Chirality driven self-sorting in supramolecular assemblies of π-conjugated systems
Bhawani
Narayan

Department of Natural Sciences, Huston-Tillotson University, 900 Chicon Street, Austin, TX-78702, USA. E-mail: bhawanin91@gmail.com
First published on 16th October 2025
Abstract
Self-sorting, the process by which molecules selectively recognize and assemble with identical or complementary partners, forms the basis of the formation of complex, functional supramolecular architectures in natural systems. Inspired by nature's precision, π-conjugated systems have been engineered to self-assemble in solution via synergistic non-covalent interactions, enabling functional nanoarchitectures for organic electronics, photovoltaics, and chiroptical devices. However, achieving control over molecular ordering in these dynamic assemblies remains challenging due to kinetic traps and competing energetic states. Chirality-driven self-sorting has emerged as a powerful strategy, utilizing enantiomeric recognition to enable homochiral supramolecular polymerization. This feature article reviews key advances in chirality-controlled self-assembly of π-conjugated donors and acceptors, elucidates mechanisms that enable narcissistic and social self-sorting, and discusses innovative characterization methodologies for probing these complex systems.
Introduction
Self-sorting is the formation of assemblies of similar (identical) molecules through a process of self-recognition.1 Molecules can be programmed to self-sort to form pure assemblies with long range ordering in multicomponent systems to deliver specialized functions with high efficiency.2 Self-recognition may occur through the assistance of one or more complementary synergistic non-covalent interactions, leading to stable self-assemblies of molecules with very high association constants. Spontaneous self-sorting of molecular components in nature has been observed to be assisted by non-covalent forces, for instance, hydrogen-bonding in the complementary pairing of nitrogenous bases in DNA,3 wherein adenine pairs (sorts) with thymine and guanine pairs (sorts) with cytosine, leading to a highly stable double-helix DNA containing all the genetic information for a lifetime and for future inheritance. Hydrophobic and electrostatic interactions assist in bringing structurally related polypeptides of α- and β-tubulin monomers, constructing the basic heterodimer unit, which further self-assembles to form one dimensional microtubule.4 This process requires precise self-recognition of complementary units followed by self-sorting of the specific heterodimers to form a supramolecular self-assembled system performing highly specialized functions. The eucaryotic cell is another multicomponent system of multiple levels of self-sorted cells forming organelles, each performing their unique function without interfering with the roles of the other organelles.5Supramolecular self-sorted systems designed in laboratory from π-conjugated systems are relatively simple, utilizing one or more non-covalent interactions working in tandem.6 Solvent assisted supramolecular polymerization of electron rich organic π-conjugated molecules (donors) and electron-deficient organic π-conjugated molecules (acceptors) have resulted in precise nanoarchitectures which have been utilized as the active components of modern organic devices, such as organic field effect transistors (OFETs),7 organic light emitting diodes (OLEDs)8 and organic solar cells.9 Control over the molecular ordering of functional organic π-conjugated donors and acceptors is desired for applications in optoelectronics. For example, segregated (orthogonal) supramolecular self-assembled stacks of π-conjugated donors and acceptors are suitable for applications in solar cells to ensure an optimum charge separation, whereas mixed (alternate) arrangement of donors and acceptors is desired for ferroelectric materials.10 The self-sorting strategy has been cleverly applied to supramolecular polymers to achieve the required donor–acceptor arrangements.11 While narcissistic self-sorting results in the separation of the donor and acceptor stacks, social self-sorting results in the alternate arrangement of donor and acceptor molecules.
Self-sorting in supramolecular polymers is challenging due to the dynamic nature of the assemblies.12 The solvent assisted self-assembly of monomers of small organic π-conjugated molecules goes through rapid equilibrium of assembly and disassembly before attaining the final thermodynamically stable self-assembled stacks. The kinetically stable conformations make room for further local energy minimum which makes the attainment of the final state even more challenging. However, thermodynamically stable self-sorted stacks have been achieved through intelligent incorporation of multiple non-covalent interactions that rigidify the conformation of the resulting stacks. The introduction of hydrogen bonding moieties in the molecular design,13 hydrophobic effects in case of self-sorting in aqueous self-assembly,14 charge-transfer interactions resulting in social self-sorting of donors and acceptors15 and more recently, introduction of chirality driven self-sorting has resulted in functional self-sorted assemblies.16
Enantiomeric self-sorting involves the incorporation of rigid, enantiomerically pure chiral cores.17 The presence of these moieties ensures sufficient energy difference between the plausible diastereomeric states, to result in homochiral supramolecular polymerization. The most well-studied class of enantiomerically pure self-sorted assemblies of organic molecules are the discrete assemblies of coordination compounds containing chiral groups.18 Self-recognition in monomers of small π-conjugated molecules is rarely reported, because of two major reasons. Firstly, most of the chiral organic molecules have chiral side chains, which are far from the self-assembling core and therefore, fail to provide a strong chirality mismatch or enantioselectivity during the process of self-assembly. The second reason is the lack of characterization methods to probe stereospecific supramolecular polymerization. Despite these challenges, there have been several reports of enantioselective supramolecular polymerization in small molecules, where innovative scientific techniques have been developed to prove self-sorting in these assemblies (vide infra). Furthermore, recent surges in chirality driven self-sorted assemblies of functional organic π-conjugated molecules have resulted in the development of highly promising organic materials with exceptional electronic and chiroptical properties.19
This feature article primarily focuses on the unique method of homochiral supramolecular polymerization (chirality driven self-sorting) in solvent assisted supramolecular assemblies of small, organic functional π-conjugated molecules. It gives a deep view of the systems investigated so far and presents the author's perspective on these systems. The article also highlights the applications of the self-sorted assemblies that have been proven and those that are promising. Apart from the primary focus, it provides a broad overview of the other strategies by which self-sorting has been achieved in these dynamic assemblies.
Early investigations of chirality driven self-sorting
The historical discovery of Louis Pasteur's tartaric acid crystals 1 with opposite handedness (Fig. 1a) followed by separation of enantiomers through hand-picking was the first ever formalized artificial chiral resolution experiment.20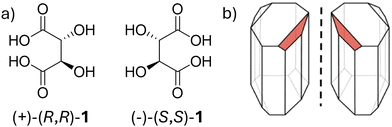 | ||
| Fig. 1 (a) Chemical structures of tartaric acid enantiomers. (b) Hemihedral crystals of double sodium–ammonium tartarates. The facets (in red) aided in visualizing and resolving the enantiomers. (b) Reproduced from ref. 20 with permission from John Wiley and Sons, copyright 2021. | ||
Through a process of homochiral recognition, the double sodium–ammonium salt of tartaric acid crystallizes into conglomerates, with each crystal having a visible tiny facet on one of its edges either oriented to the left or to the right (Fig. 1b). Even though Pasteur's discovery is considered serendipitous, it is often good to recall that “chance always favours the prepared minds.”
The molecular recognition of synthetic molecules in multicomponent complex systems was first investigated by Lehn and coworkers.21 They explored the co-assembly of two tris-bipyridine ligands (2 and 3, Fig. 2) that were previously shown to self-assemble independently in the presence of Ni(II) and Cu(I) ions into triple and double helicates, respectively. When a mixture containing 2 equivalents of ligand 3, 3 equivalents of ligand 2, 3 equivalents of Cu+, and 3 equivalents of Ni2+ was subjected to suitable conditions, only the corresponding double and triple helicates precipitated in quantitative yields. Analytical techniques, including fast atom bombardment (FAB) mass spectrometry and 1H NMR spectroscopy, provided compelling evidence for the selective recognition of tetrahedrally coordinated Cu(I) ions by ligand 3 and octahedrally coordinated Ni(II) ions by ligand 2. These observations ruled out the formation of crossover, mixed, or undesired species within the system.
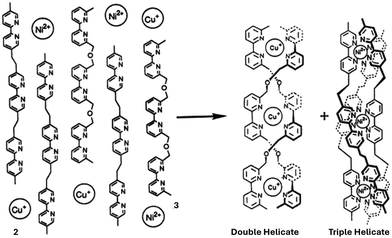 | ||
| Fig. 2 Formation of double and triple helicates of tris-bipyridine ligands through molecular recognition in a multicomponent system consisting of a mixture of 2, 3 Cu(I) and Ni(II) ions. Reproduced from ref. 21 with permission from Proc. Natl. Acad. Sci. U. S. A., copyright 1993. | ||
This phenomenon was originally termed “self-recognition,” describing the discrimination of like from unlike and self from non-self. It reflects the intrinsic ability of ligand strands to preferentially bind their cognate metal ion templates even in complex mixtures. In subsequent studies, Isaacs and co-workers proposed the more general term “self-sorting” to capture such recognition events in multicomponent systems.22 Self-sorting now denotes the high-fidelity organization of molecular and ionic components within mixtures. This concept has gained widespread acceptance in the supramolecular chemistry community. It serves as a fundamental principle in designing sophisticated, hierarchically organized architecture.
Homochiral supramolecular polymerization in π-conjugated enantiomeric self-assemblies
Ishida and Aida reported the formation of large homochiral assemblies of an S-shaped monomer 4 in solution.23 For the first time, stereoselective supramolecular polymerization was achieved by using the high enantioselectivity in hydrogen-bonding interactions of a xylylene-bridged bis(cyclic dipeptide) (Fig. 3a).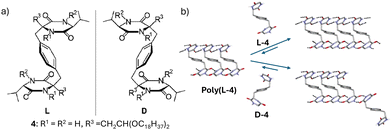 | ||
| Fig. 3 (a) Molecular structures of enantiomeric ‘S’-shaped bis(cyclic dipeptide). (b) Schematic representation of stereoselective polymerization of 4. (b) Reproduced from ref. 23 with permission from American Chemical Society, copyright 2002. | ||
The ‘S’-shaped conformation of the monomer arises from the aromatic groups “hovering over” the diketopiperadine ring due to dipole–dipole interactions. In aprotic solutions of medium polarity (CHCl3), these cyclic dipeptides exhibit extensive antiparallel multiple H-bonding possessing “S” and anti-“S” shaped (NH → CO) → (CO → NH) sequences (Fig. 3b). The racemic cyclic dipeptide undergoes homochiral supramolecular polymerization through a process of enantioselective self-recognition, forming large assemblies, enabling isolation of enantiomers by size exclusion chromatography (SEC) coupled with ultraviolet (UV) and circular dichroism (CD) detectors. The translation of optical purity of the cyclic dipeptide into molecular weight distribution enabled the characterization of self-sorted conglomerates in solution.
The homochiral supramolecular polymerization of C4- and C3-symmetric bowl-shaped chiral macrocyclic monomers 5 and 6, respectively, into self-sorted columnar assemblies was characterized by the Aida group in a similar manner using size-exclusion chromatography coupled with UV and CD detectors (Fig. 4).24 The self-sorting behaviour arises from the energy difference of +2.3 kcal mol−1 of the heterochiral dimeric core over the homochiral dimeric core, as supported by computational calculations. The macrocycles underwent homochiral supramolecular polymerization in a solvent mixture of CHCl3–C6H12 (1/1 in v/v). The equimolar mixture was CD silent with the Δε values falling into a linear trend when plotted against varying %ee. The process of self-sorting was further supported by SEC-UV and SEC-CD traces, wherein a polymeric assembly with the smallest molecular weight was observed at a 50/50 (0% ee) mixture of both the enantiomeric monomers.
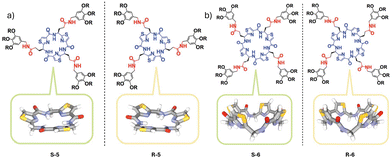 | ||
| Fig. 4 Chemical structures of (a) C3- and (b) C4-symmetric macrocyclic monomers 5 and 6 and their schematic representation as bowls to guide the eye. Reproduced from ref. 24 with permission from Royal Society of Chemistry, copyright 2010. | ||
The lack of established experimental methods in characterizing self-sorted supramolecular assemblies in solution provides opportunities for researchers to adopt multiple innovative methods of characterization. In 2005, Stupp and coworkers reported the self-sorting of racemic triblock molecules known as dendron rodcoils (Fig. 5a) through homochiral recognition of molecules during self-assembly.25 The formation of gels of 7 in acetonitrile at 0.25 wt% indicated self-assembly of the monomers. Mirror image CD spectra of the gel samples along with formation of ribbonlike structures of opposite handedness was indicative of expression of supramolecular chirality on surface. The CD silent racemic mixture and AFM images showing similar helical features with left- and right-handed ribbons with the same periodicity enabled them to characterize the self-sorted enantiomers (Fig. 5b). The homochiral self-assembly of the dendron rodcoils is driven by head-to head hydrogen bonding interactions among the dendritic segments and π–π stacking interactions between the rodlike segments.
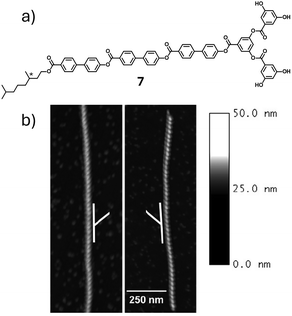 | ||
| Fig. 5 (a) Chemical structures of 7. The chiral centre has been marked with an asterix. (b) Atomic force microscopy (AFM) images of the mirror image nanostructures of the enantiomers formed upon self-assembly of 7 in acetonitrile. The horizontal and vertical scale bars are identical for both images. The white lines indicate handedness. (b) Reproduced from ref. 25 with permission from American Chemical Society, copyright 2005. | ||
In 2011, Meijer et al. presented detailed results on mixing of different porphyrin molecules in supramolecular assemblies.26 Zinc coordinated enantiomeric tetraphenyl porphyrins with gallic side chains alkylated with R-/S-3,7 dimethyloctyloxy side chains; R-Zn (8) and S-Zn (9) formed self-sorted assemblies in non-polar solvents (Fig. 6a and b). The reason for the self-sorting was attributed to the presence of 12 chiral side chains. The chiral functionalities cause a high mismatch penalty (MMP) of 3.4 kJ mol−1 and a helix reversal penalty (HRP) of 5.6 kJ mol−1, preventing the formation of heterochiral stacks from a mixture of enantiomeric monomers. High mismatch penalty prevents the monomers of the opposite chirality from co-assembling in an enantiomerically pure supramolecular aggregate, thus promoting the process of self-sorting. The absence of chiral amplification along with a linear trend in the plot of net helicity versus enantiomeric excess indicates homochiral supramolecular polymerization. Similar narcissistic self-sorting is observed upon co-assembly of 8 and copper coordinated porphyrin of opposite chirality (S-Cu, 10) with an even higher MMP of 11.2 kJ mol−1. However, upon diluting the enantiomeric stacks by structural intrusion of an achiral monomer, A-Cu (11, containing octyl side chains), the MMP comes down to 2.6 kJ mol−1. The reduction in MMP facilitated the process of co-assembly and brought down the possibility of self-sorting. In tricomponent stacks of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 (12.5
11 (12.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5
7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, ee = 25% at 20% sergeant); the 8 sergeant dominates the helical sense of the co-aggregate.
80, ee = 25% at 20% sergeant); the 8 sergeant dominates the helical sense of the co-aggregate.
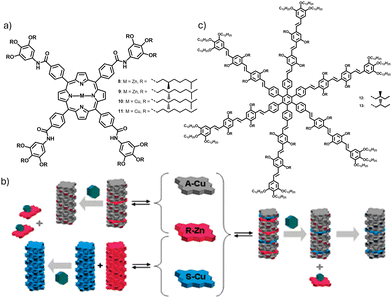 | ||
| Fig. 6 (a) Molecular structures of amide functionalized porphyrins 8–11. (b) Schematic illustration of metal complexation, enantiomeric self-sorting and co-stacking during the process of self-assembly. (c) Molecular structure of 12 and 13 with 24 stereogenic centres. (b) Reproduced from ref. 26 with permission from American Chemical Society, copyright 2011. | ||
Another instance of homochiral recognition was observed in the self-assembly of two enantiomerically pure hexa(oligo (p-phenylene vinylene))-substituted benzenes, 12 and 13 having 24 stereocenters (Fig. 6c).27 The molecules formed two different types of assemblies, A and B in pure methylcyclohexane (MCH) and in a mixture of MCH/toluene (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively. The self-assembled kinetically stable configuration A converted to the more thermodynamically stable assembly B in the individual assemblies in 6 days, while the co-assembled stacks showed narcissistic self-sorting. Upon heating the co-stacks and cooling them to obtain thermodynamically stable arrangement of enantiomers, the monomers rearranged. However, homochiral recognition was still observed in the thermodynamically stable stacks. Following the method established by Aida and coworkers,23,24 size exclusion chromatography confirmed the presence of the homochiral supramolecular stacks.
1), respectively. The self-assembled kinetically stable configuration A converted to the more thermodynamically stable assembly B in the individual assemblies in 6 days, while the co-assembled stacks showed narcissistic self-sorting. Upon heating the co-stacks and cooling them to obtain thermodynamically stable arrangement of enantiomers, the monomers rearranged. However, homochiral recognition was still observed in the thermodynamically stable stacks. Following the method established by Aida and coworkers,23,24 size exclusion chromatography confirmed the presence of the homochiral supramolecular stacks.
Our research group introduced the concept of chirality driven self-sorting for the precise organization of π-conjugated donors (D) and acceptors (A) to construct segregated/alternate D–A stacks.16 We incorporated enantiomerically pure trans-1,2-bis(amido) cyclohexane as a strong chiral motif to drive the process of homochiral supramolecular polymerization. The strong hydrogen bonding between the bis(amido) cyclohexane cores in non-polar solvents facilitated enantioselectivity during the process of self-assembly.28 We chose 1,5 dialkoxy naphthalene (DAN) derivative as the donor molecule and naphthalelene diimide (NDI) as the acceptor molecule and functionalized them to the bischromophoric cores to probe the chirality control on the D–A organization. We synthesized four molecules to understand the self-sorting mechanism, RR-NDI (14), SS-NDI (15), RR-DAN (16) and SS-DAN (17); the stereochemical nomenclature based on the stereochemistry of the trans-1,2-bis(amido)cyclohexane core (Fig. 7a). The D–A stacks of 16 and 14 (similar stereochemistry) demonstrate social self-sorting. This is evident by a deep red solution formed upon mixing the two derivatives along with the occurrence of a charge-transfer band in the absorption spectra, characteristic of NDI-DAN alternate arrangement in supramolecular self-assembled stacks.29 On the other hand, narcissistic self-sorting was observed upon mixing 15 with 16, evident from the colourless solution and the absence of charge-transfer band. To ensure thermodynamically stable self-assemblies and to avoid kinetic traps (if any), all the solutions were heated to monomeric state and then cooled at a temperature gradient of 1 K min−1. Upon monitoring the absorption band at 399 nm for individual self-assemblies, we observed a cooperative mechanism of self-assembly. This information was useful in understanding the narcissistic self-sorting of 15 from a tricomponent mixture of 14 + 15 + 16, wherein 14 and 16 formed charge-transfer complexation and 15 clearly segregated out to form its individual assemblies in a cooperative manner, suggesting auto resolution of assemblies of 15 in a multicomponent mixture (Fig. 8a and b). The strong preference for self-recognition behaviour is majorly due to the strong trans-1,2-bis(amido) cyclohexane core which provides an energy penalty of 15.1 kcal mol−1 in the heterochiral dimer compared to the homochiral dimer of a model with the long swallow tails in the actual molecules replaced by methyl groups in the computational model.
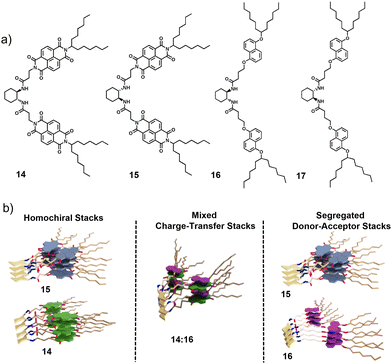 | ||
| Fig. 7 (a) Chemical structures of the NDI acceptors and DAN donors 14–17. (b) Schematic representation of the formation of self-sorted assemblies of the acceptors, mixed charge-transfer stacks upon D–A complexation of the same chiralities and segregated D–A stacks upon co-assembly of donors and acceptors of opposite chiralities. (b) Reproduced from ref. 16 with permission from John Wiley and Sons, copyright 2015. | ||
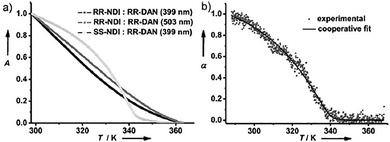 | ||
| Fig. 8 Autoresolution of 15 from a tricomponent mixture of 14 + 15 + 16 followed by mechanistic investigations by plotting (a) the absorption changes at the CT wavelength (503 nm) and NDI wavelength (399 nm) in bicomponent mixtures. (b) Experimentally obtained data of 15 (CD intensity monitored at 364 nm), overlayed with a fitted data, indicating cooperative mechanism of supramolecular polymerization and suggesting its segregation in a tri-component mixture. Reproduced from ref. 16 with permission from John Wiley and Sons, copyright 2015. | ||
In another work, we utilized the self-sorting behaviour induced by the trans-1,2-bis(amido) cyclohexane core in obtaining pure enantiomeric self-sorted solution state assemblies exhibiting circularly polarized luminescence (CPL) with high luminescence dissymmetry factor (glum) compared to many other CPL active solution state self-assembled systems reported so far.30 CPL, being the emission analogue of CD, is an important tool to investigate the chiroptical properties of excited state assemblies.31 In general, obtaining decent CPL values in self-assembled solutions of luminescent dyes is challenging majorly because of two factors. Firstly, the process of self-assembly of fluorescent monomers causes aggregation caused quenching, and secondly, organic molecules have low intrinsic glum values. To address these challenges, our molecular design involved the functionalization of enantiomerically pure trans-1,2-bis(amido) cyclohexane cores with octyl tail appended cyanostilbenes (Fig. 9a). Cyanostilbene chromophores are well known for restriction of intramolecular rotation (RIR), yielding highly emissive assemblies upon self-assembly, in a phenomenon coined as ‘aggregation induced enhanced emission (AIEE)’.32 The strong chiral motif with strong core- to- core hydrogen bonding in non-polar solvents ensured the formation of enantiopure assemblies. As discussed earlier, many of the chiral solution state co-assemblies exhibit the phenomenon of chiral amplification through the sergeants and soldiers’ rule or the majority rule, in which the enantiomer present in excess governs the chirality of the overall co-assembly.33 The CPL values in these cases would be much lower than the actual potential of the luminescent chromophores involved, as the enantiopurity will never reach unity. The incorporation of self-sorting phenomenon helps in the maximum extraction of luminescence from organic dyes in chiroptical solution state self-assemblies.
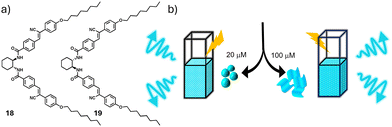 | ||
| Fig. 9 (a) Chemical structures of 18 and 19. (b) Schematic representation of CPL active solutions of 18 and 19 containing self-assembled nanostructures. (b) Reproduced from ref. 30 with permission from John Wiley and Sons, copyright 2020. | ||
The molecules RR-CSB (18) and SS-CSB (19), RR and SS indicating the stereochemical configuration of the trans-1,2-bis(amido) cyclohexane cores appended to cyanostilbene dye molecules self-assembled in non-polar solvent composition TCE/MCH (99/1).30 At a concentration of 25 μM, the individual molecules self-assemble into vesicles with CPL active assemblies (Fig. 9b). Remarkable morphological transformation into 2-D sheets was observed upon investigating self-assemblies at higher concentration (100 μM). These assemblies exhibited glum values of 3.1 × 10−3 and 2.4 × 10−3 respectively, indicating better ordered molecules with stronger excitonic coupling than the monomeric state (glum = 1.1 × 10−4). At an equimolar concentration, the co-assemblies formed supramolecular racemic conglomerates, which we further investigated through spectroscopic and mechanistic investigations. Though in totality, the equimolar mixture exhibited CPL inactive assemblies, it can be considered a quiescent stage with a remarkable latent potential. Resolution of the self-sorted conglomerates of 18/19 would yield enantiopure CPL to the utmost potential of the self-assembled chromophores.
Applications of stereoselective polymerization
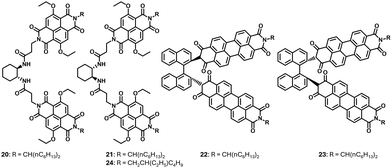
Our group had demonstrated a decade ago that the concept of homochiral recognition can be successfully utilized for the precise organization of π-conjugated chiral donors and acceptors.16 Since then, several research groups have applied this concept to obtain the desired D–A arrangements for realizing various photophysical properties. It is really encouraging to see the rapid development of this concentrated area of research in such a short time.Kawai and coworkers reported “almost absolute enantioselective recognition of a chiral perylenediimide (PDI) molecule by chiral supramolecular nanofibers of a bichromophoric naphthalenediimide (NDI) derivative”.34 Working on our earlier molecular design of bischromophoric NDI, the researchers introduced ethoxy groups in the core of the NDI, making the molecule highly emissive. These molecules (20 and 21) exhibited homochiral recognition and self-sorted when self-assembled in non-polar solutions to form fibrous nanostructures, similar to our earlier reported system. Being chiral and emissive, these narcissistic assemblies were CPL active. Enantiomerically pure perylenebisimides (PBI) 22 and 23 were then co-assembled with the core-substituted NDI derivative. Interestingly, enantioselective energy-transfer was observed when the core-substituted NDI and PBI were of the same chirality, and no energy transfer was observed when the guest PBI of opposite chirality interacted with NDI. Moreover, the CPL wavelength shifted to longer wavelengths because of energy transfer. This is the first report of enantioselectively sensitized CPL occurring in self-sorted systems.
At the same time, George and co-workers reported the visualization of supramolecular polymers by chirality-controlled energy transfer.35 Working on our earlier molecular design of bischromophoric NDI acceptors, which were established to exhibit homochiral supramolecular polymerization, the researchers synthesized emissive core substituted NDIs functionalized to enantiomerically pure RR-/SS-trans-1,2-bis(amido) cyclohexane cores. The donor molecule was NDI substituted in the core with ethoxy groups (SS-NDI-OEt), 24 whereas the acceptor molecules were NDIs substituted in the core with one ethoxy and one isopropylamine group (RR/SS-NDI-OEtiPa; 25 and 26 respectively) (Fig. 10a).
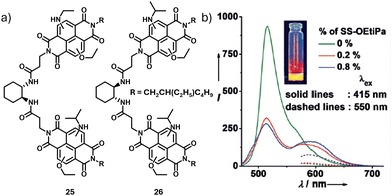 | ||
| Fig. 10 (a) Molecular structures of red emissive acceptors 25 and 26. (b) Co-assembly of 24 and 25 resulting in quenching of donor emission and enhancement of the acceptor emission, showing FRET. (b) Reproduced from ref. 35 with permission from John Wiley and Sons, copyright 2017. | ||
In nonpolar MCH rich solutions, the donors self-assembled to form fibres which were green emissive (λ = 516 nm). The acceptor molecules upon self-assembly yielded red emissive fibres (λ = 641 nm). An unprecedented chirality driven energy transfer was observed when the acceptor was co-assembled with the donor. 24 exhibited energy transfer when co-assembled with 25 (Fig. 10b). Upon mixing with the acceptors of opposite chirality, self-sorting was observed. The chiral recognition was extremely efficient, with just 0.2 mol% acceptor quenching the donor emission. For the first time, co-assembly and self-sorting of the molecules were distinguished both spectroscopically and visually through fluorescence microscopy.
Duan and coworkers reported enantioselective assembly process between a chiral energy donor and two enantiomeric energy acceptors, resulting in chirality-controlled energy transfer and enantioselective triplet–triplet annihilation upconversion (TTA-UC).36 Using a chiral Pd(II) octaethylporphyrin derivative PdOEP-LG12 (27) and anthracene derived enantiomerically pure acceptors, namely RA (28) and SA (29), triplet–triplet annihilation (TTA) based photon upconversion, further manifesting as enantioselective upconverted CPL was found to occur between the D–A pair of compatible chirality. However, the D–A pair of opposite chirality exhibit enantiosegregation, resulting in poor upconversion (Fig. 11a and b).
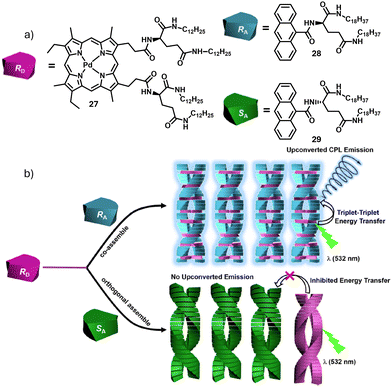 | ||
| Fig. 11 (a) Chemical structures of chiral energy donor 27 and enantiomeric acceptors 28 and 29 and their schematic block representations. (b) schematic illustration of co-assembly of D–A pair of compatible chirality, resulting in energy transfer and enantiosegregation in the D–A pair of opposite chirality. Reproduced from ref. 36 with permission from American Chemical Society, copyright 2021. | ||
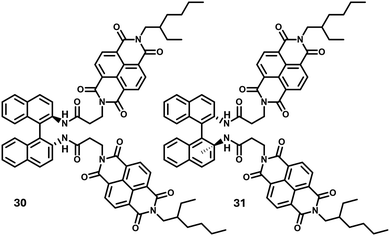
Our design strategy modified with (±)-1,1′-binaphthyl-2,2′-diamine instead of trans-1,2-bis(amido) cyclohexane cores exhibited unprecedented stereoselective seed induced living supramolecular polymerization (LSP) using chiral seeds, to synthesize precision 1D assemblies with controlled length and narrow dispersity (polydispersity index, PDI ∼ 1.08).37 These molecules, 30 and 31, showed strong self-recognition abilities and pathway complexity. While the enantiomeric seeds of the same chirality showed an on-pathway (consecutive pathway) towards supramolecular homopolymers, heterochiral seeding led to the formation of supramolecular conglomerates. In a consecutive work by the same group, the heterochiral seeding was found to provide secondary nucleation in kinetic assemblies which was characteristic of a surface-catalyzed secondary nucleation triggered supramolecular polymerization.38
Following up on our work on bischromophoric cyanostilbenes, replacing the cyanostilbenes with pyromellitic diimides as the chromophores yielded circularly polarized room-temperature phosphorescence (CPP). PMMA films obtained by processing the solutions of the bischromophoric phosphors substituted with heavy atom functionalized pyromellitic diimides, 32 and 33, show mirror-imaged cyan CPP with a high phosphorescence quantum yield (18% in air and 46% under vacuum) and a significant luminescence dissymmetry factor (|glum| = 4.0 × 10−3) (Fig. 12a and b).39
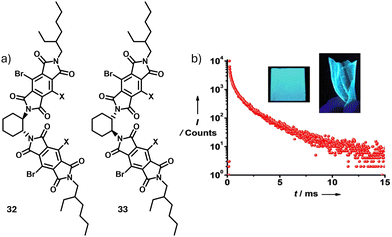 | ||
| Fig. 12 (a) Molecular structures of 32 and 33. (b) Time resolved fluorescence measurements of 32, indicating room temperature phosphorescence. Inset are the photographs PMMA coated films on glass substrate and as free-standing sheets, taken under UV light. (b) Reproduced from ref. 39 with permission from John Wiley and Sons, copyright 2022. | ||
Mechanistic insights into chirality driven self-sorting
During the dynamic process of stereoselective polymerization, enantiomers experience solvent–solvent interactions and solute–solute interactions. The delicate balance of these attractive forces is maintained in the formation of nanostructures which are stable in solution state. The homochiral narcissistic self-sorting of enantiomers involves the formation of a homomeric dimer, which forms the basis of a supramolecular helix. The stereochemistry of the chiral molecules plays a vital role in deciding the stability of the homomeric dimer. During the process of social self-sorting, additional secondary interactions, for e.g., charge-transfer interactions play a vital role in stabilizing the seed complex.16 It is important that the undergoing a social self-sorting have a low mismatch penalty to facilitate co-assembly during the process of social self-sorting.26 The helix reversal penalty of self-sorted systems is considerably high to rule out the processes of chiral amplification.26 The energy landscape of a solution-assisted supramolecular self-sorting of π-conjugated chromophores might have several intermediate kinetic states. The kinetic states might convert to the more stable thermodynamic states upon long standing or upon thermal annealing. In non-equilibrium systems, pathway complexities between the various kinetic states makes the attainment of the thermodynamically stable state even more challenging.2Insights into the energies of the homochiral and heterochiral dimers is a very reliable method of predicting chirality driven self-sorting. Predictive computational methods are often used to calculate the relative energies of the dimeric states. The energy of the heterochiral dimer of trans-1,2-bis(amido) cyclohexane core was found to be 5.1 kcal mol−1 over the homochiral dimer at B3LYP/6-31g; indicative of self-sorting.16 The hydrogens on the cyclohexane ring and the amide group were found to be in anti-periplanar conformation with respect to each other in the homochiral dimer whereas they were found to be in syn-periplanar conformation with respect to each other in the heterochiral dimer. Attaching π-conjugated naphthalene diimide chromophores facilitated π-stacking and hydrophobic interactions, increasing the energy difference between the heterochiral and homochiral dimers to 15 kcal mol−1.16 In a similar fashion the homochiral dimeric core of R-6 and S-6 was favoured over the heterochiral core by 2.3 kcal mol−1, determined by energy minimization and calculation based on M06-2X/6-31G**//B3LYP/6-31G* methods.24
Würthner and coworkers have studied the impact of molecular flexibility on binding strength and self-sorting of chiral π-surfaces using macrocyclic perylenebisimides (PBIs).40 Two series of chiral PBIs comprising oligoethylene glycol bridges of different lengths at the 1,7 bay positions were synthesized and their atropo-enantiomers (P and M enantiomers) were resolved. Single crystal X-ray diffraction studies along with detailed spectroscopic investigations revealed the preference of homochiral dimerization (PP/MM) over heterochiral dimerization (PM) (Fig. 13). The binding constants of the homochiral dimer (KD = 2800 M−1) was found to be higher than the binding constant of the heterochiral dimers (KD = 400 M−1); resulting in the formation of 93% homochiral and 7% heterochiral mixtures in the thermodynamically equilibrated state. The greater π-stacking interactions in the homochiral polymers makes them energetically favourable. Moreover, it was found that the degree of self-sorting in homologous molecules decreased with increase in the length of the spacer. Flexible scaffolds bearing longer bridging units were shown to demonstrate more planarized π-scaffolds upon self-assembly by means of an induced-fit mechanism leading to higher binding strength but lower enantioselectivity.
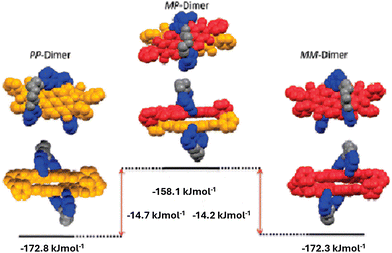 | ||
| Fig. 13 Energy diagram of the various diastereomers of chiral perylenebisimides (PBIs). Reproduced from ref. 40 with permission from American Chemical Society, copyright 2011. | ||
Introducing complementary non-covalent forces assists in the attainment of the thermodynamically stable self-sorted state and increases the energy differences between the heterochiral and the homochiral polymers. Aida and co-workers used four complementary H-bonding interactions in the supramolecular polymerization of 4 resulting in a large polymer.23 The core-to core H-bonding in trans-1,2-bis(amido) cyclohexane scaffold enables the strong homochiral polymerization in molecules 14–21,16,30,3424–26,34,353037 and 31.37 Extended π–π aromatic interactions and hydrophobicity assist in the homochiral polymerization of molecules 8–13.26,27 Despite the major role of enantiomeric recognition in the process of stereoselective polymerization, complementary synergistic non-covalent interactions strongly assist the process of chirality driven self-sorting in π-conjugated systems in solutions.22 Additionally, the factors affecting general self-sorting process, namely temperature, concentration and equilibrium constants of the molecules determine the likelihood of enantiomeric self-sorting.22
Methods of characterization of homochiral supramolecular self-assemblies
The lack of established methods of characterization of chirality driven self-sorting in supramolecular assemblies of π-conjugated systems paves way for researchers to employ innovative methods of characterization which are scientifically acceptable. The underlying goal of all the methods is to rule out the possibility of co-assembly in enantiomeric self-assembled mixtures in solutions. The process of self-sorting majorly results in the formation of supramolecular conglomerates. But homochiral recognition might also be observed in supramolecular racemates consisting of blocks of enantiomers, overall making up a supramolecular stack. This might result when the MMP is considerably low.To rule out the possibility of chiral amplification through the majority rules, the enantiomers are mixed in varying compositions ranging from 0% ee to 100% ee.16 Ensuring that the solutions are in their thermodynamically stable states removes the possibilities of any kinetic traps. In most cases, the solutions are heated to disassemble the co-stacks and cooled at very slow rates to ensure thermodynamic aggregates. The CD spectra of the self-sorted enantiomeric mixtures appear as a summation of the CD intensities of the individual enantiomers. The CD data can be visualized in a more accurate manner by plotting the net helicity/anisotropy factor against the % ee. The net helicity/anisotropy factor is more reliable than the CD intensity as it is free of linear dichroism (LD) contribution. A linear trend in this plot confirms self-sorted assemblies. In case the self-assembled solutions are CPL active, the net helicity/anisotropy factor extracted from the CPL data can be plotted against % ee. A linear trend in the data indicates self-sorting in the excited state assemblies.
Another method of characterization relies on the mechanistic investigations. Cooperative assemblies in the case of self-sorting of bischromophoric NDIs,16 porphyrins26 and OPVs27 have been characterized through this method. A cooperative self-assembly pattern has a nucleation phase where dimers/trimers exist. These oligomers begin rapidly growing into supramolecular polymers once they are cooled down to the elongation temperature (Te). This transition temperature depends on concentration for an enantiomeric monomer at a constant solvent composition and can be obtained by monitoring CD/UV at a particular wavelength in the cooling curve of a self-assembly. As the concentration is lowered, the Te is lowered. A plot of Teversus increasing % ee appears as a V-shaped curve with the minimum Te occurring at equimolar concentrations. In self-sorted conglomerates, the minimum Te at this point should match with the Te of the pure enantiomer at half the concentration. In some cases, melting temperature (Tm) has been utilized in the place of Te. The Tm obtained from the heating curves of the self-assembled solutions can be utilized if there is no hysteresis in the heating curves.
In our work, we carried out an interesting experiment to further demonstrate the auto resolution of an enantiomeric self-assembly from tricomponent D–A mixtures.1615 (acceptor of opposite chirality) self-sorted in the mixture of 14 + 15 + 16, while the donors and acceptors of same chirality formed charge transfer complexes with alternate D–A arrangement. The formation of self-sorted assemblies of 15 could be followed by monitoring the absorption changes at 399 nm, indicating Te same as that of pure self-assemblies of 15.
Self-sorted assemblies of large molecular masses can be characterized using size exclusion chromatography. Aida research group employed this technique extensively in the characterization of homochiral supramolecular polymers obtained from the self-sorting of 4, 5 and 6.23,24 Another instance of the use of size exclusion chromatography was in the characterization of homochiral assemblies of large OPVs 12 and 13, equipped with 24 stereocenters.27 In case of hydrogen bonded large enantiomeric assemblies, a broad chromatogram is obtained. Upon dilution the chromatogram becomes sharper and narrower with longer retention times. Coupled with CD, this technique can render information of which enantiomer is undergoing polymerization, based on the sign of the CD signal.
The formation of mirror image nanostructures in the case of enantiomeric assemblies of dendron rod-coils driven by chirality and multiple hydrogen bonding has been useful in characterizing the process of homochiral polymerization.25 The increase in solution viscosity and gelation in polar aprotic solvent (acetonitrile) indicated the formation of solution state assembly. Upon detailed examination of the nanostructures through atomic force microscopy (AFM), mirror image nanostructures were observed, indicating self-sorted assemblies. CD measurements supplemented the data obtained from these experiments to prove the process of stereoselective supramolecular polymerization.
More recently, photophysical phenomena, such as energy transfer,34,35 CPL30 and CPP39 which occur because of the enantiomeric recognition, work as secondary characterization tools to confirm the process of self-sorting. Visualization of the self-sorting process in solution through fluorescence imaging has emerged as a powerful tool to characterize self-sorted assemblies.
Conclusions
This feature article highlights the power of chirality-driven self-sorting as a design principle for achieving homochiral supramolecular polymerization in π-conjugated systems. By designing synthetic systems with enantiomeric recognition, unprecedented control over the molecular organization of donors and acceptors has been achieved, enabling functional assemblies with remarkable optoelectronic and chiroptical properties. Such assemblies circumvent the kinetic traps inherent to dynamic supramolecular systems, leading to thermodynamically stable architectures. The integration of strong chiral motifs, such as trans-1,2-bis(amido)cyclohexane cores, has proven particularly effective in driving narcissistic and social self-sorting. These strategies have facilitated the emergence of highly emissive, CPL-active assemblies and facilitated precise control of energy transfer processes, offering new directions for organic electronics and photonic applications. Despite these advances, challenges remain in understanding the mechanistic insights and in the characterization of stereoselective self-assembly, necessitating innovative spectroscopic and computational methodologies. Future exploration of self-sorting in multicomponent systems and under non-equilibrium conditions may unlock access to adaptive and responsive supramolecular materials. Multicomponent chiral systems offer more choices for its components to exhibit social/narcissistic self-sorting within the pool of molecules, based on the complementary attractive intermolecular interactions. Introducing targeted complementary attractive interactions, such as charge-transfer interactions, electrostatic or dipolar interactions might improve the predictability of enantiomeric self-sorting. However, challenges remain in achieving controlled self-sorting of components in multicomponent systems. The dynamic regulation of self-sorting in non-equilibrium systems could provide unprecedented insights into self-sorting in living systems at the cellular level. Non-equilibrium systems exhibit pathway complexities and have several kinetically trapped transient states. The continuous energy dissipation and complex orchestration of molecules working in tandem pose significant challenges to achieve programmable stereoselective supramolecular assemblies in a reproducible manner in these systems. Thermal annealing of samples might be helpful in avoiding a few kinetic traps; however, the assistance of predictive computational methodologies might still be required to completely understand these complicated synthetic systems. With the advent of artificial intelligence/machine learning (AI/ML) in predicting complicated systems, we are not very far from achieving control over stereoselective self-sorting in artificial systems. Improving the scalability and the translation of these principles into applicative devices represents an exciting frontier for functional materials science. Ultimately, chirality-driven self-sorting exemplifies how supramolecular chemistry can harness nature's precision to engineer complexity and function at the nanoscale.Author contributions
Bhawani Narayan conceptualized, drafted and edited this article.Conflicts of interest
There are no conflicts to declare.Data availability
Data sharing is not applicable to this article as no primary research results, software, or code was included, and no new data were generated for this feature article.Notes and references
- M. M. Safont-Sempere, G. Fernández and F. Würthner, Chem. Rev., 2011, 111, 5784 CrossRef CAS PubMed; C. Rest, M. J. Mayoral and G. Fernández, Int. J. Mol. Sci., 2013, 14, 1541 CrossRef PubMed; A. Ghosh and M. Schmittel, Beilstein J. Org. Chem., 2020, 16, 2831 CrossRef PubMed.
- K. Aratsu, R. Takeya, B. R. Pauw, M. J. Hollamby, Y. Kitamoto, N. Shimizu, H. Takagi, R. Haruki, S. Adachi and S. Yagai, Nat. Commun., 2020, 11, 1623 CrossRef CAS PubMed; P. Xing and Y. Zhao, Acc. Chem. Res., 2018, 51, 2324 CrossRef PubMed.
- J. D. Watson and F. H. C. Crick, Nature, 1953, 171, 737 CrossRef CAS PubMed.
- E. Nogales, S. G. Wolf and K. H. Downing, Nature, 1998, 391, 199 CrossRef CAS PubMed; B. Gigant, Cell, 2000, 102, 809 CrossRef PubMed.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Mol. Bio. Cell, Garland Science, New York, 4th edn, 2002 Search PubMed; H. Lodish, A. Berk, P. Matsudaira, C. A. Kaiser, M. Krieger, M. P. Scott, S. L. Zipursky and J. Darnell, Mol. Cell Biology, 5th edn, 2004, W.H. Freeman, New York Search PubMed.
- Z. He, W. Jiang and C. A. Schalley, Chem. Soc. Rev., 2015, 44, 779 RSC; C. H. Chen, L. C. Palmer and S. I. Stupp, Soft Matter, 2021, 17, 3902 RSC.
- B. Narayan, S. P. Senanayak, A. Jain, K. S. Narayan and S. J. George, Adv. Funct. Mater., 2013, 23, 3053 CrossRef CAS; J. Yin, Y. Zhou, T. Lei and J. Pei, Angew. Chem., Int. Ed., 2011, 50, 6320 CrossRef.
- Y.-T. Tsai, G. Raffy, H.-F. Liu, B.-J. Peng, K.-P. Tseng, L. Hirsch, A. Del Guerzo, D. M. Bassani and K.-T. Wong, Mater. Chem. Front., 2020, 4, 845 RSC.
- T. Ghosh, J. S. Panicker and V. C. Nair, Polymers, 2017, 9, 112 CrossRef CAS; X. Zhou, W. Tang, P. Bi, L. Yan, X. Wang, W.-K. Wong, X. Hao, B. S. Ong and X. Zhu, J. Mater. Chem. A, 2018, 6, 14675 RSC.
- A. S. Tayi, A. Kaeser, M. Matsumoto, T. Aida and S. I. Stupp, Nat. Chem., 2015, 7, 281 CrossRef CAS PubMed.
- H. Kar and S. Ghosh, Isr. J. Chem., 2019, 59, 881 CrossRef CAS.
- A. R. A. Palmans and E. W. Meijer, Angew. Chem., Int. Ed., 2007, 46, 8948 CrossRef CAS PubMed; T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813 CrossRef.
- K. Sugiyasu, S. Kawano, N. Fujita and S. Shinkai, Chem. Mater., 2008, 20, 2863 CrossRef CAS; P. Jonkheijm, N. Stutzmann, Z. J. Chen, D. M. de Leeuw, E. W. Meijer, A. Schenning and F. Würthner, J. Am. Chem. Soc., 2006, 128, 9535 CrossRef; M. R. Molla, A. Das and S. Ghosh, Chem. – Eur. J., 2010, 16, 10084 CrossRef PubMed; S. Onogi, H. Shigemitsu, T. Yoshii, T. Tanida, M. Ikeda, R. Kubota and I. Hamachi, Nat. Chem., 2016, 8, 743 CrossRef; M. Lista, J. Areephong, N. Sakai and S. Matile, J. Am. Chem. Soc., 2011, 133, 15228 CrossRef.
- X. Zhang, Z. Chen and F. Würthner, J. Am. Chem. Soc., 2007, 129, 4886 CrossRef CAS.
- A. de la Escosura, M. V. Martínez-Díaz, P. Thordarson, A. E. Rowan, R. J. M. Nolte and T. Torres, J. Am. Chem. Soc., 2003, 125, 12300 CrossRef CAS PubMed; M. Yoshizawa, J. Nakagawa, K. Kurnazawa, M. Nagao, M. Kawano, T. Ozeki and M. Fujita, Angew. Chem., Int. Ed., 2005, 44, 1810 CrossRef.
- B. Narayan, K. K. Bejagam, S. Balasubramanian and S. J. George, Angew. Chem., Int. Ed., 2015, 54, 13053 CrossRef CAS PubMed.
- M. de Loos, J. van Esch, R. M. Kellogg and B. L. Feringa, Angew. Chem., Int. Ed., 2001, 40, 613 CrossRef CAS PubMed; W. Makiguchi, J. Tanabe, H. Yamada, H. Iida, D. Taura, N. Ousaka and E. Yashima, Nat. Commun., 2015, 6, 7236 CrossRef PubMed.
- L. J. Prins, J. Huskens, F. de Jong, P. Timmerman and D. N. Reinhoudt, Nature, 1999, 398, 498 CrossRef CAS; H. Jędrzejewska, M. Wierzbicki, P. Cmoch, K. Rissanen and A. Szumna, Angew. Chem., Int. Ed., 2014, 53, 13760 CrossRef PubMed.
- M. Hecht, P. Leowanawat, T. Gerlach, V. Stepanenko, M. Stolte, M. Lehmann and F. Würthner, Angew. Chem., Int. Ed., 2020, 59, 17084 CrossRef CAS.
- L. Pasteur, Ann. Chim. Phys., 1853, 38, 437 Search PubMed; G. Vantomme and J. Crassous, Chirality, 2021, 33, 597 CrossRef CAS PubMed.
- R. Kramer, J.-M. Lehn and A. Marquisrigault, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 5394 CrossRef CAS.
- A. X. Wu and L. Isaacs, J. Am. Chem. Soc., 2003, 125, 4831 CrossRef CAS PubMed.
- Y. Ishida and T. Aida, J. Am. Chem. Soc., 2002, 124, 14017 CrossRef CAS PubMed.
- K. Sato, Y. Itoh and T. Aida, Chem. Sci., 2014, 5, 136 RSC.
- B. W. Messmore, P. A. Sukerkar and S. I. Stupp, J. Am. Chem. Soc., 2005, 127, 7992 CrossRef CAS.
- F. Helmich, M. M. J. Smulders, C. C. Lee, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2011, 133, 12238 CrossRef CAS.
- M. Wolffs, J. L. J. van Velthoven, X. Lou, R. A. A. Bovee, M. Pouderoijen, J. L. J. van Dongen, A. P. H. J. Schenning and E. W. Meijer, Chem. – Eur. J., 2012, 18, 15057 CrossRef CAS.
- F. S. Schoonbeek, J. H. van Esch, R. Hulst, R. M. Kellogg and B. L. Feringa, Chem. – Eur. J., 2000, 6, 2633 CrossRef CAS PubMed; M. de Loos, J. van Esch, R. M. Kellogg and B. L. Feringa, Angew. Chem., Int. Ed., 2001, 40, 613 CrossRef.
- M. A. Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor and S. V. Bhosale, Chem. Rev., 2016, 116, 11685 CrossRef CAS PubMed; M. R. Molla, A. Das and S. Ghosh, Chem. – Eur. J., 2010, 16, 10084 CrossRef PubMed.
- S. Sarkar, B. Narayan and S. J. George, ChemNanoMat, 2020, 6, 1169 CrossRef CAS.
- J. P. Riehl and F. S. Richardson, Chem. Rev., 1986, 86, 1 CrossRef CAS; Circularly Polarized Luminescence of Isolated Small Organic Molecules, ed. T. Mori, Springer, Singapore, 1st edn, 2020 Search PubMed; Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2019, 31, 1900110 Search PubMed; J. Kumar, T. Nakashima and T. Kawai, J. Phys. Chem. Lett., 2015, 6, 3445 CrossRef PubMed; Circular Dichroism: Principles and Applications, ed. N. Berova, K. Nakanishi and R. W. Woody, Wiley-VCH, New York, 2nd edn, 2000 Search PubMed.
- L. Zhu and Y. Zhao, J. Mater. Chem. C, 2013, 1, 1059 RSC; Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC; B.-K. An, D.-S. Lee, J.-S. Lee, Y.-S. Park, H.-S. Song and S. Y. Park, J. Am. Chem. Soc., 2004, 126, 10232 CrossRef CAS PubMed.
- F. García, R. Gómez and L. Sánchez, Chem. Soc. Rev., 2023, 52, 7524 RSC; M. Liu, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304 CrossRef CAS; P. Xing and Y. Zhao, Acc. Chem. Res., 2018, 51, 2324 CrossRef PubMed.
- R. Sethy, J. Kumar, R. Métivier, M. Louis, K. Nakatani, N. M. T. Mecheri, A. Subhakumari, K. G. Thomas, T. Kawai and T. Nakashima, Angew. Chem., Int. Ed., 2017, 56, 15053 CrossRef CAS PubMed.
- A. Sarkar, S. Dhiman, A. Chalishazar and S. J. George, Angew. Chem., Int. Ed., 2017, 56, 13767 CrossRef CAS PubMed.
- D. Yang, J. Han, Y. Sang, T. Zhao, M. Liu and P. Duan, J. Am. Chem. Soc., 2021, 43, 13259 CrossRef PubMed.
- S. Sarkar, A. Sarkar and S. J. George, Angew. Chem., Int. Ed., 2020, 59, 19841 CrossRef CAS PubMed.
- S. Sarkar, A. Sarkar, A. Som, S. S. Agasti and S. J. George, J. Am. Chem. Soc., 2021, 143, 11777 CrossRef CAS PubMed.
- S. Garain, S. Sarkar, B. C. Garain, S. K. Pati and S. J. George, Angew. Chem., Int. Ed., 2022, 61, e202115773 CrossRef CAS PubMed.
- M. M. Safont-Sempere, P. Osswald, M. Stolte, M. Grüne, M. Renz, M. Kaupp, K. Radacki, H. Braunschweig and F. Würthner, J. Am. Chem. Soc., 2011, 133, 9580 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |