature, while the leaching potential of PAHs increased. However, less research has been done on the leaching of PAHs from biochar with different pyrolysis temperatures and feedstock types.
With regard to the pollution characteristics of PAHs in regional stormwater runoff, optimisation of filler selection is essential to enhance the removal efficiency. Future research can concentrate on the preparation of composite fillers (e.g., combination of BSM and biochar) and low leachability biochar. In addition, the synergistic removal efficacy of the biochar-enhanced bioretention system on dissolved and particulate PAHs needs to be verified through pilot experiments to promote practical applications.
4.5 Climate and seasonal variations
Climate change directly impacts the pollutant removal performance of constructed bioretention cells by altering precipitation frequency and intensity. Empirical support for this is provided by ref. 76, which evaluated the removal efficiency of typical PAHs such as naphthalene and anthracene in bioretention cells under varying rainfall intensities (light, moderate, and heavy rain). The results indicate that removal rates under heavy rain conditions were significantly reduced, irrespective of the type of the filler material. In addition to hydrological conditions, temperature variation constitutes a crucial factor within climatic and seasonal evolution. Generally, temperature fluctuations exert a relatively minor influence on the adsorption processes of PAHs within bioretention cells, yet they significantly impact the biodegradation process.60 Microbial activity and metabolic rates are highly dependent on temperature conditions: within suitable ranges, elevated temperatures typically promote microbial degradation of PAHs. Conversely, in low-temperature environments, microbial activity becomes constrained, leading to diminished biodegradation efficiency.5. Effect of PAH accumulation on bioretention cell performance
5.1 Effects on plant growth
PAHs can affect plant growth and primary metabolic processes (e.g., photosynthesis). Photosynthesis is considered a susceptible marker of plant stress.86 It has been found that estimation of the photosynthetic rate is beneficial for evaluating the potential toxic impacts of exogenous pollutants, including PAHs, on plants.87 PAHs inhibit photosynthesis in plants, mainly by interfering with the electron transport chain and reducing PSII action.88–90 For example, R. S. Tomar et al.,86 found that the process of wheat photosynthesis was inhibited by fluoranthene and that the inhibitory effect of fluoranthene on the dark reaction was more pronounced than that on the light reaction. G. J. Ahammed et al.,91 found that the rate of CO2 assimilation (14 µmol m−2 s−1), stomatal conductance (0.2 mmol m−2 s−1), and intercellular CO2 concentration (275 µmol mol−1) were lower than those of the control. PAHs inhibit several growth stages in plants. R. S. Tomar et al.,86 showed that fluorescent anthracene resulted in a significant reduction in the length of roots and shoots of wheat seedlings 5 days after germination (20% reduction in root length and 18% reduction in stem length). Accumulation of PAHs also reduces biomass production in plants. G. J. Ahammed et al.,91 found that the lowest phenanthrene concentration (30 µM) in a variety of vegetables exposed to different concentrations of phenanthrene reduced biomass production. G. J. Ahammed et al.,91 compared the biomass production of several vegetables exposed to different phenanthrene concentrations and found that at the lowest phenanthrene concentration (30 µM), stem dry weights of Chinese cabbage, cucumber and lettuce were reduced by 28, 19 and 17%, respectively, and that biomass decreased as the concentration of phenanthrene increased. In addition, the accumulation of PAHs induces oxidative stress through the overproduction of reactive oxygen species, which may cause cellular damage and death (Fig. 3).925.2 Effects on microorganisms
Microorganisms are one of the sensitive indicators of response to PAH contamination in bioretention cells.21 The entry of PAHs into the bioretention cells will have a large impact on microbial functions and community structure. In particular, PAHs affect processes such as carbon, nitrogen, sulphur and phosphorus cycling in microbial systems.93 G. Chai et al.,22 found that the relative abundance of nitrifying bacteria (i.e., Nitrosomonas and Nitrospiraclea) was lower and continued to decrease over time in the pyrene-exposed group. The accumulation of PAHs also caused a reduction in microbial community diversity.78 Y. Zhu et al.,93 found that in aerobic environments, PAHs were more toxic to most C, N, P, and S cycling functional microorganisms, whereas in anaerobic environments, PAHs probably enhanced the activity of PAH-degrading bacteria with SO42−, CO2/HCO3− and NO3− as electron acceptors to varying degrees, thus promoting coupled sulfur reduction, methanogenesis and denitrification by PAH-degrading bacteria. The long-term accumulation of PAHs also led to a significant reduction in the diversity of the microbial community and selective pressure to screen out dominant populations with degradation functions (such as Ascomycetes, Mycobacteria, and Thick-walled Mycobacteria), which altered the structural characteristics of the community.11Once accumulated in the filler, PAHs affect not only the soil microbial community structure but also vital microbial functions.94,95 Studies have shown that microbial functions are more sensitive to environmental perturbations than the community structure.96 So far, most of the microbial studies on PAH removal in bioretention cells have been on the community structure and diversity, while microbial functions have been less studied.
5.3 Effects on the removal of other pollutants
The accumulation of PAHs in bioretention cells not only affects their own purification efficacy, but also interferes with the removal process of other pollutants through multiple mechanisms. It was found that 100 mg L−1 naphthalene or anthracene mono-treatment inhibited wheat seedling growth, while the co-existence of microplastics (MPs) could partially counteract the toxicity of PAHs by reducing oxidative stress and protecting photosynthetic efficiency.97 S. H. Liu et al.,98 demonstrated that organic pollutants and heavy metals in fillers co-exist as compound contaminants, interacting through competitive adsorption, redox processes, and microbial functional inhibition. The occurrence of PAHs in the bioretention system would lead to a decrease in the nitrogen removal capacity of the system.21 In addition, the occurrence of PAHs in bioretention cells inhibits the nitrogen removal rate of the system. G. Chai et al.,22 found that the removal efficiency of total nitrogen in the bioretention cells showed a gradual increase without the addition of pyrene. However, when the system was exposed to pyrene contamination, its total nitrogen removal performance showed a significant decrease. The experimental data showed that the average effluent total nitrogen concentration in the pyrene-exposed group reached 3.35–3.66 mg L−1, while the control group maintained a relatively stable effluent total nitrogen concentration (1.78–2.83 mg L−1). In contrast, the effect of PAHs on phosphorus removal was more limited, mainly because phosphorus retention mainly relies on adsorption and precipitation, while microbial uptake only accounts for a small part, so the accumulation of PAHs has a smaller effect on the overall total phosphorus removal performance of the bioretention cells.20 There are fewer studies on the interaction mechanism between PAHs and other pollutants and the effect on PAH removal during co-pollution. To sum up, the impact of PAHs on bioretention cells is complex and may limit their purification efficacy, but in some cases may also enhance it.6. Conclusions
With the rapid development of industrialisation and urbanisation, PAHs, as a typical persistent organic pollutant, have received increasing attention for their environmental behaviour and ecological risks. Under the flushing effect of rainfall runoff, PAHs accumulated in the environment will migrate and diffuse with surface runoff, thus posing a potential threat to the water environment. Bioretention cells can make PAHs less hazardous by retaining them. It is essential to clarify the pollution sources of PAHs in urban runoff and their environmental attribution, which is a key link to improve the effectiveness of urban water environment management. Analyses of existing studies revealed that:(1) The main sources of PAHs in stormwater runoff include traffic emissions, industrial activities, residential heating, and atmospheric deposition, with concentrations typically on the ng and µg scale. Average PAH concentrations are higher in cities than in suburban and rural areas. In urban areas, city centres have the highest average PAH concentrations among different functional zones, and average PAH concentrations are usually higher in industrial and commercial areas than in other functional areas such as residential areas. PAHs tend to bind to colloids, dissolved organic matter, or suspended particles in stormwater runoff and are eventually deposited in sediments. The occurrence of PAHs in stormwater runoff can be categorised as net deposition, equilibrium and net diffusion.
(2) PAHs in stormwater are mainly adsorbed on particles into the bioretention cells. The removal of PAHs is achieved by adsorption on fillers, biodegradation, plant adsorption and volatilisation, with adsorption and biodegradation being the main removal mechanisms. In the short term, adsorption is the main mechanism for removing PAHs in the bioretention cells, and the adsorption capacity of the filler for highly hydrophobic PAHs is better than that for low hydrophobicity PAHs. In the long term, PAHs enriched in the bioretention cells will be further removed by biodegradation. The plant and soil environments promote the removal of PAHs by indirectly regulating the activity of enzymes related to the decomposition of PAHs, mainly by influencing the microbial community in the soil. In contrast to adsorption, microbial biodegradation of low hydrophobic PAHs is usually stronger than that of high hydrophobic PAHs.
(3) Factors affecting the removal of PAHs mainly include hydrophobic properties of PAHs, plant type, microorganisms and filler parameters and selection. In bioretention cells, hydrophobic PAHs are more likely to be adsorbed by the filler through hydrophobic interaction due to their higher Kow. The removal efficiency of PAHs varies among different types of plants and the removal efficiency of PAHs is mainly related to the root system, and the key to successful inter-root remediation depends on the appropriate partnership of plants and microorganisms with degradation capacity. Microorganisms achieve efficient degradation by metabolising PAHs as carbon and energy sources. In addition, filler parameters (filler particle size, filler water content, and filler temperature) and selection affect the removal of PAHs. The average removal of PAHs by different types of fillers exceeded 80%. Most of the biochar fillers showed an increase and then a decrease in PAH content with increasing pyrolysis temperature.
(4) PAHs can affect plant growth, oxidative stress, and primary metabolic processes (e.g., photosynthesis). PAHs cause a reduction in the diversity of microbial communities and the selection of dominant populations with degrading functions, and affect processes such as cycling of carbon, nitrogen, sulphur, and phosphorus in microbial systems. For other pollutants, PAHs interfere with the removal of other pollutants through multiple mechanisms.
(5) In practical applications, the construction of the filter layer is fundamental. It is recommended to use highly efficient and cost-effective materials (such as biochar) in combination with BSM to ensure pollutant removal efficiency while controlling costs. For plant selection, priority should be given to native, well-adapted, and stress-resistant species to reduce costs. Additionally, deep-rooted and shallow-rooted plants should be cultivated together to create a vertical root spatial structure, enhancing long-term purification performance through strengthened rhizosphere degradation and reducing maintenance requirements. Furthermore, long-term operational maintenance measures must be implemented, including regular inspection of filter media clogging, monitoring effluent quality changes, and timely replacement or regeneration of surface media when adsorption saturation or performance decline occurs. This ensures the stable operation of the bioretention cells throughout its entire lifecycle.
7. Challenges and future research directions
Although bioretention cells have shown potential for removing PAHs in stormwater runoff, their practical application still faces several key challenges that limit further improvements in treatment performance and engineering-scale deployment. The primary challenges currently include:(1) The migration and transformation behaviour of PAHs within the system is complex, with degradation pathways yet to be fully elucidated, potentially leading to the accumulation of toxic intermediates.
(2) The functionality of biochar as an adsorbent material is significantly influenced by its feedstock and pyrolysis conditions, and may pose a risk of PAH leaching during long-term operation.
(3) Research into the degradation capacity of microbial communities towards PAHs remains insufficient, limiting process optimisation based on microbial regulation.
(4) In actual stormwater runoff, PAHs frequently coexist with other pollutants. Their combined pollution effects and the mechanisms influencing PAH removal have yet to be systematically elucidated.
To address the aforementioned challenges, future research should focus on the following innovative directions:
(1) It is necessary to conduct an in-depth analysis of the environmental behaviour and degradation mechanisms of PAHs within bioretention systems. Firstly, the relative contributions of different pathways (such as the dioxygenase and P450 monooxygenase pathways) to total degradation should be quantified, clarifying the primary pathways operating within bioretention basins under specific environmental conditions (e.g., alternating wet and dry states and varying depths). Secondly, integrating non-targeted analysis with stable isotope tracing techniques should elucidate PAH degradation pathways and intermediate product formation patterns, providing theoretical foundations for mitigating secondary pollution risks.
(2) Novel biochar materials should be developed by optimising adsorption properties through controlled pyrolysis conditions and surface modifications. Assessing PAH leaching potential from biochar under diverse environmental conditions will support safe material application.
(3) Research at the microbial functional level should be intensified to elucidate the roles of key functional genes, enzymes, and metabolic pathways in PAH degradation, thereby providing novel strategies for enhancing system performance through microbial augmentation.
(4) Attention must be directed towards PAH removal mechanisms in composite pollution scenarios, with a focus on investigating interactions between PAHs and typical pollutants such as heavy metals and microplastics, and their impact on the comprehensive removal efficacy of bioretention systems. This will facilitate the development of synergistic purification technologies suitable for complex water quality conditions.
Conflicts of interest
There are no conflicts to declare.Data availability
All data relevant to our study are available within the manuscript.Supplementary information is available. See DOI: https://doi.org/10.1039/d5em00440c.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 52070152). The authors also appreciate Shiyi Hu at the Instrument Analysis Center of Xi'an University of Architecture and Technology (China) for assistance.Notes and references
- H. I. Abdel-Shafy and M. S. M. Mansour, A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation, Egypt. J. Pet., 2016, 25(1), 107–123 CrossRef.
- A. B. Patel, S. Shaikh, K. R. Jain, C. Desai and D. Madamwar, Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches, Front. Microbiol., 2020, 11, 562813 CrossRef.
- E. Reizer, B. Viskolcz and B. Fiser, Formation and growth mechanisms of polycyclic aromatic hydrocarbons: A mini-review, Chemosphere, 2022, 291, 132793 CrossRef CAS PubMed.
- L. Zhang, L. Yang, Q. Zhou, X. Zhang, W. Xing and Y. Wei, et al., Size distribution of particulate polycyclic aromatic hydrocarbons in fresh combustion smoke and ambient air: A review, J. Environ. Sci., 2020, 88, 370–384 CrossRef CAS PubMed.
- J. L. Crane, Source Apportionment and Distribution of Polycyclic Aromatic Hydrocarbons, Risk Considerations, and Management Implications for Urban Stormwater Pond Sediments in Minnesota, USA, Arch. Environ. Contam. Toxicol., 2014, 66(2), 176–200 CrossRef CAS PubMed.
- N. David, J. E. Leatherbarrow, D. Yee and L. J. McKee, Removal Efficiencies of a Bioretention System for Trace Metals, PCBs, PAHs, and Dioxins in a Semiarid Environment, J. Environ. Eng., 2015, 141(6), 04014092 CrossRef.
- S. K. Prajapati and B. D. Tripathi, Biomonitoring seasonal variation of urban air polycyclic aromatic hydrocarbons (PAHs) using Ficus benghalensis leaves, Environ. Pollut., 2008, 151(3), 543–548 CrossRef CAS PubMed.
- Z. Zhang, J. Li, Y. Li, L. Zhao and X. Duan, Accumulation of polycyclic aromatic hydrocarbons in the road green infrastructures of sponge city in Northwestern China: Distribution, risk assessments and microbial community impacts, J. Cleaner Prod., 2022, 350, 131494 CrossRef.
- G. Gbeddy, P. Egodawatta, A. Goonetilleke, E. Akortia and E. T. Glover, Influence of photolysis on source characterization and health risk of polycyclic aromatic hydrocarbons (PAHs), and carbonyl-, nitro-, hydroxy- PAHs in urban road dust, Environ. Pollut., 2021, 269, 116103 CrossRef CAS PubMed.
- A. Jafarzadeh, A. Matta, S. V. Moghadam, K. K. Vadde, S. Dessouky and J. Hutchinson, et al., Assessing the removal of heavy metals and polycyclic aromatic hydrocarbons and occurrence of metal resistance genes and antibiotic resistance genes in a stormwater bioretention system, Chemosphere, 2024, 364, 143043 CrossRef CAS PubMed.
- Y. J. Li, Y. T. Shi, Z. L. zhi, L. Y. fang, J. P. Wang and L. J. ke, Study of polycyclic aromatic hydrocarbons accumulation in bioretention facilities and its influence on microbial community structure, Environ. Sci. Pollut. Res., 2023, 30(44), 100165–100187 CrossRef CAS PubMed.
- T. N. T. Nguyen, M. K. Park, J. M. Son and S. D. Choi, Spatial distribution and temporal variation of polycyclic aromatic hydrocarbons in runoff and surface water, Sci. Total Environ., 2021, 793, 148339 CrossRef CAS.
- C. H. Shi, B. B. He, J. L. Zhao, Y. H. Liu and A. Liu, Characterising polycyclic aromatic hydrocarbons in road dusts and stormwater in urban environments, Environ. Monit. Assess., 2024, 196(9), 791 CrossRef CAS PubMed.
- M. A. Mallah, L. Changxing, M. A. Mallah, S. Noreen, Y. Liu and M. Saeed, et al., Polycyclic aromatic hydrocarbon and its effects on human health: An overeview, Chemosphere, 2022, 296, 133948 CrossRef CAS.
- M. Brzezinski, L. Martin, K. Simpson, K. Lu, N. Gan and C. Huang, et al., Photodegradation enhances the toxic effect of anthracene on skin, J. Hazard. Mater., 2024, 471, 134386 CrossRef CAS PubMed.
- B. Bukowska, K. Mokra and J. Michałowicz, Benzo[a]pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity, Int. J. Mater. Sci., 2022, 23(11), 6348 CAS.
- K. Sun, Y. Song, F. He, M. Jing, J. Tang and R. Liu, A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics, Sci. Total Environ., 2021, 773, 145403 CrossRef CAS PubMed.
- K. Björklund and L. Li, Removal of organic contaminants in bioretention medium amended with activated carbon from sewage sludge, Environ. Sci. Pollut. Res., 2017, 24(23), 19167–19180 CrossRef PubMed.
- C. J. Mitchell, A. D. Jayakaran and J. K. McIntyre, Biochar and fungi as bioretention amendments for bacteria and PAH removal from stormwater, J. Environ. Manage., 2023, 327, 116915 CrossRef CAS.
- D. Q. Wang, G. D. Chai, J. Q. Shan, Z. J. Yang, H. E. Li and J. K. Li, et al., Impact of pyrene on pollutant removal and microbial enzyme activities in bioretention systems, IOP Conf. Ser. Earth Environ. Sci., 2018, 191, 012108 CrossRef.
- Z. Zhang, J. Li, H. Wang, Y. Li and X. Duan, Impact of co-contamination by PAHs and heavy metals on micro-ecosystem in bioretention systems with soil, sand, and water treatment residuals, J. Cleaner Prod., 2023, 383, 135417 CrossRef CAS.
- G. Chai, D. Wang, J. Shan, C. Jiang, Z. Yang and E. Liu, et al., Accumulation of high-molecular-weight polycyclic aromatic hydrocarbon impacted the performance and microbial ecology of bioretention systems, Chemosphere, 2022, 298, 134314 CrossRef CAS PubMed.
- G. H. LeFevre, P. J. Novak and R. M. Hozalski, Fate of Naphthalene in Laboratory-Scale Bioretention Cells: Implications for Sustainable Stormwater Management, Environ. Sci. Technol., 2012, 46(2), 995–1002 CrossRef CAS PubMed.
- Z. Han, J. Xiong, J. Zhou, Z. Wang, T. Hu and J. Xu, Microplastics removal from stormwater runoff by bioretention cells: A review, J. Environ. Sci., 2025, 154, 73–90 CrossRef CAS PubMed.
- C. Pies, B. Hoffmann, J. Petrowsky, Y. Yang, T. A. Ternes and T. Hofmann, Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils, Chemosphere, 2008, 72(10), 1594–1601 CrossRef CAS PubMed.
- M. B. Yunker, R. W. Macdonald, R. Vingarzan, R. H. Mitchell, D. Goyette and S. Sylvestre, PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition, Org. Geochem., 2002, 33(4), 489–515 CrossRef CAS.
- K. Ravindra, R. Sokhi and R. Vangrieken, Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation, Atmos. Environ., 2008, 42(13), 2895–2921 CrossRef CAS.
- R. J. De La Torre-Roche, W. Y. Lee and S. I. Campos-Díaz, Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: Analysis of a potential problem in the United States/Mexico border region, J. Hazard. Mater., 2009, 163(2–3), 946–958 CrossRef CAS.
- W. Zhang, S. Zhang, C. Wan, D. Yue, Y. Ye and X. Wang, Source diagnostics of polycyclic aromatic hydrocarbons in urban road runoff, dust, rain and canopy throughfall, Environ. Pollut., 2008, 153(3), 594–601 CrossRef CAS.
- G. Vuković, M. A. Urošević, M. Pergal, M. Janković, Z. Goryainova and M. Tomašević, et al., Residential heating contribution to level of air pollutants (PAHs, major, trace, and rare earth elements): a moss bag case study, Environ. Sci. Pollut. Res., 2015, 22(23), 18956–18966 CrossRef.
- M. Tobiszewski and J. Namieśnik, PAH diagnostic ratios for the identification of pollution emission sources, Environ. Pollut., 2012, 162, 110–119 CrossRef CAS.
- K. Arole, M. Velhal, M. Tajedini, P. G. Xavier, E. Bardasz and M. J. Green, et al., Impacts of particles released from vehicles on environment and health, Tribol. Int., 2023, 184, 108417 CrossRef CAS.
- B. K. Lee and T. T. T. Dong, Effects of road characteristics on distribution and toxicity of polycyclic aromatic hydrocarbons in urban road dust of Ulsan, Korea, J. Hazard. Mater., 2010, 175(1–3), 540–550 CrossRef CAS.
- P. R. N. Fernandes, S. D. A. Soares, R. F. Nascimento, J. B. Soares and R. M. Cavalcante, Evaluation of Polycyclic Aromatic Hydrocarbons in Asphalt Binder Using Matrix Solid-Phase Dispersion and Gas Chromatography, J. Chromatogr. Sci., 2009, 47(9), 789–793 CAS.
- J. Su, P. Gao, S. J. Laux, L. Q. Ma and T. G. Townsend, Contribution of Asphalt Products to Total and Bioaccessible Polycyclic Aromatic Hydrocarbons, Int. J. Environ. Res., 2019, 13(3), 499–509 CrossRef CAS.
- M. U. Ali, L. Siyi, B. Yousaf, Q. Abbas, R. Hameed and C. Zheng, et al., Emission sources and full spectrum of health impacts of black carbon associated polycyclic aromatic hydrocarbons (PAHs) in urban environment: A review, Crit. Rev. Environ. Sci. Technol., 2021, 51(9), 857–896 CrossRef.
- C. Yuan, A. P. Davis, D. Kaya and B. V. Kjellerup, Distribution and biodegradation potential of polycyclic aromatic hydrocarbons (PAHs) accumulated in media of a stormwater bioretention, Chemosphere, 2023, 336, 139188 CrossRef CAS.
- Y. Xu, H. Li, X. Zhang, X. Bai, L. Wu and C. Tan, et al., Removal, migration, and distribution of naphthalene in bioretention facilities: the influences of particulate matter, Environ. Sci. Pollut. Res., 2023, 30(16), 46940–46949 CrossRef CAS.
- L. Herngren, A. Goonetilleke, G. A. Ayoko and M. M. M. Mostert, Distribution of polycyclic aromatic hydrocarbons in urban stormwater in Queensland, Australia, Environ. Pollut., 2010, 158(9), 2848–2856 CrossRef CAS PubMed.
- C. Wang, S. Wu, S. Zhou, H. Wang, B. Li and H. Chen, et al., Polycyclic aromatic hydrocarbons in soils from urban to rural areas in Nanjing: Concentration, source, spatial distribution, and potential human health risk, Sci. Total Environ., 2015, 527–528, 375–383 CrossRef CAS PubMed.
- K. Ciarkowska, F. Gambus, J. Antonkiewicz and T. Koliopoulos, Polycyclic aromatic hydrocarbon and heavy metal contents in the urban soils in southern Poland, Chemosphere, 2019, 229, 214–226 CrossRef CAS.
- T. Mihankhah, M. Saeedi and A. Karbassi, Contamination and cancer risk assessment of polycyclic aromatic hydrocarbons (PAHs) in urban dust from different land-uses in the most populated city of Iran, Ecotoxicol. Environ. Saf., 2020, 187, 109838 CrossRef CAS.
- Z. Wu, Y. Duan, L. Liu, L. Xu, X. Yao and X. Chen, Characteristics of polycyclic aromatic hydrocarbons in the soils of different functional areas of a typical industrial capital city, Taiyuan, Shanxi province, China, J. Soils Sediments, 2023, 23(3), 1315–1331 CrossRef CAS.
- Y. Jiang, U. J. Yves, H. Sun, X. Hu, H. Zhan and Y. Wu, Distribution, compositional pattern and sources of polycyclic aromatic hydrocarbons in urban soils of an industrial city, Lanzhou, China, Ecotoxicol. Environ. Saf., 2016, 126, 154–162 CrossRef CAS PubMed.
- M. J. Ehigbor, C. M. A. Iwegbue, O. I. Eguavoen, G. O. Tesi and B. S. Martincigh, Occurrence, sources and ecological and human health risks of polycyclic aromatic hydrocarbons in soils from some functional areas of the Nigerian megacity, Lagos, Environ. Geochem. Health, 2020, 42(9), 2895–2923 CrossRef CAS.
- Y. Sun, Z. Xie, K. Wu, J. Lan, T. Li and D. Yuan, Speciation, distribution and migration pathways of polycyclic aromatic hydrocarbons in a typical underground river system in Southwest China, J. Hydrol., 2021, 596, 125690 CrossRef CAS.
- S. Zhang, X. Xing, H. Yu, M. Du, Y. Zhang and P. Li, et al., Fate of polycyclic aromatic hydrocarbon (PAHs) in urban lakes under hydrological connectivity: A multi-media mass balance approach, Environ. Pollut., 2025, 366, 125556 CrossRef CAS PubMed.
- H. Liu, F. Qiu, M. Gao, Y. Che, C. Tan and Z. Zhang, et al., Migration and adsorption of naphthalene in road-deposited sediments from stormwater runoff: Impact of the particle size, Sci. Total Environ., 2023, 904, 166673 CrossRef CAS PubMed.
- X. Duan, J. Li and Y. Li, The fate of three typical persistent organic pollutants in bioretention columns as revealed by stable carbon isotopes, Chemosphere, 2023, 334, 138996 CrossRef CAS.
- C. J. DiBlasi, H. Li, A. P. Davis and U. Ghosh, Removal and Fate of Polycyclic Aromatic Hydrocarbon Pollutants in an Urban Stormwater Bioretention Facility, Environ. Sci. Technol., 2009, 43(2), 494–502 CrossRef CAS PubMed.
- N. Esfandiar, R. Suri and E. R. McKenzie, Simultaneous removal of multiple polycyclic aromatic hydrocarbons (PAHs) from urban stormwater using low-cost agricultural/industrial byproducts as sorbents, Chemosphere, 2021, 274, 129812 CrossRef CAS.
- N. Esfandiar and E. R. McKenzie, Bioretention soil capacity for removing nutrients, metals, and polycyclic aromatic hydrocarbons; roles of co-contaminants, pH, salinity and dissolved organic carbon, J. Environ. Manage., 2022, 324, 116314 CrossRef CAS.
- A. P. Schwab and C. L. Dermody, Pathways of polycyclic aromatic hydrocarbons assimilation by plants growing in contaminated soils, in Advances in Agronomy, Elsevier, 2021, pp. 193–250, Available from: https://linkinghub.elsevier.com/retrieve/pii/S0065211321000353 Search PubMed.
- X. Zhan, M. Zhu, Y. Shen, L. Yue, J. Li and J. L. Gardea-Torresdey, et al., Apoplastic and symplastic uptake of phenanthrene in wheat roots, Environ. Pollut., 2018, 233, 331–339 CrossRef CAS PubMed.
- J. Zhu, R. Chen, C. Huang, J. Wang and X. Zhan, Exogenous auxin alters the polycyclic aromatic hydrocarbons apoplastic and symplastic uptake by wheat seedling roots, Environ. Pollut., 2024, 343, 123112 CrossRef CAS.
- S. Li, Z. Jiang and S. Wei, Interaction of heavy metals and polycyclic aromatic hydrocarbons in soil-crop systems: The effects and mechanisms, Environ. Res., 2024, 263, 120035 CrossRef CAS PubMed.
- M. Lu, Z. Z. Zhang, X. L. Su, Y. X. Xu, X. J. Wu and M. Zhang, Effect of copper on in vivo fate of BDE-209 in pumpkin, J. Hazard. Mater., 2013, 262, 311–317 CrossRef CAS PubMed.
- A. K. Haritash and C. P. Kaushik, Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review, J. Hazard. Mater., 2009, 169(1–3), 1–15 CrossRef CAS PubMed.
- C. Chen, Z. Zhang, P. Xu, H. Hu and H. Tang, Anaerobic biodegradation of polycyclic aromatic hydrocarbons, Environ. Res., 2023, 223, 115472 CrossRef CAS PubMed.
- C. Akdeniz, Z. H. Yu and E. Passeport, Adsorption and desorption of naphthalene in bioretention cells under cold climate conditions, Ecol. Eng., 2021, 169, 106308 CrossRef.
- M. C. Leroy, M. Legras, S. Marcotte, V. Moncond'huy, N. Machour and F. Le Derf, et al., Assessment of PAH dissipation processes in large-scale outdoor mesocosms simulating vegetated road-side swales, Sci. Total Environ., 2015, 520, 146–153 CrossRef CAS PubMed.
- Z. Zhang, J. Li, Y. Li, L. Zhao and X. Duan, Accumulation of polycyclic aromatic hydrocarbons in the road green infrastructures of sponge city in Northwestern China: Distribution, risk assessments and microbial community impacts, J. Cleaner Prod., 2022, 350, 131494 CrossRef.
- S. L. Afegbua and L. C. Batty, Effect of single and mixed polycyclic aromatic hydrocarbon contamination on plant biomass yield and PAH dissipation during phytoremediation, Environ. Sci. Pollut. Res., 2018, 25(19), 18596–18603 CrossRef CAS PubMed.
- A. C. Agnello, M. Bagard, E. D. Van Hullebusch, G. Esposito and D. Huguenot, Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation, Sci. Total Environ., 2016, 563–564, 693–703 CrossRef CAS PubMed.
- S. Eskandary, A. Tahmourespour, M. Hoodaji and A. Abdollahi, The synergistic use of plant and isolated bacteria to clean up polycyclic aromatic hydrocarbons from contaminated soil, J. Environ. Health Sci. Eng., 2017, 15(1), 12 CrossRef CAS PubMed.
- W. Aprill and R. C. Sims, Evaluation of the use of prairie grasses for stimulating polycyclic aromatic hydrocarbon treatment in soil, Chemosphere, 1990, 20(1–2), 253–265 CrossRef CAS.
- S. Gitipour, G. A. Sorial, S. Ghasemi and M. Bazyari, Treatment technologies for PAH-contaminated sites: a critical review, Environ. Monit. Assess., 2018, 190(9), 546 CrossRef PubMed.
- X. Zhang, Y. Li, D. Ouyang, J. Lei, Q. Tan and L. Xie, et al., Systematical review of interactions between microplastics and microorganisms in the soil environment, J. Hazard. Mater., 2021, 418, 126288 CrossRef CAS PubMed.
- A. H. El-Sheikh, A. P. Newman, H. Al-Daffaee, S. Phull, N. Cresswell and S. York, Deposition of anatase on the surface of activated carbon, Surf. Coat. Technol., 2004, 187(2–3), 284–292 CrossRef CAS.
- A. Naeem, P. Westerhoff and S. Mustafa, Vanadium removal by metal (hydr)oxide adsorbents, Water Res., 2007, 41(7), 1596–1602 CrossRef CAS PubMed.
- S. Lamichhane, K. C. Bal Krishna and R. Sarukkalige, Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review, Chemosphere, 2016, 148, 336–353 CrossRef CAS.
- C. N. Owabor, S. E. Agarry, B. V. Ayodele, I. S. Udeh and E. Ehiosun, Comparative Study of the Adsorption and Desorption Behavior of Single and Multi-Ring Aromatics in Sediment Fractions, Adv. Chem. Eng. Sci., 2013, 03(01), 67–73 CrossRef.
- A. Parajulee, Y. D. Lei, A. Kananathalingam, D. S. McLagan, C. P. J. Mitchell and F. Wania, The transport of polycyclic aromatic hydrocarbons during rainfall and snowmelt in contrasting landscapes, Water Res., 2017, 124, 407–414 CrossRef CAS PubMed.
- J. K. McIntyre, J. W. Davis, J. P. Incardona, J. D. Stark, B. F. Anulacion and N. L. Scholz, Zebrafish and clean water technology: Assessing soil bioretention as a protective treatment for toxic urban runoff, Sci. Total Environ., 2014, 500–501, 173–180 CrossRef CAS.
- J. K. McIntyre, R. C. Edmunds, B. F. Anulacion, J. W. Davis, J. P. Incardona and J. D. Stark, et al., Severe Coal Tar Sealcoat Runoff Toxicity to Fish Is Prevented by Bioretention Filtration, Environ. Sci. Technol., 2016, 50(3), 1570–1578 CrossRef CAS PubMed.
- N. Esfandiar, R. Suri and E. R. McKenzie, Evaluation of sorbent amendments used with stormwater management practices to remove contaminants: Impacts of rainfall intensity and antecedent dry periods, Sci. Total Environ., 2024, 906, 167766 CrossRef CAS PubMed.
- K. Zhang, A. Randelovic, D. Page, D. T. McCarthy and A. Deletic, The validation of stormwater biofilters for micropollutant removal using in situ challenge tests, Ecol. Eng., 2014, 67, 1–10 CrossRef.
- X. Duan, J. Li, Y. Li, Y. Xu, H. Chai and S. Chao, Removal, accumulation, and micro-ecosystem impacts of typical POPs in bioretention systems with different media: A runoff infiltration study, Sci. Total Environ., 2024, 946, 174278 CrossRef CAS PubMed.
- A. Brennan, E. Moreno Jiménez, J. A. Alburquerque, C. W. Knapp and C. Switzer, Effects of biochar and activated carbon amendment on maize growth and the uptake and measured availability of polycyclic aromatic hydrocarbons (PAHs) and potentially toxic elements (PTEs), Environ. Pollut., 2014, 193, 79–87 CrossRef CAS PubMed.
- C. Yao, B. Wang, J. Zhang, M. Faheem, Q. Feng and M. Hassan, et al., Formation mechanisms and degradation methods of polycyclic aromatic hydrocarbons in biochar: A review, J. Environ. Manage., 2024, 357, 120610 CrossRef CAS PubMed.
- S. Pathak, A. K. Sakhiya, A. Anand, K. K. Pant and P. Kaushal, A state-of-the-art review of various adsorption media employed for the removal of toxic Polycyclic aromatic hydrocarbons (PAHs): An approach towards a cleaner environment, J. Water Proc. Eng., 2022, 47, 102674 CrossRef.
- L. Kong, B. Song, T. Zhang, K. Gao and J. Liu, Effects of soil organic matter on biochar application in developing the biodegradation potentials of polycyclic aromatic hydrocarbons (PAHs), Appl. Soil Ecol., 2021, 167, 104046 CrossRef.
- X. Chen, L. Yang, S. C. B. Myneni and Y. Deng, Leaching of polycyclic aromatic hydrocarbons (PAHs) from sewage sludge-derived biochar, Chem. Eng. J., 2019, 373, 840–845 CrossRef CAS.
- A. Jafarzadeh, A. Matta, S. V. Moghadam, S. Dessouky, J. Hutchinson and V. Kapoor, Field performance of two stormwater bioretention systems for treating heavy metals and polycyclic aromatic hydrocarbons from urban runoff, J. Environ. Manage., 2024, 370, 123080 CrossRef CAS PubMed.
- D. Ekanayake, P. Loganathan, M. A. H. Johir, J. Kandasamy and S. Vigneswaran, Enhanced Removal of Nutrients, Heavy Metals, and PAH from Synthetic Stormwater by Incorporating Different Adsorbents into a Filter Media, Water, Air, Soil Pollut., 2021, 232(3), 96 CrossRef CAS.
- R. S. Tomar and A. Jajoo, Fluoranthene, a polycyclic aromatic hydrocarbon, inhibits light as well as dark reactions of photosynthesis in wheat (Triticum aestivum), Ecotoxicol. Environ. Saf., 2014, 109, 110–115 CrossRef CAS.
- X. D. Huang, B. J. McConkey, T. S. Babu and B. M. Greenberg, Mechanisms of photoinduced toxicity of photomodified anthracene to plants: Inhibition of photosynthesis in the aquatic higher plant Lemna gibba (duckweed), Environ. Toxicol. Chem., 1997, 16(8), 1707–1715 CAS.
- M. Kummerová, M. Barták, J. Dubová, J. Tříska, E. Zubrová and Š. Zezulka, Inhibitory Effect of Fluoranthene on Photosynthetic Processes in Lichens Detected by Chlorophyll Fluorescence, Ecotoxicology, 2006, 15(2), 121–131 CrossRef.
- R. Singh Tomar and A. Jajoo, A quick investigation of the detrimental effects of environmental pollutant polycyclic aromatic hydrocarbon fluoranthene on the photosynthetic efficiency of wheat (Triticum aestivum), Ecotoxicology, 2013, 22(8), 1313–1318 CrossRef CAS.
- R. Singh-Tomar and A. Jajoo, Alteration in PS II heterogeneity under the influence of polycyclic aromatic hydrocarbon (fluoranthene) in wheat leaves (Triticum aestivum), Plant Sci., 2013, 209, 58–63 CrossRef CAS PubMed.
- G. J. Ahammed, M. M. Wang, Y. H. Zhou, X. J. Xia, W. H. Mao and K. Shi, et al., The growth, photosynthesis and antioxidant defense responses of five vegetable crops to phenanthrene stress, Ecotoxicol. Environ. Saf., 2012, 80, 132–139 CrossRef CAS PubMed.
- A. Krzyszczak, M. Dybowski, I. Jośko, M. Kusiak, M. Sikora and B. Czech, The antioxidant defense responses of Hordeum vulgare L. to polycyclic aromatic hydrocarbons and their derivatives in biochar-amended soil, Environ. Pollut., 2022, 294, 118664 CrossRef CAS.
- Y. Zhu, Y. Xu, J. Xu, P. Meidl and Y. He, Contrasting response strategies of microbial functional traits to polycyclic aromatic hydrocarbons contamination under aerobic and anaerobic conditions, J. Hazard. Mater., 2023, 454, 131548 CrossRef CAS.
- X. Li, C. Qu, Y. Bian, C. Gu, X. Jiang and Y. Song, New insights into the responses of soil microorganisms to polycyclic aromatic hydrocarbon stress by combining enzyme activity and sequencing analysis with metabolomics, Environ. Pollut., 2019, 255, 113312 CrossRef CAS.
- Y. Liu, Y. H. Huang, H. Lü, H. Li, Y. W. Li and C. H. Mo, et al., Persistent contamination of polycyclic aromatic hydrocarbons (PAHs) and phthalates linked to the shift of microbial function in urban river sediments, J. Hazard. Mater., 2021, 414, 125416 CrossRef CAS PubMed.
- Y. Gao, J. Ding, M. Yuan, N. Chiariello, K. Docherty and C. Field, et al., Long-term warming in a Mediterranean-type grassland affects soil bacterial functional potential but not bacterial taxonomic composition, npj Biofilms Microbiomes, 2021, 7(1), 17 CrossRef CAS.
- A. R. Khan, Z. Ulhassan, G. Li, J. Lou, B. Iqbal and A. Salam, et al., Micro/nanoplastics: Critical review of their impacts on plants, interactions with other contaminants (antibiotics, heavy metals, and polycyclic aromatic hydrocarbons), and management strategies, Sci. Total Environ., 2024, 912, 169420 CrossRef CAS PubMed.
- S. H. Liu, G. M. Zeng, Q. Y. Niu, Y. Liu, L. Zhou and L. H. Jiang, et al., Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review, Bioresour. Technol., 2017, 224, 25–33 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |


