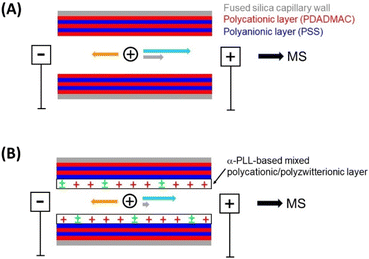
Adjusting the electroosmotic flow for CE separation of proteins by using poly(α-L-lysine)-based mixed polycationic/polyzwitterionic multilayer coatings†
Henry
Frick
ab,
Laura
Dhellemmes
c,
Alisa
Höchsmann
a,
Laurent
Leclercq
 c,
Christian
Neusüβ
c,
Christian
Neusüβ
 a,
Hervé
Cottet
a,
Hervé
Cottet
 c and
Norbert
Schaschke
c and
Norbert
Schaschke
 *a
*a
aFaculty of Chemistry, Aalen University, Beethovenstraße 1, D-73430 Aalen, Germany. E-mail: norbert.schaschke@hs-aalen.de
bDepartment of Chemistry, Bielefeld University, Bielefeld, Germany
cIBMM, University of Montpellier, CNRS, ENSCM, Montpellier, France
First published on 3rd June 2025
Abstract
Capillary electrophoresis (CE) is a robust, selective and highly efficient technique for the analysis of peptides and (intact) proteins. The anionic and/or hydrophilic character of the fused silica surface generally leads to protein adsorption by electrostatic interaction or H-bonding. Successive multiple ionic-polymer layer (SMIL) coatings are often used to limit adsorption and to improve the repeatability of migration times. Besides these adsorption phenomena, the electroosmotic flow (EOF) also has a strong influence on the resolution. Here, the frequently used and efficient cationic SMIL coating agent poly(α-L-lysine) (α-PLL) is modified for the systematic modulation of the EOF. In particular, by converting stepwise the ε-amino functions of this polycation into carboxamides, the total number of positive charges decreases, leading to reduced EOF. To simultaneously keep the analyte–surface interactions as small as possible, carboxylic acids bearing a zwitterionic functionality based structurally on a sulfobetaine motif were developed. Using the water-soluble and highly hydrolysis-resistant condensation agent 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM), the degree of functionalization could be adjusted between 8 and 99%, depending on the applied stoichiometry. The obtained set of mixed polycationic/polyzwitterionic polymers based on the α-PLL scaffold were investigated as the outermost layers of the SMIL coatings, clearly showing that the EOF decreases depending on the degree of functionalization while maintaining high efficiency.
Introduction
For the analysis of complex mixtures of peptides or proteins, capillary electrophoresis (CE) is a frequently used separation technique. CE has a variety of distinct advantages compared to liquid chromatography: high separation efficiencies, small sample volumes and fast analysis times.1,2 The separation mechanism in CE is orthogonal to reversed phase-high performance liquid chromatography (RP-HPLC). While the separation in RP-HPLC is primarily based on the hydrophobic interaction between the analyte and the stationary phase, in CE, the different electrophoretic mobilities of the analytes are exploited for separation.3 CE is also an environmentally friendly technique. Only small amounts of organic chemicals and reagents are required. In particular, the coupling of CE with mass spectrometry (MS) analysis makes it a powerful tool in the field of protein analytics, which has been highlighted in a series of studies.4–6The anionic and/or hydrophilic character of the fused silica surface generally leads to protein adsorption by electrostatic interaction or H-bonding.7 Today, the best way to circumvent the protein adsorption for CE-MS analysis is the coating of the capillary. Based on the mode of attachment, one can distinguish between dynamic and permanent capillary wall coatings.8,9 Besides neutral coatings,10 mostly cationic coatings as a single layer11–16 or in multiple ionic polymer layers (SMILs)17,18 have been used for highly efficient separation of peptides and proteins.
Katayama et al. have developed SMIL coatings in 1998.19 First, the silanol groups are deprotonated with NaOH. Then, a polycation is adhered to the inner capillary surface, followed by a polyanion, which is adsorbed by electrostatic interaction with the cationic layer. These steps can be repeated several times. Thus, SMIL coatings are generated by alternating polycationic and polyanionic layers with either a negatively or positively charged surface as the outermost layer. A broad range of polycations have been developed, such as poly(diallyldimethylammonium) chloride (PDADMAC),20 polyarginine,21 chitosan,22,23 ε-poly(L-lysine) (ε-PLL)18 and α-PLL.24 Among the possible polyanions, dextran sulfate (DS)25 and poly(sodium-4-styrenesulfonate) (PSS)18 are often used. Acidic separation conditions are often preferred in CE-MS to facilitate ionization under positive electrospray conditions. In this case, the outermost layer should be positively charged, typically applying three or five homopolymer layers.26,27 In the last few years, different parameters, such as the number and the thickness of the polyelectrolyte layers, were investigated for CE and CE-MS separation of proteins using SMIL coated capillaries.26,28–32 Besides reduced adsorption, the coating is important to control the electroosmotic flow (EOF) in order to achieve the best resolution by counterbalancing the analyte mobility.33
In this work, we present novel mixed polycationic/polyzwitterionic polymers used as the outermost layer interacting with the protein analytes (Fig. 1). Sulfobetaine moieties with their anti-adsorptive properties have a net charge of zero and are introduced via an amide bond into the polycation. Thus, the adjustment of the EOF is achieved by replacing a defined number of positive charges of the polycation by introducing zwitterionic building blocks. The best polycations for SMIL coatings, however, are mostly polymers containing quaternary or tertiary amino groups and thus cannot be derivatized with the typical coupling reagents. Therefore, this work is focused on the functionalisation of α-PLL, one of the few polymers with primary amino groups and good properties as a cationic layer for SMIL coatings.18,24,26
Experimental
Materials
N,N-Dimethylglycine ethyl ester, 4-(dimethylamino)butyric acid hydrochloride, N-methylmorpholine (NMM), and DMTMM were purchased from Tokyo Chemical Industry (TCI). Methyl 3-(dimethylamino)propionate was purchased from Sigma-Aldrich. 1,3-Propanesultone was purchased from Thermo Fisher Scientific. α-PLL hydrochloride (average MW = 66.000 Da) with a polydispersity index (PDI) between 1.0 and 1.2 was purchased from Alamanda Polymers. 2-[4-(2-Hydroxy-ethyl)piperazine-1-yl]ethane sulfonic acid (HEPES) and acetic acid were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). The model proteins, carbonic anhydrase I from human erythrocytes (CA, purity not indicated by the supplier), myoglobin from equine skeletal muscle (Myo, purity ≥95%), ribonuclease A from bovine pancreas (RNAse A, purity ≥60%), β-lactoglobulin A from bovine milk (β-lac A, purity ≥90%), and lysozyme from chicken egg white (Lyz, purity ≥90%) were purchased from Sigma-Aldrich (Saint-Quentin Fallavier, France). Ultrapure water was obtained using a MilliQ system from Millipore (Molsheim, France). PDADMAC (Mr 4 × 105–5 × 105, 20% w/w in water) was purchased from Sigma-Aldrich (Lyon, France). PSS (Mr 7 × 104) was purchased from Acros Organics (Geel, Belgium). All other solvents and chemicals were obtained from commercial sources and used as received unless otherwise noted. Dialysis tubes (D-Tube™ Dialyzers, MWCO 3.5 kDa) were purchased from Merck KGaA. LoBind® tubes were purchased from Eppendorf.NMR spectroscopy
NMR spectra were recorded using a Bruker DRX 500 spectrometer at room temperature. Chemical shifts are reported in parts per million (ppm) calibrated using a residual non-deuterated solvent as the internal reference. All samples were dissolved in D2O (99.95% deuteration).CE-MS analysis of zwitterions 4–9
CE-MS analysis was performed using an Agilent 7100 CE coupled to a compact mass spectrometer from Bruker. Separation conditions are provided in the ESI.†Size exclusion chromatography
SEC analyses were performed using an Agilent 1260 quaternary pump at a flow rate of 0.5 mL min−1. 150 mM phosphate buffer (pH 6.8) with 750 mM NaCl was used as the mobile phase and eluted using a 7.8 × 300 mm Agilent AdvanceBio SEC column (with a guard), and the elution of the polymers was monitored with a refractive index detector (RID). The temperature of the column and the RID was set at 35 °C.Capillary electrophoresis
Electrophoretic separations were performed using an Agilent 7100 CE system. Fused silica capillaries of 50 μm in diameter and 60 cm total length (51.5 cm to the detector) were used. For most of the coatings, the electric fields applied during the separations were between −30 kV and −10 kV. Negative polarity was used due to the strong anodic (reversed) EOF and counter-electroosmotic migration of the positively charged proteins. Positive polarity was used for the coatings with a low EOF magnitude compared to protein electrophoretic mobilities. The capillary was flushed for 5 min with the BGE between each run. First, 0.001% DMF in 2 M acetic acid was injected for 3 s at 30 mbar. DMF was used as a neutral marker for EOF determination. The EOF is given as an average obtained on 25 runs ± one standard deviation. Then, the protein mixture was injected for 6 s at 30 mbar. The cassette and tray temperatures were set at 25 °C and the detection wavelength was 214 nm. Plate numbers per meter N/l were calculated using CEval software available at [https://echmet.natur.cuni.cz/]34 based on the following equation: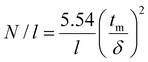 | (1) |
Capillary coating procedure
The background electrolyte (BGE) was 2 M acetic acid (pH 2.2), while the construction buffer was made from 20 mM HEPES and 10 mM NaOH (pH 7.4). PDADMAC, PSS and the polyzwitterion solutions were made by dissolving each coating agent in 3 g L−1 of HEPES buffer at least one night before the first use. The polycation and zwitterion solutions were kept in the freezer while the polyanions could be kept in the fridge. The protein mixture was prepared from the stock solutions of proteins stored individually in water at a higher concentration. 100 μL aliquots were prepared by mixing 10 μL of each protein solution with 50 μL of 2 M acetic acid so that each protein was at a final concentration of 0.2 g L−1 in BGE. The protein sample was placed in an oven for 30 min at 37 °C in order to reach more stable protein conformations.35 Before coating, the silica capillary was preconditioned by flushing with 1 M NaOH for 10 min and then with water for 5 min and HEPES for 10 min. Next, PDADMAC was flushed for 7 min, HEPES for 3 min, PSS for 7 min, HEPES for 3 min, and so on, until 4 layers were formed. The polyzwitterion was flushed for 7 min as the outermost layer. Then, HEPES was flushed for 3 min, and after 5 min, ultrapure water was flushed for 3 min and BGE for 10 min. Analyses were performed after standing for 10 min. All flushes were performed at 930 mbar. SMILs are designated as (A/B)i, where A refers to the polycation, B refers to the polyanion, and i refers to the number of bilayers.Synthesis of zwitterions
To generate the free amine, 8.0 g of hydrochloride was partitioned between EtOAc and 2 M NaOH and extracted again with a copious amount of EtOAc. The combined organic layers were washed with brine and dried over MgSO4. The solvent was removed under reduced pressure to give a colourless liquid which was used without purification (yield: 3.7 g, 57%).
3-[(2-Ethoxy-2-oxoethyl)(dimethyl)azaniumyl]propane-1-sulfonate (4). Yield: 7.4 g (94%); 1H NMR (500 MHz, D2O): δ = 1.19 (t, J = 7.2 Hz, 3 H), 2.14 (m, 2 H), 2.87 (t, J = 7.1 Hz, 2 H), 3.21 (s, 6 H), 3.63 (m, 2 H), 4.20 (q, J = 7.2 Hz, 4 H); 13C NMR (125 MHz, D2O): 12.5, 17.7, 46.6, 51.2, 61.0, 62.9, 63.2, 164.5; CE-MS (ESI) m/z calcd for [M + H]+ 254.098, found [M + H]+ 254.107; m/z calcd for [2M + H]+ 507.196, found [2M + H]+ 507.205; m/z calcd for [3M + Na]+ 782.283, found [3M + Na]+ 782.282.
3-((3-Methoxy-3-oxopropyl)dimethylamino)propane-1-sulfonate (5). Yield: 7.5 g (93%); 1H NMR (500 MHz, D2O): δ = 2.12 (m, 2 H), 2.88 (m, 4 H), 3.02 (s, 6 H), 3.38 (m, 2 H), 3.59 (t, J = 7.5 Hz, 2 H), 3.63 (s, 3 H); 13C NMR (125 MHz, D2O): 17.6, 27.0, 46.6, 50.1, 52.3, 58.7, 62.0, 171.3; CE-MS (ESI) m/z calcd for [M + H]+ 254.098, found [M + H]+ 254.107; m/z calcd for [2M + H]+ 507.196, found [2M + H]+ 507.205; m/z calcd for [3M + Na]+ 782.283, found [3M + Na]+ 782.282.
3-((4-Methoxy-4-oxobutyl)dimethylammonio)propane-1-sulfonate (6). Yield: 7.4 g (91%); 1H NMR (500 MHz, D2O): δ = 1.96 (m, 2 H), 2.10 (m, 2 H), 2.40 (t, J = 7.0 Hz, 2H), 2.86 (t, J = 7.1 Hz, 2 H), 3.00 (s, 6 H), 3.25 (m, 2 H), 3.36 (m, 2 H), 3.59 (s, 3 H); 13C NMR (125 MHz, D2O): 16.9, 17.5, 29.3, 46.7, 50.1, 51.8, 61.6, 62.3, 174.4; CE-MS (ESI) m/z calcd for [M + H]+ 268.121, found [M + H]+ 268.123; m/z calcd for [2M + H]+ 535.243, found [2M + H]+ 535.235; m/z calcd for [3M + Na]+ 824.364 found [3M + Na]+ 824.328.
3-((Carboxymethyl)dimethylammonio)propane-1-sulfonate (7). Yield: 4.5 g (96%); 1H NMR (500 MHz, D2O): δ = 2.12 (m, 2 H), 2.86 (t, J = 7.1 Hz, 2 H), 3.18 (s, 6 H), 3.61 (m, 2 H), 4.11 (s, 2 H); 13C NMR (125 MHz, D2O): 17.7, 46.7, 50.9, 61.3, 63.0, 166.6; CE-MS (ESI) m/z calcd for [M + H]+ 226.074, found [M + H]+ 226.076; m/z calcd for [2M + H]+ 451.148, found [2M + H]+ 451.143.
3-((2-Carboxyethyl)dimethylammonio)propane-1-sulfonate (8). Yield: 3.6 g (94%); 1H NMR (500 MHz, D2O): δ = 2.13 (m, 2 H), 2.86 (m, 4 H), 3.02 (s, 6 H), 3.39 (m, 2 H), 3.57 (t, J = 7.2 Hz, 2 H); 13C NMR (125 MHz, D2O): 17.6, 27.1, 46.6, 50.1, 58.9, 62.0, 172.6; CE-MS (ESI) m/z calcd for [M + H]+ 240.090, found [M + H]+ 240.091; m/z calcd for [2M + H]+ 479.180, found [2M + H]+ 479.172; m/z calcd for [3M + H]+ 718.270, found [3M + H]+ 718.251.
3-((3-Carboxypropyl)dimethylammonio)propane-1-sulfonate (9). Yield: 3.3 g (86%); 1H NMR (500 MHz, D2O): δ = 1.97 (m, 2 H), 2.12 (m, 2 H), 2.39 (t, J = 7.0 Hz, 2 H), 2.87 (t, J = 7.1 Hz, 2 H), 3.01 (s, 6 H), 3.26 (m, 2 H), 3.38 (m, 2 H); 13C NMR (125 MHz, D2O): 16.9, 17.5, 29.4, 46.7, 50.1, 61.7, 62.4, 175.8; CE-MS (ESI) m/z calcd for [M + H]+ 254.212, found [M + H]+ 254.107; m/z calcd for [2M + H]+ 507.196, found [2M + H]+ 507.204; m/z calcd for [3M + H]+ 760.636, found [3M + H]+ 760.318.
General procedure for the functionalization of α-PLL
In a 5 mL LoBind® tube, α-PLL hydrochloride (10 mg) was dissolved in H2O/MeOH (800 μL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). In another 2 mL LoBind® tube, the zwitterion was dissolved in H2O/MeOH (200 μL, 1
v). In another 2 mL LoBind® tube, the zwitterion was dissolved in H2O/MeOH (200 μL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). After the addition of 2 equivalents of NMM, the reaction mixture was stirred for 5 min at room temperature. The deprotonated zwitterion and the coupling reagent DMTMM were added to the α-PLL solution in the desired stoichiometric ratio (see Table 1) under vigorous stirring. The reaction was allowed to proceed overnight at room temperature, followed by dialysis against 0.5 M sodium perchlorate for 48 h. The dialyzed polymer was lyophilized for 48 h.
v). After the addition of 2 equivalents of NMM, the reaction mixture was stirred for 5 min at room temperature. The deprotonated zwitterion and the coupling reagent DMTMM were added to the α-PLL solution in the desired stoichiometric ratio (see Table 1) under vigorous stirring. The reaction was allowed to proceed overnight at room temperature, followed by dialysis against 0.5 M sodium perchlorate for 48 h. The dialyzed polymer was lyophilized for 48 h.
| Entry | Polymera | Applied equiv. of zwitterion/DMTMM/NMM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
Degree of functionalizationb (%) | N/lc (103 plates per m) |
|---|---|---|---|---|
| a Reactions were performed twice, yielding almost the same degree of functionalization, respectively. Exemplary, this is shown for polymers 10b (a low degree of functionalization) and 12d (a high degree of functionalization). b Determined by 1H NMR (500 MHz) using D2O as a solvent. c Separation efficiencies of Lyz obtained on PDADMAC/PSS SMILs with different final layers. Data are shown for −10 kV runs except for polymer 10g, which is shown for −20 kV. | ||||
| 1 | 10a | 0.5 | 8 | 316 |
| 2 | 10ba | 1.0 | 17 | — |
| 3 | 10bb | 1.0 | 19 | 193 |
| 4 | 10c | 1.5 | 29 | 202 |
| 5 | 10d | 1.75 | 38 | 234 |
| 6 | 10e | 2.0 | 50 | 182 |
| 7 | 10f | 3.0 | 71 | Not applicable |
| 8 | 10g | 4.0 | 88 | 104 |
| 9 | 11a | 0.5 | 17 | — |
| 10 | 11b | 1.0 | 25 | 173 |
| 11 | 11c | 2.0 | 25 | — |
| 12 | 12a | 0.25 | 11 | 316 |
| 13 | 12b | 0.5 | 31 | 188 |
| 14 | 12c | 0.75 | 51 | 293 |
| 15 | 12da | 1.0 | 60 | — |
| 16 | 12db | 1.0 | 63 | 49 |
| 17 | 12e | 2.0 | 99 | 44 |
| 18 | PDADMAC | — | — | 367 |
Results and discussion
Synthesis of zwitterionic building blocks
The zwitterionic motif consists of a quaternary amine and a sulfonic acid function (Fig. 2). The difference between these molecules lies in the length of the alkyl chain between the carboxylic acid and the quaternary amino function. Such molecules with zwitterionic characters have been known for a long time in biochemistry as buffer agents.36 Also for SMIL coatings, polyzwitterionic polymers are used to reduce the adsorption of proteins onto the wall of fused silica capillaries.37–39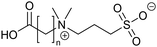 | ||
| Fig. 2 Chemical structures of zwitterions 7–9 used for the functionalization of α-PLL (7: n = 1, 8: n = 2, and 9: n = 3). | ||
Sultones, cyclic esters of sulfonic acids, are versatile substances that react under mild conditions with a variety of nucleophiles under ring-opening, yielding the corresponding alkylsulfonic acids.40 We took advantage of this particular reactivity of sultones for the synthesis of zwitterions 7–9 (Scheme 1). Due to severe solubility problems of the carboxylic acid salts of these ω-dimethylamino acids in acetone and, furthermore, to avoid side reactions originating from the nucleophilic character of the carboxylate function during the quaternalization reaction,41 the corresponding methyl or ethyl esters 1–3 were utilized as nucleophiles. The ring-opening reactions of 1,3-propanesultone with nucleophiles 1–3 result directly in the formation of tert-alkylammonium sulfonates 4–6, respectively. Furthermore, it turned out that these tert-alkylammonium sulfonates 4–6 precipitate from acetone during the reaction and, thus, can be easily isolated by filtration as pure compounds in excellent yields.
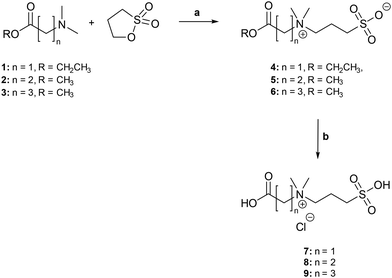 | ||
| Scheme 1 Synthesis of zwitterions 7–9. Reagents and conditions: (a) acetone, 50 °C, overnight, 4: 94%, 5: 93%, 6: 91%; (b): 6 M HCl, 70 °C, 24 h, 7: 96%, 8: 94%, 9: 86%. | ||
The needed methyl ester 3 was prepared from 4-(dimethylamino)butyric acid hydrochloride. For this, the amino acid was quantitatively converted into its corresponding methyl ester in the presence of methanol and an excess amount of SOCl2.42 Eventually, by treating the thus obtained hydrochloride with 2 M NaOH, the free amine 3 could be isolated by extracting it into ethyl acetate.
The three synthesized zwitterionic molecules 4–6 are protected as esters. Thus, to generate free carboxylic acid functions, first of all, saponification with NaOH was investigated. Due to serious difficulties in isolating fully water-soluble acids 7–9 free from salts after the saponification process, we decided to employ acid hydrolysis (Scheme 1). Using 6 M HCl at 70 °C, cleavage of the esters proceeded smoothly and the zwitterionic building blocks 7–9 could be isolated free from by-products without any problems as confirmed by means of CE-MS and NMR spectroscopy. After the successful synthesis of the zwitterionic molecules 7–9, we could proceed with the preparation of mixed cationic/zwitterionic polymers by derivatization of the ε-amino functions of the lysine residues of α-PLL.
Functionalization of α-PLL with zwitterions 7–9
Due to the fact that both α-PLL and zwitterions 7–9 are almost exclusively water soluble, it was necessary to find a coupling chemistry for the functionalization of the polymer that shows a high degree of water tolerance. Among the coupling reagents typically used for amide bond formation that combine both water solubility and a high extent of hydrolysis resistance, the recently developed 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM) is most suitable.43–45 Compared to the 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide-hydrochloride (EDC)/N-hydroxysuccinimide (NHS) or N-hydroxysulfo-succinimide (sulfo-NHS) system, which is frequently used for labelling or crosslinking peptides, proteins or biomolecular probes in water,46–48 DMTMM has significantly better stability in water. While EDC has a half-life of 3.9 h in water at a pH of 5.0,49 DMTMM is 24 h stable in water without any decomposition by demethylation or hydrolysis.43 Moreover, DMTMM is more efficient and easier to handle than the EDC/NHS system.45,50,51Initially, for the functionalization of α-PLL, a pre-activation approach was chosen. For this, the zwitterions 7–9 were transformed with DMTMM into the corresponding triazine esters and then reacted with the completely deprotonated α-PLL, respectively. It turned out that under these reaction conditions, a gel-like solid was formed immediately after the addition of the active ester, preventing the formation of any condensation product. This behaviour of α-PLL in basic aqueous solutions at higher concentrations is in good agreement with previous observations made by Hull et al.52 and later confirmed by Dos et al.,53 both of which investigated α-PLL by 15N NMR spectroscopy. They found that α-PLL forms a gel-like solid which is built up by α-helices that assemble into higher aggregates in the pH range from 9.2 to 11.4.
To circumvent these severe solubility problems with α-PLL arising under basic aqueous conditions, a stepwise liberation of the ε-amino function in terms of a titration approach was selected (Scheme 2). For this, the condensing agent DMTMM provides the prerequisites. In this one-pot approach, the carboxylates of zwitterions 7–9 which were obtained upon treatment with two equivalents of NMM and α-PLL hydrochloride were dissolved in MeOH/H2O (v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the desired stoichiometric ratio to adjust the degree of functionalization, followed by DMTMM. Under these conditions, first of all, the carboxylates of zwitterions 7–9 are readily converted by DMTMM into their corresponding triazine esters. Along the formation of this active ester, NMM acts as a leaving group and deprotonates in situ in a stepwise manner the ε-amino functions of α-PLL hydrochloride, which then react immediately with the active ester to give carboxamides. Thus, under these one-pot reaction conditions, the coupling reaction with DMTMM proceeds completely under neutral conditions and no additional base is necessary to deprotonate the ε-amino function of α-PLL hydrochloride. Therefore, one does not run into aggregation problems caused by the helix formation of α-PLL. The initial attempts to purify the thus obtained mixed polycationic/polyzwitterionic polymers by dialysis were not successful. Even after 48 h by dialysis against 0.001 M hydrochloric acid or 1 M acetate buffer, the excess of zwitterion could not be removed completely. To break up the strong ionic interactions between the non-functionalized protonated ε-amino function of α-PLL and the sulfonate residues of the excess zwitterions, only dialysis for 48 h against sodium perchlorate was successful.
1) in the desired stoichiometric ratio to adjust the degree of functionalization, followed by DMTMM. Under these conditions, first of all, the carboxylates of zwitterions 7–9 are readily converted by DMTMM into their corresponding triazine esters. Along the formation of this active ester, NMM acts as a leaving group and deprotonates in situ in a stepwise manner the ε-amino functions of α-PLL hydrochloride, which then react immediately with the active ester to give carboxamides. Thus, under these one-pot reaction conditions, the coupling reaction with DMTMM proceeds completely under neutral conditions and no additional base is necessary to deprotonate the ε-amino function of α-PLL hydrochloride. Therefore, one does not run into aggregation problems caused by the helix formation of α-PLL. The initial attempts to purify the thus obtained mixed polycationic/polyzwitterionic polymers by dialysis were not successful. Even after 48 h by dialysis against 0.001 M hydrochloric acid or 1 M acetate buffer, the excess of zwitterion could not be removed completely. To break up the strong ionic interactions between the non-functionalized protonated ε-amino function of α-PLL and the sulfonate residues of the excess zwitterions, only dialysis for 48 h against sodium perchlorate was successful.
 | ||
Scheme 2 Functionalization of α-PLL. Reagents and conditions: (a): Zwitterions 7–9/DTMMM/NMM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 0.25–4 equiv.); H2O/MeOH (1 2; 0.25–4 equiv.); H2O/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v 1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v), rt, overnight and (b): dialysis against NaClO4, 48 h. v), rt, overnight and (b): dialysis against NaClO4, 48 h. | ||
This one-pot condensation approach between the ε-amino groups of α-PLL and the carboxyl groups of zwitterionic molecules 7–9 yields a set of mixed polycationic/polyzwitterionic polymers with different degrees of functionalization (10a–g, 11a–c, and 12a–e), which are summarized in Table 1. The degree of functionalization was determined from the ratio of the integrals of the signals of the α-CH protons of the lysine residues of α-PLL and the α-CH2 protons of the respective spacer amino acid (degree of functionalization = [I(α-CH2 X)/(I(α-CH Lys) × 2)] × 100%, X = Gly, β-alanine, and γ-aminobutyric acid) by 1H NMR spectroscopy. The obtained data clearly show that the achieved degree of functionalization depends on both the applied excess of the activated zwitterion for the coupling reaction and the length of the respective spacer amino acid. In particular, in the case of zwitterion 7, the degree of functionalization increases stepwise from 8% (Table 1, entry 1, polymer 10a) to 88% (Table 1, entry 8, polymer 10g). Similar results were obtained with the zwitterion 9 and to a lesser extent with the zwitterion 8. The expected influence of the length of the spacer amino acid on the functionalization efficiency shows the comparison of zwitterions 7 (spacer: glycine) and 9 (spacer: γ-aminobutyric acid) clearly. While 4.0 equiv. of activated zwitterion 7 lead only to a functionalization of 88% (Table 1, entry 8), 2.0 equiv. of activated zwitterion 9 are completely sufficient for an almost complete functionalization of α-PLL (Table 1, entry 17). Surprisingly, the activated zwitterion 8 (spacer: β-alanine) shows in comparison with the activated zwitterions 7 and 9 a different functionalization behaviour. It turned out that the degree of functionalization stops at 25% (Table 1, entry 10 vs. entry 11).
Characterization of the α-PLL-based mixed polycationic/poly-zwitterionic polymers by NMR spectroscopy and SEC
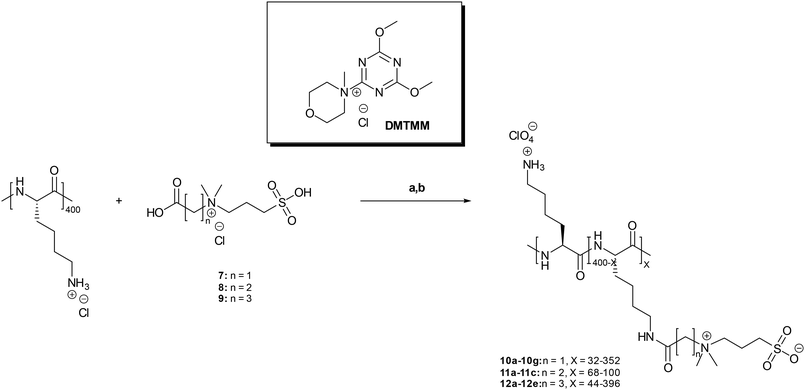
Due to the fact that an almost complete coverage of the degree of side chain functionalization of α-PLL was obtained using the zwitterion 9 for the derivatisation step, we chose this series of polymers (compounds 12a–12e) to investigate in detail the impact of the functionalization on the structure of the α-PLL scaffold by 1H NMR spectroscopy. For this, we selected α-PLLs with 31% (12b), 63% (12db) and 99% (12e) side-chain functionalizations as prominent representatives. Fig. 3 shows the 1H NMR spectra of these polymers. Based on a combination of COSY and NOESY spectra, all signals in the 1H NMR spectra could be assigned. First of all, the inspection of the spectra shows that the complexity reflecting the fact that the functionalization occurs randomly decreases with increasing degree of the functionalization of the ε-NH2 functions of the side chains of α-PLL from 12b over 12db to 12e as the end point which represents a completely functionalized species. A measure for the increasing degree of functionalization are the ε-CH2 protons of the lysine side chains with a chemical shift at 3.23 ppm. The intensity of this signal decreases from 12b to 12db and has disappeared completely in the case of 12e. Upon transformation of the ε-NH2 function into the corresponding carboxamide, the signal of the ε-CH2 protons of the lysine side chains shifts towards 3.35 ppm. Expectedly, for the complete functionalization, the signal of ε-CH2 near the free amino function at 3.23 ppm disappears completely and only the new signal at 3.35 ppm was observed. | ||
| Fig. 3 1H NMR spectra (500 MHz, D2O) of α-PLL functionalized with zwitterion 9. (A) Structures of polymers 12a–12e, X = 44–396. (B) 31%, (C) 63%, and (D) 99% functionalization. | ||
Because of the difficulties in removing the excess amount of 9 used in the functionalization step (vide supra) and thus to completely exclude non-covalent interactions between the ε-NH2 functions of the side chains of α-PLL and the sulfonate residues of 9 and to corroborate the covalent amide linkage, a 2D-NOESY experiment of 12e was carried out. As expected for an amide linkage, the NOE between the α-CH2 GABA protons at 2.50 ppm of the zwitterionic segment and the ε-CH2 protons of the lysine side chain at 3.34 ppm could be detected (see the ESI†).
In addition to the characterization of the functionalized α-PLLs 12b, 12db, and 12e by 1D and 2D 1H NMR, size exclusion chromatography (SEC) was also performed. We used both aqueous acetate buffer (1 M, pH 4.65) and aqueous phosphate buffer (150 mM, pH 6.8) as eluents with different concentrations of sodium chloride as additive which were demonstrated in the literature as suitable eluents for the SEC analysis of α-PLL.54,55 Surprisingly, when acetate buffer (1 M, pH 4.65) was used for the elution of the functionalized polymers, no peaks were detected. Based on these findings, phosphate buffer (150 mM, pH 6.8) with different concentrations of sodium chloride was used. Interestingly, with low concentrations of sodium chloride (250 mM and 400 mM), only α-PLLs with a high degree of side chain substitutions (12db: 63% and 12e: 99%) could be eluted as very broad peaks, probably due to their strong interactions with the SEC phase. With higher salt concentrations (750 mM), the interaction with the SEC matrix could be further reduced and polymer 12b with 31% functionalization could also be eluted (Fig. 4). It turned out that the elution time decreases from 11.8 min in the case of 12b to 11.2 min in the case of 12e with increasing degree of functionalization.
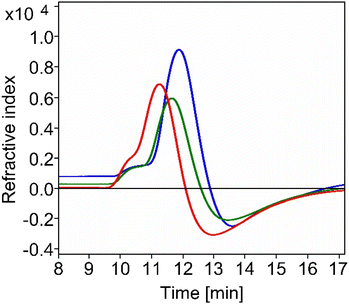 | ||
Fig. 4 SEC analysis of the functionalized α-PLLs 12b ( ), 12db ( ), 12db ( ), and 12e ( ), and 12e ( ). Eluent: 150 mM phosphate buffer with 750 mM NaCl at pH = 6.8; flow rate: 0.5 mL min−1; temperature: 35 °C. ). Eluent: 150 mM phosphate buffer with 750 mM NaCl at pH = 6.8; flow rate: 0.5 mL min−1; temperature: 35 °C. | ||
Polyzwitterionic coatings in CE
Previous work has shown that very high separation efficiencies could be obtained with (PDADMAC/PSS)2.5 coatings under optimized conditions.35 Nevertheless, the plate height still appeared to increase slightly with the electric field, an effect that has been attributed to analyte adsorption and coating inhomogeneity.56 Furthermore, the best resolution in CE is obtained when the EOF is close to the mobility of the proteins of interest along with minimized protein adsorption. Therefore, we applied the novel mixed zwitterionic/cationic polymers as a final layer in a 5-layer SMIL approach, as shown in Fig. 1.Protein separations were carried out on each modified SMIL, leading to the plate numbers provided in Table 1. The classical (PDADMAC/PSS)2.5 coating led to the most efficient separations, followed closely by polymers 10a and 12a. Overall, the polymers with low percentages of derivatization lead to satisfactory efficiencies, while the ones that are highly derivatized (above 60%) result in much poorer performance. Fig. 5 shows the impact of the percentage of α-PLL derivatization on the EOF. The EOF decreases as the amount of zwitterion present in the coating increases, which is coherent since the overall surface charge decreases. The type of polymer, 10 to 12, does not seem to have much influence on both the separation efficiency and the magnitude of the EOF. The 71% derivatized polymer, 10f, was not able to analyze any proteins because it generated a too low EOF to transport the proteins to the detector. Similarly, the two most derivatized polymers, 10g and 12e, led to EOF magnitudes that were lower than the proteins’ electrophoretic mobilities, and so the separations had to be carried out with a positive polarity, while all the other coatings were used with a negative polarity.
Coatings containing inner layers of polyzwitterions instead of PDADMAC (as in (polyzwitterion/PSS)2.5) were also tested but resulted in an unstable film and poor migration time repeatability (data not shown). When the polyzwitterion was used in two layers with PDADMAC as the first layer (as in PDADMAC1(PSS/polyzwitterion)2), the stability improved and the separation performance was similar to those of (PDADMAC/PSS)2polyzwitterion1 coatings, with small modifications in the EOF. This is coherent with studies that find that the impact of the innermost and outermost layers is more important than that of the intermediate layers of a SMIL.57 These coatings were not further investigated and only the results obtained with SMILs terminating with a layer of polyzwitterion were included in this work.
Therefore, introducing polymers with different percentages of derivatization allows for the precise control of the EOF. A similar approach using polycations modified with poly(ethylene glycol) (PEG) as the outermost layer of SMIL coatings was recently presented, enabling highly suppressed EOF as low as 2 TU and good separation efficiency (200 × 103 plates per m for Lyz).24 The polyzwitterionic coating with up to 50% derivatization could reduce the EOF by just 4 TU but led to more efficient separation (Table 1). Nevertheless, this is already interesting for certain applications, namely in CE-MS, where better resolution can be achieved with even a slightly lower EOF.58 Polycations leading to reduced EOFs such as a quaternized diethylaminoethyl dextran (DEAEDq) are sought after for use in CE-MS applications. Indeed, a DEADq/poly(L-lysine citramide) (PLC) SMIL generating an EOF of −43.3 TU was able to separate glycoforms of ribonuclease B.18 Hence, SMILs modified with polyzwitterions offer an interesting alternative to these methods.
Conclusions
Based on α-PLL, which is frequently used as the outermost cationic layer in SMIL coatings to suppress analyte–surface interactions in CE separations of proteins, a series of mixed polycations/polyzwitterions were synthesized. The zwitterionic functionalities structurally based on sulfobetaines were grafted by amide linkages using the ε-amino functions of the polymer as attachment points. DMTMM as a water-soluble and highly hydrolysis resistant condensation agent was the key for the successful functionalization of α-PLL in water/methanol mixtures as solvents, which also completely suppresses the undesired formation of aggregates. The degree of functionalization can be fully controlled by the applied stoichiometry for each polymer. Using these novel mixed polycationic/polyzwitterionic polymers as the outermost layer in SMIL coatings, efficient separation is obtained. Moreover, it could be clearly demonstrated that the higher the degree of zwitterionic functionalization, the more the EOF decreases. These findings should give access to tailor-made conditions for the challenging separation of charge variants of therapeutic antibodies as well as any other separation issues of closely related proteins/proteoforms.Author contributions
Henry Frick: conceptualization, data curation, formal analysis, investigation, validation, and writing – original draft. Laura Dhellemmes: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, and writing – original draft. Alisa Höchsmann: investigation, data curation, formal analysis, and writing – review & editing. Laurent Leclercq: conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, and writing – review & editing. Christian Neusüβ: funding acquisition, methodology, project administration, supervision, validation, and writing – review & editing. Hervé Cottet: conceptualization, funding acquisition, methodology, project administration, supervision, validation, and writing – review & editing. Norbert Schaschke: conceptualization, funding acquisition, methodology, project administration, supervision, validation, and writing – review & editing.Data availability
All data are available either in the manuscript or in its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the DFG (grant: SCHA 1012/4-1).References
- J. S. Creamer, N. J. Oborny and S. M. Lunte, Anal. Methods, 2014, 6, 5427–5449 RSC.
- G. K. E. Scriba and A. Psurek, Methods Mol. Biol., 2008, 384, 483–506 CAS.
- M. Pelzing and C. Neusüss, Electrophoresis, 2005, 26, 2717–2728 CrossRef CAS PubMed.
- A. Staub, D. Guillarme, J. Schappler, J.-L. Veuthey and S. Rudaz, J. Pharm. Biomed. Anal., 2011, 55, 810–822 CrossRef CAS PubMed.
- S. S. Zhao and D. D. Y. Chen, Electrophoresis, 2014, 35, 96–108 CrossRef CAS PubMed.
- J. Hernández-Borges, C. Neusüss, A. Cifuentes and M. Pelzing, Electrophoresis, 2004, 25, 2257–2281 CrossRef PubMed.
- T. Wehr, R. Rodríguez-Díaz and M. Zhu, Capillary electrophoresis of proteins, Marcel Dekker, New York, 1999 Search PubMed.
- J. Horvath and V. Dolník, Electrophoresis, 2001, 22, 644–655 CrossRef CAS PubMed.
- V. Dolník, Electrophoresis, 2004, 25, 3589–3601 CrossRef PubMed.
- F. H. Navarro, J. E. Gómez, J. H. Espinal and J. E. Sandoval, Anal. Chim. Acta, 2016, 948, 104–112 CrossRef CAS PubMed.
- E. Córdova, J. Gao and G. M. Whitesides, Anal. Chem., 1997, 69, 1370–1379 CrossRef PubMed.
- J. Znaleziona, D. Drahoňovský, B. Drahoš, J. Ševčík and V. Maier, J. Sep. Sci., 2016, 39, 2406–2412 CrossRef CAS PubMed.
- J. P. Quirino, J. Sep. Sci., 2017, 40, 4835–4838 Search PubMed.
- R. Sebastiano, M. E. Mendieta, N. Contiello, A. Citterio and P. G. Righetti, Electrophoresis, 2009, 30, 2313–2320 CrossRef CAS PubMed.
- S. Ullsten, A. Zuberovic and J. Bergquist, Methods Mol. Biol., 2008, 384, 631–646 CAS.
- N. Hamidli, M. Andrasi, C. Nagy and A. Gaspar, J. Chromatogr. A, 2021, 1654, 462448 CrossRef CAS PubMed.
- F. Kitagawa, M. Kamiya, Y. Okamoto, H. Taji, S. Onoue, Y. Tsuda and K. Otsuka, Anal. Bioanal. Chem., 2006, 386, 594–601 CrossRef CAS PubMed.
- L. Leclercq, M. Morvan, J. Koch, C. Neusüβ and H. Cottet, Anal. Chim. Acta, 2019, 1057, 152–161 CrossRef CAS PubMed.
- H. Katayama, Y. Ishihama and N. Asakawa, Anal. Chem., 1998, 70, 2254–2260 CrossRef CAS PubMed.
- Q. Liu, F. Lin and R. A. Hartwick, J. Chromatogr. Sci., 1997, 35, 126–130 CAS.
- R. W. Chiu, J. C. Jimenez and C. A. Monnig, Anal. Chim. Acta, 1995, 307, 193–201 CrossRef CAS.
- X. Huang, Q. Wang and B. Huang, Talanta, 2006, 69, 463–468 CrossRef CAS PubMed.
- L. Vitali, F. Della Betta, A. C. O. Costa, F. A. S. Vaz, M. A. L. Oliveira, J. P. Vistuba, V. T. Fávere and G. A. Micke, Talanta, 2014, 123, 45–53 CrossRef CAS PubMed.
- S. Roca, L. Leclercq, P. Gonzalez, L. Dhellemmes, L. Boiteau, G. Rydzek and H. Cottet, J. Chromatogr. A, 2023, 1692, 463837 CrossRef CAS PubMed.
- R. Haselberg, G. J. de Jong and G. W. Somsen, Anal. Chim. Acta, 2010, 678, 128–134 CrossRef CAS PubMed.
- S. Bekri, L. Leclercq and H. Cottet, J. Chromatogr. A, 2015, 1399, 80–87 CrossRef CAS PubMed.
- R. Haselberg, G. J. de Jong and G. W. Somsen, J. Sep. Sci., 2009, 32, 2408–2415 CrossRef CAS PubMed.
- R. Nehmé, C. Perrin, H. Cottet, M.-D. Blanchin and H. Fabre, Electrophoresis, 2009, 30, 1888–1898 CrossRef PubMed.
- T. W. Graul and J. B. Schlenoff, Anal. Chem., 1999, 71, 4007–4013 CrossRef CAS.
- S. T. Dubas and J. B. Schlenoff, Macromolecules, 1999, 32, 8153–8160 CrossRef CAS.
- S. L. Clark, M. F. Montague and P. T. Hammond, Macromolecules, 1997, 30, 7237–7244 CrossRef CAS.
- S. T. Dubas and J. B. Schlenoff, Macromolecules, 2001, 34, 3736–3740 CrossRef CAS.
- A. Höchsmann, L. Dhellemmes, L. Leclercq, H. Cottet and C. Neusüβ, Electrophoresis, 2025, 46, 279–289 CrossRef PubMed.
- Web.natur.cuni.cz/gas, ECHMET, https://echmet.natur.cuni.cz/, (accessed 14 December 2023).
- L. Dhellemmes, L. Leclercq, A. Höchsmann, C. Neusüβ, J.-P. Biron, S. Roca and H. Cottet, J. Chromatogr. A, 2023, 1695, 463912 CrossRef CAS PubMed.
- N. E. Good, G. D. Winget, W. Winter, T. N. Connolly, S. Izawa and R. M. Singh, Biochemistry, 1966, 5, 467–477 CrossRef CAS PubMed.
- W.-H. Kuo, M.-J. Wang, H.-W. Chien, T.-C. Wei, C. Lee and W.-B. Tsai, Biomacromolecules, 2011, 12, 4348–4356 CrossRef CAS PubMed.
- F. Cao, L. Tan, L. Xiang, S. Liu and Y. Wang, J. Biomater. Sci., Polym. Ed., 2013, 24, 2058–2070 CrossRef CAS PubMed.
- L. Chen, L. Tan, S. Liu, L. Bai and Y. Wang, J. Biomater. Sci., Polym. Ed., 2014, 25, 766–785 CrossRef CAS PubMed.
- S. Mondal, Chem. Rev., 2012, 112, 5339–5355 CrossRef CAS PubMed.
- M. Borzenkov, N. Mitina, V. Lobaz and O. Hevus, J. Surfactants Deterg., 2015, 18, 133–144 CrossRef CAS.
- St. Guttmann and R. A. Boissonnas, Helv. Chim. Acta, 1958, 41, 1852–1867 CrossRef CAS.
- M. Kunishima, C. Kawachi, J. Monta, K. Terao, F. Iwasaki and S. Tani, Tetrahedron, 1999, 55, 13159–13170 CrossRef CAS.
- A. Falchi, G. Giacomelli, A. Porcheddu and M. Taddei, Synlett, 2000, 275–277 CrossRef CAS.
- M. Kunishima, C. Kawachi, K. Hioki, K. Terao and S. Tani, Tetrahedron, 2001, 57, 1551–1558 CrossRef CAS.
- A. Samavati, Z. Samavati, M. Velashjerdi, A. F. Ismail, M. H. D. Othman, G. Eisaabadi B, M. S. Abdullah, M. Bolurian and M. Bolurian, Chem. Eng. J., 2021, 420, 127655 CrossRef CAS PubMed.
- N. Olsson, P. James, C. A. K. Borrebaeck and C. Wingren, Mol. Cell. Proteomics, 2012, 11, 342–354 CrossRef PubMed.
- A. K. Bergfeld, O. M. T. Pearce, S. L. Diaz, T. Pham and A. Varki, J. Biol. Chem., 2012, 287, 28865–28881 CrossRef CAS PubMed.
- M. A. Gilles, A. Q. Hudson and C. L. Borders, Anal. Biochem., 1990, 184, 244–248 CrossRef CAS PubMed.
- A. Li, Y. Ye, P. Gong, B. Xiao and B. Jiang, J. Appl. Polym. Sci., 2023, 140, e53318 CrossRef CAS.
- M. D'Este, D. Eglin and M. Alini, Carbohydr. Polym., 2014, 108, 239–246 CrossRef PubMed.
- W. E. Hull, H. R. Kricheldorf and M. Fehrle, Biopolymers, 1978, 17, 2427–2443 CrossRef CAS.
- A. Dos, V. Schimming, S. Tosoni and H.-H. Limbach, J. Phys. Chem. B, 2008, 112, 15604–15615 CrossRef CAS PubMed.
- W. Vayaboury, O. Giani, H. Collet, A. Commeyras and F. Schué, Amino Acids, 2004, 27, 161–167 CrossRef CAS PubMed.
- K. Sato, C. E. Farquhar, J. Rodriguez and B. L. Pentelute, J. Am. Chem. Soc., 2023, 145, 12992–12997 CrossRef CAS PubMed.
- L. Dhellemmes, L. Leclercq, L. Lichtenauer, A. Höchsmann, M. Leitner, A. Ebner, M. Martin, C. Neusüβ and H. Cottet, Anal. Chem., 2024, 96, 11172–11180 CrossRef CAS PubMed.
- L. Dhellemmes, L. Leclercq, H. Frick, A. Höchsmann, N. Schaschke, C. Neusüβ and H. Cottet, J. Chromatogr. A, 2024, 1720, 464802 CrossRef CAS PubMed.
- C. Huhn, R. Ramautar, M. Wuhrer and G. W. Somsen, Anal. Bioanal. Chem., 2010, 396, 297–314 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR and HR-MS spectra of compounds 4–9 and 1H–1H NOESY NMR spectrum of polymer 12e. See DOI: https://doi.org/10.1039/d5py00122f |
| This journal is © The Royal Society of Chemistry 2025 |